Abstract.
The invasion of Toxoplasma gondii tachyzoites into the host cell results in extensive host cell signaling activation/deactivation that is usually regulated by the phosphorylation/dephosphorylation. To elucidate how T. gondii regulates host cell signal transduction, the comparative phosphoproteome of stable isotope labeling with amino acids in cell culture–labeled human foreskin fibroblast cells was analyzed. The cells were grouped (Light [L], Medium [M], and Heavy [H] groups) based on the labeling isotope weight and were infected with T. gondii for different lengths of time (L: 0 hour; M: 2 hours; and H: 6 hours). A total of 892 phosphoproteins were identified with 1,872 phosphopeptides and 1,619 phosphorylation sites. The M versus L comparison revealed 694 significantly regulated phosphopeptides (436 upregulated and 258 downregulated). The H versus L comparison revealed 592 significantly regulated phosphopeptides (146 upregulated and 446 downregulated). The H versus M comparison revealed 794 significantly regulated phosphopeptides (149 upregulated and 645 downregulated). At 2 and 6 hours post-T. gondii infection, the most predominant host cell reactions were cell cycle regulation and cytoskeletal reorganization, which might be required for the efficient invasion and multiplication of T. gondii. Similar biological process profiles but different molecular function categories of host cells infected with T. gondii for 2 and 6 hours, which suggested that the host cell processes were not affected significantly by T. gondii infection but emphasized some differences in specific cellular processes at this two time points. Western blotting verification of some significantly regulated phosphoprotein phosphorylation sites was consistent with the mass spectra data. This study provided new insights into and further understanding of pathogen–host interactions from the host cell perspective.
INTRODUCTION
Toxoplasmosis has been regarded as one of the major neglected parasitic infections in the United States.1 Toxoplasma gondii is an intracellular apicomplexan parasite that can infect almost every type of warm-blooded vertebrate nucleated cell. Toxoplasma gondii ubiquitously distributes and infects approximately one-third of the world population.2 Although T. gondii infection in healthy people is generally asymptomatic and usually induces a self-limited disease, infection in specific groups of individuals may result in severe outcomes. For example, primary infection of pregnant women may cause congenital fetal deformities, abortion or stillbirth, and infection of immune-compromised patients may result in encephalitis or disseminated infection.3
Acute T. gondii infection begins with entry into the host cell, which is accompanied by the formation of the parasitophorous vacuole (PV) for residence and development. The parasite extensively and delicately modulates the host cell to facilitate its parasitism. The parasite multiplies within the host cell and gains nutrients until egress from the host cell to begin the next cycle of infection.4 Cell signal transduction is prominently modulated by the posttranslational modification of signaling proteins, which influence and control their enzymatic activity, conformation, protein–protein interactions, and cellular localization.5 Protein phosphorylation is one of the most important posttranslational modifications and occurs through the addition of a phosphate (PO43−) group to a protein. This process affects approximately one-third of proteins, and the reversible phosphorylation of proteins regulates almost all aspects of cell life.5,6 Phosphorylation turns many enzymes on and off, thereby altering their functions and activities, and plays a significant role in a wide range of cellular processes.6
Toxoplasma gondii infection triggers extensive host cell signal transduction involved in the regulation of antiapoptosis,7 apoptosis,8,9 cell immunity,10 cell-cycle control,11 calcium regulation,12 cytoskeletal reorganization,13,14 and all other cellular processes. Phosphoproteomic analysis of Plasmodium falciparum schizonts and T. gondii tachyzoites by liquid chromatography-tandem mass spectrometry (LC-MS/MS) revealed a large number of unknown phosphorylation sites in both parasites; this analysis also provided abundant clues for the role of phosphorylation in host–pathogen interactions.15 To date, phosphoproteomic investigations of the host–parasite interaction have been focused on the parasite rather than the host cell.
Recently, our knowledge about the role of protein phosphorylation in cell signaling has accumulated rapidly due to the emergence of new technologies and increased sensitivity in phosphoproteome analysis. Recent advances in phosphopeptide isolation, such as immobilized metal affinity chromatography, TiO2, and phosphoramidate chemistry followed by LC-MS/MS, have enabled system-level studies of the proteins regulated by phosphorylation and allowed detailed analyses of the phosphoproteomes of many uni- and multicellular organisms.16,17 We report here an extensive phosphoproteome of human foreskin fibroblast (HFF) cells infected by T. gondii. The data set will be helpful for the understanding of the manipulation of the host cell by T. gondii and the roles played by different phosphoproteins in host–pathogen interactions.
METHODS
Toxoplasma gondii tachyzoites culture and isotope labeling of HFF cells.
Toxoplasma gondii RH strain tachyzoites were cultured under standard conditions in HFFs obtained from American Type Culture Collection in complete Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 1% heat-inactivated fetal calf serum (HyClone). The stable isotope labeling with amino acids in cell culture (SILAC) kit and the isotope-labeled amino acids of 12C6-L-Lys·2HCl, 12C6-L-Arg·HCl, 13C6-L-Lys·2HCl, 13C6-L-Arg·HCl, 13C615N2-L-Lys·2HCl, and 13C615N4-L-Arg·HCl were purchased from Pierce. Three groups of HFFs were cultured for six generations (doublings) in Heavy (H), Medium (M), and Light (L) SILAC medium supplemented with 13C615N4-L-Arg·HCl/13C615N2-L-Lys·2HCl, 13C6LArg·HCl/13C6-L-Lys·2HCl, and L-Arg-HCl/L-Lys-2HCl, respectively, following the method reported by Olsen and others.5 Cells labeled with the heavy weight Lys and Arg were named the H group, with the medium weight Lys and Arg were named the M group, and with the normal weight Lys and Arg were named the L group. The H, M, and L groups of cells were prepared in triplicate, and the infection of the host cells with T. gondii, harvest of the cells and phosphoproteome analysis were performed separately.
Infection of HFF cells with T. gondii RH tachyzoites.
The M and H cell groups were infected with the same number of RH tachyzoites for different time at a multiplicity of infection (parasite/cell) of 10:1. M was infected for 2 hours, H was infected for 6 hours, and L was cultured without parasite infection as the uninfected control (0 hour).
Sample preparation and MS.
After infection, the unrecruited tachyzoites and culture medium were washed off with phosphate-buffered saline (PBS) for three times. All three groups of cells were harvested with cell scrapers and sent to the Beijing Genomic Institute for total protein extraction and subsequent analysis.
Protein extraction.
All cells from the H, M, and L groups were ground to powder in liquid nitrogen. In total, 500 μl of 1% sodium dodecyl sulfate (SDS) with 1 mM phenylmethanesulfonyl fluoride (PMSF), 2 mM ethylenediaminetetraacetic acid, 10 mM NaF, 5 mM β-glycerophosphate, and 100 mM sodium pyrophosphate were added to the cell powder, and the mixture was incubated on ice for 5 minutes; dithiothreitol was added to a final concentration of 10 mM. The cell lysate was sonicated at 200 W for 15 minutes, and the supernatant was collected after centrifugation at 25,000 × g for 20 minutes. The supernatant was incubated at 56°C for 1 hour. Iodoacetamide was added to the cell lysate at a final concentration of 55 mM and kept in the dark for 45 minutes to block the cysteines. After centrifugation (25,000 × g for 20 minutes at 4°C), the supernatant was collected and precipitated by adding 4 × volumes of chilled acetone at –20°C overnight. The precipitate was washed with 1 mL of chilled acetone for a minimum of 30 minutes and then centrifuged at 25,000 × g for 20 minutes at 4°C. The supernatant was discarded, and the precipitate was air-dried. Then, the pellet was dissolved in 300 μL of 0.5 M tetraethylammonium bromide. The dissolved pellet was sonicated at 200 W for 15 minutes, and the supernatant was collected and quantified after centrifugation at 25,000 × g for 20 minutes at 4°C. This supernatant was kept as the original total protein.
Protein digestion.
Two milligrams of total protein from each sample (H, M, and L groups) was digested with Trypsin Gold in a ratio of protein/trypsin = 20:1 at 37°C for 4 hours. Trypsin Gold was added to each sample again in a ratio of protein/trypsin = 20:1 and digested for another 4 hours.
Strong cation-exchange chromatography fractionation.
Strong cation-exchange chromatography (SCX) fractionation was performed with a LC-20AB HPLC Pump system (Shimadzu, Kyoto, Japan). The peptide mixtures were reconstituted with 4 mL of buffer A (25 mM NaH2PO4 in 25% acetonitrile [ACN], pH 2.7) and loaded onto a 4.6 × 250 mm Ultremex SCX column containing 5-μm particles (Phenomenex). The peptides were eluted at a flow rate of 1 mL/minute with a gradient of buffer A for 10 minutes, 5–60% buffer B (25 mM NaH2PO4 and 1 M KCl in 25% ACN, pH 2.7) for 14 minutes, and 60–100% buffer B for 1 minute. The system was maintained at 100% buffer B for 2 minutes before equilibrating with buffer A for 10 minutes before the next injection. The eluted peptides were pooled into 10 fractions, desalted with a Strata X C18 column (Phenomenex) and vacuum-dried.
TiO2 enrichment of phosphopeptides.
The dried peptides from each fraction were reconstituted in a solution containing 65% ACN and 2% trifluoroacetic acid (TFA), and then saturated with glutamic acid (20 mg/mL, pH 2.0–2.5). The peptide solution was added to 500 μg of equilibrated TiO2 (GL Science, Saitama, Japan) in a 1.5-mL Eppendorf tube and incubated for 20 minutes with end-over-end rotation at 37°C. The column was washed once with 65% ACN and 0.5% TFA (pH 2.0–3.5) and 65% ACN and 0.1% TFA (pH 2.0–3.5). The peptides were eluted once with 1.1% NH4OH solution in 50% ACN and once with 3% NH4OH solution in 50% ACN (diluted from a 25% NH4OH solution). The two eluates were combined and vacuum-dried.
Analytical separation of phosphopeptides with the NanoLC platform.
A splitless nanoACQuity (Waters Milford, MA) system was coupled to the TripleTOF for analytical separation. The system uses microfluidic traps and nanofluidic columns packed with Symmetry C18 (5 μm, 180 μm × 20 mm) for online trapping and desalting and nanofluidic columns packed with BEH130 C18 (1.7 μm, 100 μm × 100 mm) for analytical separation. The solvents were purchased from Thermo Fisher Scientific (San Jose, CA) and were composed of water/ACN/formic acid (A, 98:2:0.1% and B, 2:98:0.1%). The phosphopeptides were loaded, and trapping and desalting were performed at a flow rate of 2 μL/minute for 15 minutes with 99% mobile phase A. Analytical separation was established by maintaining 2% B for 1 minute at a flow rate of 300 nL/minute. During the next 60 minutes, a linear gradient to 35% B occurred in 35 minutes and then increased to 60% in 5 minutes. Following the peptide elution window, the gradient was increased to 80% B in 4 minutes and maintained for 5 minutes. The initial chromatographic conditions were restored in 11 minutes.
Data acquisition with the TripleTOF 5600 System.
Data acquisition was performed with a TripleTOF 5600 System (AB SCIEX, Concord, Ontario, Canada) fitted with a Nanospray III source (AB SCIEX) and a pulled quartz tip as the emitter (New Objectives, Woburn, MA). The data were acquired using an ion spray voltage of 2.5 kV, curtain gas of 30 psi, nebulizer gas of 15 psi, and an interface heater temperature of 150°C. The mass spectrometer (MS) was operated with a resolution power greater than or equal to 30,000 full width half maximum for the time of flight (TOF) MS scans. For information-dependent acquisition, survey scans were acquired in 250 ms and as many as 40 product ion scans were collected if exceeding a threshold of 120 counts per second and with a 2+ to 5+ charge state. The total cycle time was fixed to 2.29 seconds. Four time bins were summed for each scan at a pulser frequency value of 11 kHz through monitoring of the 40-GHz multichannel time-to-digital converter detector with four-anode/channel detection. A sweeping collision energy setting of 35 ± 5 eV was applied to all precursor ions for collision-induced dissociation. Dynamic exclusion was set for 1/2 of the peak width (∼18s); then, the precursor was refreshed off the exclusion list.
Phosphopeptides identification and quantification.
The samples were analyzed via LC-ESI-MS/MS and the resulting spectra were searched against the IPI_human (http://www.ebi.ac.uk/IPI) and ToxoDB (http://toxodb.org/toxo/) databases with ProteinPilot v4.5 (AB SCIEX, Foster City, CA). The matched peptides were filtered with a peptide identification confidence > 80% and a false discovery rate (FDR) < 0.2% for the identification of peptides and a protein FDR < 0.5% for the identification of protein using the target-decoy strategy.18 The data from triplicate samples were analyzed with the SPSS software (Chicago, IL) to remove outliers, and the mean value was obtained from the remaining two or three samples.
Phosphorylation site locations were determined with Mascot 2.4 (Matrix Science, London, UK), Percolator (http://www.sanger.ac.uk/Software/analysis/MascotPercolator/), and phosphoRS 3.1 (http://ms.imp.ac.at/?goto=phosphors). All raw data files were searched again with Mascot 2.4. Then, the search results were rescored using Percolator. The files generated by Percolator were loaded into phosphors 3.1. The phosphopeptides with phosphoRS probability were compared with the phosphopeptides identified by the ProteinPilot Software v. 4.5. The localized phosphoprotein sites were considered credible if the calculated phosphoRS site probabilities were ≥ 0.75.19
Phosphopeptide quantification was performed according to Wu's method.20 The phosphorylation peptides extracted by ProteinPilot were filtered with the following condition: peptides modified with a phosphorylated group and without uncertain information, including no quant, auto-discordant peptide type, auto-shared MS, and previously used auto-MS, and so on. The phosphopeptide ratios of each comparison were calculated as the average ratio of the related specta. We defined the extreme ratios as 1,000 when only the heavy peptides were detected and as 0.001 when only light peptides were detected in the H/L comparison. A meaningful difference in the phosphorylation level was defined as a difference greater than 2-fold between the compared groups.
Functional analysis of the phosphorylation data set.
All phosphoproteins and the significantly regulated phosphoproteins were subjected to bioinformatics analysis. The gene ontology (GO) annotation and enrichment analysis were completed using the web-based GO software (http://www.geneontology.org). A pathway analysis was performed using the web-based Kyoto Encyclopedia of Genes and Genomes (KEGG, http://www.genome.p/kegg).
Comparative analysis of phosphoproteomes from the H, M, and L HFF cell groups.
The phosphopeptides that differed by 2-fold in reads between different groups (M versus L, H versus L, and H versus M) were identified as significantly regulated. The compositions and profiles of the significantly regulated phosphopeptides from the three comparisons (M versus L, H versus L, and H versus M) were analyzed.
Verification of the different phosphorylation levels for some phosphoproteins in the H, M, and L groups.
Some significantly regulated phosphoproteins that were highly phosphorylated, including phospho-Bad-S118, phospho-MARCKS-S170, phospho-Vimentin-S42, and phospho-Vimentin-S56, were chosen to be verified by Western blotting with anti-phosphorylated protein antibodies. After infection with T. gondii for 2 or 6 hours (the uninfected cells were used as a control), the host cells were washed three times with PBS to remove the unrecruited tachyzoites and the remaining medium. All cell samples were harvested with cell scrapers. After centrifugation at 1,200 rpm for 10 minutes at 4°C, the cell precipitates were collected. Then, the precipitates were resuspended by pipetting up and down with lysis buffer supplemented with protease inhibitor, phosphatase inhibitor (Roche), and PMSF and kept on ice for 30 minutes followed by ultrasonication. After centrifugation at 14,000 × g for 15 minutes at 4°C, total protein from the supernatants of the three groups were loaded for SDS-polyacrylamide gel electrophoresis; then, the proteins were electrotransferred onto polyvinylidene fluoride (PVDF) membranes for Western blotting. The primary antibodies used in this experiment were a rabbit anti-human phospho-Bad-S118 polyclonal antibody (diluted 1:500, ABclonal), rabbit anti-human phospho-MARCKS-S170 polyclonal antibody (diluted 1:500, ABclonal), rabbit anti-human phospho-vimentin-S42 polyclonal antibody (diluted 1:250, Abcam), and rabbit anti-human actin (diluted 1:1000, Cell Signaling Technology). The secondary goat anti-rabbit IgG antibody (Santa Cruz) was diluted at 1:2,000.
RESULTS
Identification of the phosphopeptides from HFFs infected or uninfected with T. gondii tachyzoites.
Quantitative phosphoproteomics strategies using SILAC were applied to characterize the host cell signaling network affected by T. gondii infection. After six generations of cell culture in SILAC medium, the L, M, and H groups were labeled with Lys (+0)/Arg (+0), Lys (+6)/Arg (+6), and Lys (+8)/Arg (+10), respectively. The M and H groups were infected with RH tachyzoites for 2 and 6 hours, respectively, and the L group served as an uninfected control. A TiO2 column was used to enrich the phosphopeptides from the cell lysates, and a total of 10 fractions were involved in the final LC-MS/MS analysis with the TripleTOF 5600 system (Figure 1).
Figure 1.
Processes used to generate phosphoproteomic data for human foreskin fibroblasts (HFFs) infected with Toxoplasma gondii RH tachyzoites. HFF cells were grown in Dulbecco's modified Eagle's medium supplemented with one of the three different forms of lysine and arginine to label almost all the phosphoproteins with the stable N and C isotopes via the incorporation of the labeled lysine or arginine. The H, M, and L groups were infected with T. gondii at a multiplicity of infection of 10 for 6, 2, and 0 hours (uninfected), respectively. The cells were harvested and lysed, followed by alkylation with Iiodoacetamide and trypsin digestion. The phosphopeptides were separated with strong cation-exchange chromatography and enriched with a TiO2 column. Then spectrometric analysis was performed. This figure appears in color at www.ajtmh.org.
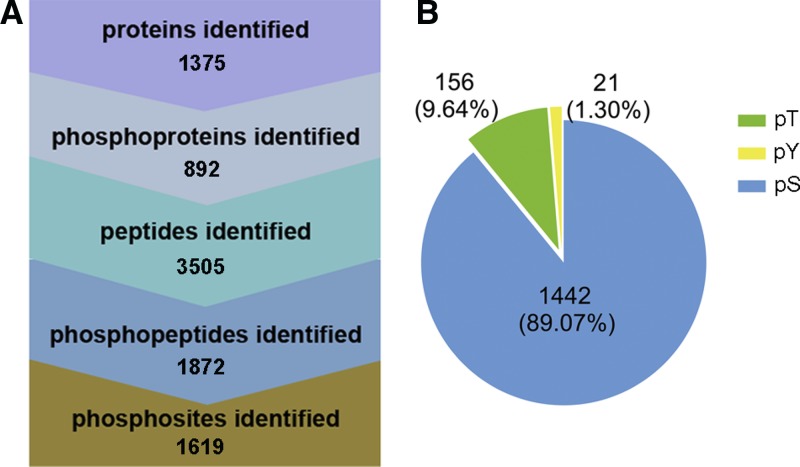
The proteomic data analysis was performed. A total of 3,505 peptides matched with 1,375 proteins were identified, among which 1,872 distinct phosphopeptides were identified and mapped to 892 phosphoproteins with 1,619 phosphorylation sites (Figure 2A, Supplemental Table 1). Because most phosphorylation events occur on serine (S), threonine (T), and tyrosine (Y), the phosphorylation frequencies of these three phosphoamino acids were more closely evaluated. Phospho-S was the most abundant and accounted for 89.07% of all phosphorylated amino acids, followed by phospho-T (9.64%) and phospho-Y (only 1.30%) (Figure 2B). This distribution of phosphorylation among the individually identified sites was very close to the previous report in HeLa cells.6
Figure 2.
Large-scale mass spectra of the phosphopeptides. (A) Identification of total proteins, phosphoproteins, total peptides, phosphopeptides, and phosphosites. A total of 1,375 proteins were identified, among which 892 phosphoproteins and 1,619 phosphorylation sites were found. (B) Distribution of the pS/pT/pY phosphoproteome. Phospho-S was the most abundant and accounted for 89.07% of all the phosphorylated amino acids, followed by phospho-T (9.64%) and phospho-Y (1.30%). This figure appears in color at www.ajtmh.org.
Comparative analysis of the phosphoproteomes from H, M, and L groups of HFFs.
Toxoplasma gondii infection is a successive process involving many biological changes of the host cell. To reveal the specific host cell phosphoproteomes at different infection phases, comparisons of M versus L, H versus L, and H versus M were performed, and the significantly regulated phosphopeptides are shown in Table 1. A total of 694 phosphopeptides matched with 410 phosphoproteins were significantly regulated in the comparison of M versus L, including 436 upregulated and 258 downregulated phosphopeptides. A total of 592 phosphopeptides matched with 393 phosphoproteins were significantly regulated in the H versus L comparison, including 146 upregulated and 446 downregulated phosphopeptides. Finally, a total of 794 phosphopeptides matched with 461 phosphoproteins were significantly regulated in the H versus M comparison, including 149 upregulated and 645 downregulated phosphopeptides (Supplemental Table 2).
Table 1.
Detailed information for the significantly regulated phosphopeptides/proteins among the three comparison groups
| Group A/group B | Significantly upregulated phosphopeptides | Significantly downregulated phosphopeptides | Total significantly regulated phosphopeptides | Total significantly regulated phosphoproteins |
|---|---|---|---|---|
| M/L | 436 | 258 | 694 | 410 |
| H/L | 146 | 446 | 592 | 393 |
| H/M | 149 | 645 | 794 | 461 |
GO enrichment analysis of the identified phosphoproteins and the significantly regulated phophoproteins.
GO analysis of the 892 identified phosphoproteins revealed that the highest proportion were categorized as “binding” in the molecular function, “cell part” and “cell” in the cellular component, and “cellular process” in the biological process (Figure 3). The GO analysis of the significantly regulated phosphoproteins among the three comparison groups was consistent with the total phosphoprotein analysis (Supplemental Figure 1). A GO analysis was performed for the 410, 393, and 461 significantly regulated phosphoproteins from the different comparisons (M versus L, H versus L, and H versus M, respectively); the top 10 GO terms were shown in Supplemental Table 3. The enriched top three terms in the cellular component category were “non-membrane-bounded organelle,” “intracellular non-membrane-bounded organelle,” and “cell leading edge” in the M versus L group, “integral to membrane,” “intrinsic to membrane,” and “membrane part” in the H versus L group, and “cell-cell junction,” “cell junction,” and “non-membrane-bounded organelle” in the H versus M group. Among the biological processes, the top three were “cell cycle,” “cell cycle phase,” and “mitotic cell cycle” in the M versus L group, “cellular macromolecular complex assembly,” “cell cycle,” and “telomere maintenance via telomere lengthening” in the H versus L group, and “intracellular receptor mediated signaling pathway,” “transcription from RNA polymerase II promoter,” and “transcription initiation from RNA polymerase II promoter” in the H versus M group. Among the molecular functions, the top three were “protein binding,” “cytoskeletal protein binding,” and “binding” in the M versus L group, “protein binding,” “actin binding,” and “cytoskeletal protein binding” in the H versus L group, “binding,” “protein binding,” and “cytoskeletal protein binding” in the H versus M group. These GO analyses suggested that some common features existed in host cell development and multiplication following T. gondii infection. However, some different biological functions of the host cell were also regulated by the significantly regulated phosphoproteins at different time points postinfection.
Figure 3.
Gene ontology (GO) enrichment analysis of the 892 identified phosphoproteins. GO analysis of the 892 identified phosphoproteins revealed that the highest proportion were categorized as “binding” in the molecular function, “cell part” and “cell” in the cellular component, and “cellular process” in the biological process. This figure appears in color at www.ajtmh.org.
Analysis of the significantly regulated KEGG pathways identified in the comparison of the significantly regulated phosphoproteins.
KEGG pathway analysis was used to identify the signaling pathways in which the phosphoproteins were involved. The enriched top10 pathways of different comparison groups were shown in Supplemental Table 4. The top three pathways enriched in the M versus L comparison were “mismatch repair,” “nucleotide excision repair,” and “amoebiasis,” in the H versus L comparison were “mismatch repair,” “DNA replication,” and “glycerophospholipid metabolism,” and in the H versus M comparison were “axon guidance,” “adrenergic signaling in cardiomyocytes,” and “notch signaling pathway” (Supplemental Table 4). Pathways related to cell cycle processes were enriched in both comparison groups, suggesting that cell cycle regulation might be one important approach to affect the invasion and multiplication of T. gondii.
Western blotting verification of some significantly regulated phosphoproteins in response to T. gondii infection.
Four phosphorylated sites, Bad-S118, MARCKS-S170, Vimentin-S42, and Vimentin-S56, were chosen for further verification by Western blotting; the results are shown in Figure 4. After 2 hours of T. gondii infection, the phosphorylation level of MARCKS-S170 in the host cells was almost unchanged, whereas Bad-S118 phosphorylation was slightly upregulated compared with the uninfected group; however, the phosphorylation of both proteins was significantly upregulated at the 6-hour infection stage to almost five times the phosphorylation level observed in the uninfected group (Figure 4 and Supplemental Table 1). The Vimentin-S42 and Vimentin-S56 phosphorylation levels were upregulated almost 2.4- and 4.9-fold, respectively, at the 2-hour infection stage compared with the uninfected group; at the 6-hour infection stage, Vimentin-S56 phosphorylation was slightly upregulated to approximately 5.6 times the phosphorylation level in the uninfected group, whereas Vimentin-S42 phosphorylation was upregulated to approximately 28.5 times the level in the uninfected group (Figure 4 and Supplemental Table 1). The phosphorylation level changes of these phosphorylation sites shown by Western blotting were totally consistent with the MS data.
Figure 4.
Verification of the phosphorylation level change of the host cell phosphoproteins induced by Toxoplasma gondii infection. Human foreskin fibroblasts cell total proteins from the three groups of T. gondii infection for 0, 2, and 6 hours (indicated as uninfected, infected for 2 hours, infected for 6 hours) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and analyzed by Western blotting with antibodies against beta-actin, Phospho-Bad-S118, Phospho-MARCKS-S170, Phospho-Vimentin-S56, and Phospho-Vimentin-S42. Beta-actin was detected as loading control. From the Western blotting result, the trend change of the phosphorylation level of different host cell phosphoprotein phosphorylation sites induced by T. gondii infection at different time points was shown.
DISCUSSION
Phosphoprotein functions are comprehensively modulated by phosphorylation/dephosphorylation in both prokaryotic and eukaryotic organisms. Protein phosphorylation plays a significant role in a wide range of cellular processes and is involved in intracellular signal transduction, which distributes signals to various parts of the cell.21–24 This study revealed changes in the phosphorylation levels of a large number of phosphoproteins involved in different cellular processes upon T. gondii infection.
A comparable study of the phosphoproteomes from intracellular tachyzoites and tachyzoites purified from host materials identified approximately 10,000 T. gondii phosphorylation sites, and provided important information on the role of phosphorylation in host–pathogen interactions from the pathogen perspective.15 In this study, we aimed to explain the role of phosphorylation in host–pathogen interactions from the perspective of the host cell. A total of 1,619 phosphorylation sites from 892 host cell phosphoproteins were identified and analyzed. Among the 1,619 phosphorylation sites, 1,442 S, 156 T, and 21 Y phosphorylation sites were detected, accounting for 89.07%, 9.64%, and 1.30% of the total sites, respectively. A large number of phosphopeptides with up- or downregulated phosphorylation levels induced by T. gondii infection were found in a comparison of the phosphor-proteomes (H versus M, H versus L, and M versus L), which suggested that protein phosphorylation was an important regulatory host cell mechanism during different phases of infection.
In the GO analysis of the significantly regulated phosphoproteins, the terms “GTPase binding,” “small GTPase binding,” and “Ran GTPase binding” were enriched in molecular function in M versus L, but were not in the top 10 in the H versus L comparison. This result suggested that host cell GTPases may play an important role at 2 hours but not at 6 hours during T. gondii infection. GTPases are a family of hydrolases that bind and hydrolyze guanosine triphosphate. The large GTPase, interferon-inducible-p47 GTPase, has been reported to specifically translocate and adhere to the parasitophorous vacuolar membrane (PVM), resulting in the stripping of the PVM and autophagic elimination of γ irradiated CPS (strain) T. gondii in primed macrophages.25 The large immunity-related GTPases such as Irga6, mediate clearance of T. gondii and are involved in the cooperative recruitment and loading of the guanine triphosphate (GTP)-bound immunity-related GTPases onto the PVM, with subsequent vesiculation and rupture of the vacuole and thereby release of vacuolar pathogens into the cytosol where they can be removed through autophagy.26 The small GTPases, including Rho, Rac, and adenosine diphosphate-ribosylation factor 6, have been reported to accumulate on the PVM and function in host cell cytoskeleton reorganization during T. gondii invasion, which facilitates PV formation and enlargement in the host cell.13,14
Interestingly, the GO term “cytoskeletal protein binding” was enriched in all comparisons (M versus L, H versus L, and H versus M), which suggested that host cell cytoskeletal reorganization was an important biological process during T. gondii infection. Consistent with the GO analysis, the pathways “adherens junction” and “regulation of actin cytoskeleton” were also enriched in the M versus L comparison in the KEGG analysis, which suggested that both adhesion and cytoskeletal reorganization played important roles during the 2-hour postinfection phase. Studies have shown that the maintenance of host cell actin cytoskeleton integrity is important for parasite invasion and successful penetration of host cells by apicomplexans; during this process, significant host cell cytoskeletal reorganization is accompanied.13,14,27
Toxoplasma gondii infection induces and maintains a “proliferation response” including dysregulation of the cell cycle in infected host cells. This response may fulfill critical growth and multiplication requirements of the parasite during intracellular residence.11 Studies have shown that infection of human cells with T. gondii inhibits cell proliferation and that T. gondii can adjust and induce the cell cycle of the infected and even neighboring host cells to enter S phase followed by an arrest toward the G2/M boundary; allows more effective recruitment of the parasite into the host cell and the enlargement of the PV.2,28–30 This inhibitory effect of T. gondii on cellular proliferation is observed within 6 hours.28 Consistent with the reported results, almost all the 10 most common GO terms enriched in the biological process in both the M versus L and H versus L comparison were closely related to the cell cycle. In addition, in the KEGG analysis, the top two enriched pathways were “mismatch repair” and “nucleotide excision repair” in the M versus L and H versus L comparison; these pathways were also associated with the cell cycle. These phosphoproteomic analysis results indicate that T. gondii infection results in dysregulation of the host cell cycle at both the 2- and 6-hour postinfection time points.
Apoptosis is crucial for the elimination of pathogen-infected host cells,2 plays indispensable roles in the regulation of the immune response,31 and inhibits pathogen growth.32 Phosphoinositide 3-kinase (PI3K) signaling directly or indirectly regulates the apoptotic pathway through the involvement of PKB/Akt.33 The PI3K-PKB/Akt pathway may be one of the major routes for the prevention of the host cell apoptosis induced by T. gondii infection.34 PKB/Akt has also been reported to promote cell survival through its ability to phosphorylate Bad.35 Although Bad cannot directly trigger apoptosis, it lowers the threshold at which apoptosis is induced.36 Consequently, the Bad protein is often referred to as a sensitizer or an enabler of apoptosis.36 The Bad phosphorylation promotes cell survival in many cell types.34 Toxoplasma gondii infection activates PI3K, and in turn PI3K stimulates PKB/Akt phosphorylation and leads to Bad phosphorylation.13 This phosphorylation inhibits Bax translocation from the cytosol to the mitochondria and thus inhibits host cell apoptosis.34 Compared with the L group (the control group), Bad phosphorylation at S118 was slightly upregulated at 2-hour postinfection by T. gondii but was significantly upregulated at 6-hour post-T. gondii infection, which suggested that T. gondii rendered host cells resistant to apoptosis at the 6-hour postinfection phase to allow its survival and multiplication in the host cell.
Cytoskeletal reorganization of the host cell is involved in every phase of T. gondii infection.14 The MARCKS has been implicated in cell adhesion, secretion, motility, and mitogenesis through the regulation of the actin cytoskeletal structure.37 In addition, MARCKS is one of the most predominant substrates for protein kinase C (PKC) and can be phosphorylated by PKC or bind to calcium-calmodulin to inhibit its association with actin and the plasma membrane, leading to its presence in the cytoplasm.38,39 Furthermore, one domain of MARCKS known as the phosphorylation site domain appears to play a key role in regulating MARCKS functions in its phosphorylated and dephosphorylated forms.40 No obvious changes in MARCKS-S170 phosphorylation levels were observed 2-hour postinfection of HFF cells by T. gondii, whereas a large upregulation was found at 6-hour postinfection. The mechanisms underlying this large change in the phosphorylation level and whether this change has biological effects require clarification.
Host cell vimentin associates with the PV within an hour after invasion, and a significant reorganization of vimentin around the PV is observed throughout the infection.41 Phosphorylation has been demonstrated to regulate the assembly/disassembly of vimentin during physiological processes such as mitosis.42 In this study, the phosphorylation level of vimentin changed at different infection time points, which suggested that the phosphorylation of different protein sites might play different roles during different infection stages. This finding also hinted that vimentin phosphorylation was important for T. gondii infection, most likely for PV formation and enlargement. Furthermore, the essential role of phosphorylation in cytoskeletal reorganization was suggested in our study by the change in the phosphorylation levels of the phosphoproteins induced by T. gondii infection, including the proteins associated with host cell cytoskeleton reorganization, such as integrin alpha, integrin beta, Caveolin, receptor tyrosine kinase, epidermal growth factor receptor 1/2, plasma membrane calcium-transporting ATPase, and protein tyrosine kinase.
A complicated host cell manipulation occurs following T. gondii infection. Spatial reorganization of host organelles and the cytoskeleton around the PV are observed following entry, followed by the manipulation of host signaling pathways and cellular pathways by T. gondii to establish an antiapoptotic environment and subvert immune cells, and so on.2 Protein phosphorylation within and beyond parasite boundaries has been confirmed and comprehensively anslysed,15 although no information is available on protein phosphorylation in host cells in response to T. gondii infection at present. Here, we confirmed that protein phosphorylation played an important role in the regulation of many cellular events and showed that different cellular processes were predominant in different infection phases. Unfortunately, only two infection time points was selected to analyze the regulation of phosphorylation levels in our study through amino acid labeling technology. The regulation of phosphorylation at different infection time points is important and warrants further exploration. In addition, the functions of the differentially regulated phosphoproteins during T. gondii infection can be tested using forward or reverse genetic approaches.
CONCLUSIONS
The comparative phosphoproteomes of SILAC-labeled HFF cells infected with T. gondii were analyzed at different time points, and the significantly regulated phosphoproteins were categorized using GO enrichment and KEGG analyses. The analysis results showed that similar biological profiles at both 2 and 6 hours postinfection, which suggested that the cellular processes of host cells at these two time points might not be significantly affected by T. gondii infection. Conversely, different functional category compositions were shown for the significantly regulated host cell phosphoproteins following T. gondii infection at the different time points, which suggested that the different phases of infection emphasized some different and specific cellular processes required for the essential infection and multiplication of T. gondii. For example, GO terms associated with the cell cycle and cytoskeleton reorganization were enriched, suggesting cell cycle changes and cytoskeletal reorganization of the host cell at both 2 and 6 hours. In addition, the GO terms associated with GTPase binding were enriched in the M versus L comparison, which suggested that the host might regulate GTPases activity to protect the cell from infection. The different phosphoproteomics profiles of the host cells infected by T. gondii at different time points provided a basis for further understanding of pathogen–host interactions. The phosphorylation of the apoptotic protein Bad will inhibit cell apoptosis, and this protein was found to be significantly phosphorylated 6 hours postinfection in our study.
Supplementary Material
Supplemental Table and Figures.
Note: Supplemental tables and figure appear at www.ajtmh.org.
REFERENCES
- 1.Hotez PJ, 2014. Neglected parasitic infections and poverty in the United States. PLoS Negl Trop Dis 8: e3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laliberte J, Carruthers VB, 2008. Host cell manipulation by the human pathogen Toxoplasma gondii. Cell Mol Life Sci 65: 1900–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss LM, Dubey JP, 2009. Toxoplasmosis: a history of clinical observations. Int J Parasitol 39: 895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng HJ, Chen XG, Lindsay DS, 2011. A review: competence, compromise, and concomitance-reaction of the host cell to Toxoplasma gondii infection and development. J Parasitol 97: 620–628. [DOI] [PubMed] [Google Scholar]
- 5.Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M, 2006. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127: 635–648. [DOI] [PubMed] [Google Scholar]
- 6.Cohen P, 2001. The role of protein phosphorylation in human health and disease. The Sir Hans Krebs Medal Lecture. Eur J Biochem 268: 5001–5010. [DOI] [PubMed] [Google Scholar]
- 7.Song KJ, Ahn HJ, Nam HW, 2012. Anti-apoptotic effects of SERPIN B3 and B4 via STAT6 activation in macrophages after infection with Toxoplasma gondii . Korean J Parasitol 50: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peixoto L, Chen F, Harb OS, Davis PH, Beiting DP, Brownback CS, Ouloguem D, Roos DS, 2010. Integrative genomic approaches highlight a family of parasite-specific kinases that regulate host responses. Cell Host Microbe 8: 208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vutova P, Wirth M, Hippe D, Gross U, Schulze-Osthoff K, Schmitz I, Luder CG, 2007. Toxoplasma gondii inhibits Fas/CD95-triggered cell death by inducing aberrant processing and degradation of caspase 8. Cell Microbiol 9: 1556–1570. [DOI] [PubMed] [Google Scholar]
- 10.Melo MB, Nguyen QP, Cordeiro C, Hassan MA, Yang N, McKell R, Rosowski EE, Julien L, Butty V, Darde ML, Ajzenberg D, Fitzgerald K, Young LH, Saeij JP, 2013. Transcriptional analysis of murine macrophages infected with different Toxoplasma strains identifies novel regulation of host signaling pathways. PLoS Pathog 9: e1003779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molestina RE, El-Guendy N, Sinai AP, 2008. Infection with Toxoplasma gondii results in dysregulation of the host cell cycle. Cell Microbiol 10: 1153–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bottova I, Sauder U, Olivieri V, Hehl AB, Sonda S, 2010. The P-glycoprotein inhibitor GF120918 modulates Ca2+-dependent processes and lipid metabolism in Toxoplasma gondii . PLoS One 5: e10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Da SC, Da SE, Cruz MC, Chavrier P, Mortara RA, 2009. ARF6, PI3-kinase and host cell actin cytoskeleton in Toxoplasma gondii cell invasion. Biochem Biophys Res Commun 378: 656–661. [DOI] [PubMed] [Google Scholar]
- 14.Na RH, Zhu GH, Luo JX, Meng XJ, Cui L, Peng HJ, Chen XG, Gomez-Cambronero J, 2013. Enzymatically active Rho and Rac small-GTPases are involved in the establishment of the vacuolar membrane after Toxoplasma gondii invasion of host cells. BMC Microbiol 13: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Treeck M, Sanders JL, Elias JE, Boothroyd JC, 2011. The phosphoproteomes of Plasmodium falciparum and Toxoplasma gondii reveal unusual adaptations within and beyond the parasites' boundaries. Cell Host Microbe 10: 410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodenmiller B, Malmstrom J, Gerrits B, Campbell D, Lam H, Schmidt A, Rinner O, Mueller LN, Shannon PT, Pedrioli PG, Panse C, Lee HK, Schlapbach R, Aebersold R, 2007. PhosphoPep-a phosphoproteome resource for systems biology research in Drosophila Kc167 cells. Mol Syst Biol 3: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villen J, Gygi SP, 2008. The SCX/IMAC enrichment approach for global phosphorylation analysis by mass spectrometry. Nat Protoc 3: 1630–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elias JE, Gygi SP, 2007. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods 4: 207–214. [DOI] [PubMed] [Google Scholar]
- 19.Taus T, Kocher T, Pichler P, Paschke C, Schmidt A, Henrich C, Mechtler K, 2011. Universal and confident phosphorylation site localization using phosphoRS. J Proteome Res 10: 5354–5362. [DOI] [PubMed] [Google Scholar]
- 20.Wu X, Tian L, Li J, Zhang Y, Han V, Li Y, Xu X, Li H, Chen X, Chen J, Jin W, Xie Y, Han J, Zhong CQ, 2012. Investigation of receptor interacting protein (RIP3)-dependent protein phosphorylation by quantitative phosphoproteomics. Mol Cell Proteomics 11: 1640–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graves JD, Krebs EG, 1999. Protein phosphorylation and signal transduction. Pharmacol Ther 82: 111–121. [DOI] [PubMed] [Google Scholar]
- 22.Marks F, 2001. Protein phosphorylation: cellular functions and therapeutic perspectives. Tenth colloquium on cellular signal transduction: DKFZ Heidelberg, 9 February 2001. J Cancer Res Clin Oncol 127: 744–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mundy J, Schneitz K, 2002. Protein phosphorylation in and around signal transduction. Trends Plant Sci 7: 54–55. [DOI] [PubMed] [Google Scholar]
- 24.Sawyer TK, Shakespeare WC, Wang Y, Sundaramoorthi R, Huang WS, Metcalf CR, Thomas M, Lawrence BM, Rozamus L, Noehre J, Zhu X, Narula S, Bohacek RS, Weigele M, Dalgarno DC, 2005. Protein phosphorylation and signal transduction modulation: chemistry perspectives for small-molecule drug discovery. Med Chem 1: 293–319. [DOI] [PubMed] [Google Scholar]
- 25.Ling YM, Shaw MH, Ayala C, Coppens I, Taylor GA, Ferguson DJ, Yap GS, 2006. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J Exp Med 203: 2063–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pawlowski N, Khaminets A, Hunn JP, Papic N, Schmidt A, Uthaiah RC, Lange R, Vopper G, Martens S, Wolf E, Howard JC, 2011. The activation mechanism of Irga6, an interferon-inducible GTPase contributing to mouse resistance against Toxoplasma gondii . BMC Biol 9: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Weiss LM, Orlofsky A, 2010. Coordinate control of host centrosome position, organelle distribution, and migratory response by Toxoplasma gondii via host mTORC2. J Biol Chem 285: 15611–15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dvorak JA, Crane MS, 1981. Vertebrate cell cycle modulates infection by protozoan parasites. Science 214: 1034–1036. [DOI] [PubMed] [Google Scholar]
- 29.Grimwood J, Mineo JR, Kasper LH, 1996. Attachment of Toxoplasma gondii to host cells is host cell cycle dependent. Infect Immun 64: 4099–4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavine MD, Arrizabalaga G, 2009. Induction of mitotic S-phase of host and neighboring cells by Toxoplasma gondii enhances parasite invasion. Mol Biochem Parasitol 164: 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams GT, 1994. Programmed cell death: a fundamental protective response to pathogens. Trends Microbiol 2: 463–464. [DOI] [PubMed] [Google Scholar]
- 32.Green DR, 2003. Overview: apoptotic signaling pathways in the immune system. Immunol Rev 193: 5–9. [DOI] [PubMed] [Google Scholar]
- 33.Duronio V, 2008. The life of a cell: apoptosis regulation by the PI3K/PKB pathway. Biochem J 415: 333–344. [DOI] [PubMed] [Google Scholar]
- 34.Quan JH, Cha GH, Zhou W, Chu JQ, Nishikawa Y, Lee YH, 2013. Involvement of PI 3 kinase/Akt-dependent Bad phosphorylation in Toxoplasma gondii-mediated inhibition of host cell apoptosis. Exp Parasitol 133: 462–471. [DOI] [PubMed] [Google Scholar]
- 35.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME, 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91: 231–241. [DOI] [PubMed] [Google Scholar]
- 36.Howells CC, Baumann WT, Samuels DC, Finkielstein CV, 2011. The Bcl-2-associated death promoter (BAD) lowers the threshold at which the Bcl-2-interacting domain death agonist (BID) triggers mitochondria disintegration. J Theor Biol 271: 114–123. [DOI] [PubMed] [Google Scholar]
- 37.Rombouts K, Lottini B, Caligiuri A, Liotta F, Mello T, Carloni V, Marra F, Pinzani M, 2008. MARCKS is a downstream effector in platelet-derived growth factor-induced cell motility in activated human hepatic stellate cells. Exp Cell Res 314: 1444–1454. [DOI] [PubMed] [Google Scholar]
- 38.Thelen M, Rosen A, Nairn AC, Aderem A, 1991. Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature 351: 320–322. [DOI] [PubMed] [Google Scholar]
- 39.Tinoco LW, Fraga JL, Anobom CD, Zolessi FR, Obal G, Toledo A, Pritsch O, Arruti C, 2014. Structural characterization of a neuroblast-specific phosphorylated region of MARCKS. Biochim Biophys Acta 1844: 837–849. [DOI] [PubMed] [Google Scholar]
- 40.Michaut MA, Williams CJ, Schultz RM, 2005. Phosphorylated MARCKS: a novel centrosome component that also defines a peripheral subdomain of the cortical actin cap in mouse eggs. Dev Biol 280: 26–37. [DOI] [PubMed] [Google Scholar]
- 41.Halonen SK, Weidner E, 1994. Overcoating of Toxoplasma parasitophorous vacuoles with host cell vimentin type intermediate filaments. J Eukaryot Microbiol 41: 65–71. [DOI] [PubMed] [Google Scholar]
- 42.Aziz A, Hess JF, Budamagunta MS, Voss JC, Fitzgerald PG, 2010. Site-directed spin labeling and electron paramagnetic resonance determination of vimentin head domain structure. J Biol Chem 285: 15278–15285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table and Figures.