Abstract.
Rickettsia australis, the etiologic agent of Queensland tick typhus (QTT), is increasingly being recognized as a cause of community-acquired acute febrile illness in eastern Australia. Changing human population demographics, climate change, and increased understanding of expanding vector distribution indicate QTT is an emerging public health threat. This review summarizes the epidemiology, pathogenesis, clinical features, treatment principles, and future directions of this disease. Increased recognition of QTT will enable consideration of and prompt treatment of R. australis infection by clinicians in Australia.
SEARCH STRATEGY AND SELECTION CRITERIA
References for this review were identified through searches of PubMed and ScienceDirect for articles published from 1946 to 2015 by use of the terms “Rickettsia australis,” “Queensland tick typhus (QTT),” “spotted fever group rickettsia,” and “tick borne disease Queensland.” Articles resulting from these searches and relevant references cited in those articles were reviewed. Articles published in English were included.
INTRODUCTION
QTT is an increasingly recognized important cause of community-acquired acute febrile illness in eastern Australia. Serological surveys have identified a larger distribution of disease along eastern coastal Australia than was originally demonstrated in the retrospective review by Derne and others.1 Rickettsia australis (QTT) is a pathogen in the Rickettsial spotted fever group (SFG), which appear to be relatively uncommon in Australia.2 Other documented pathogenic SFG rickettsiae known to cause human disease in Australia include Rickettsia honei (Flinders Island spotted fever) and R. honei subsp. marmionii (Australian spotted fever).2 These infections (and scrub typhus caused by Orientia tsutsugamushi) share similar clinical features with some overlap in geographical distribution, creating challenges for species identification and obtaining accurate epidemiological data.3 Acute Rickettsia australis infection is likely to be underrecognized, with recent evidence showing its increasing disease burden with increased recognition of severe disease, including death.1,4 Recent case series demonstrate the broad spectrum of clinical illness seen in hospitalized patients.5,6 QTT is not simply a mild, self-limiting febrile illness but an emerging public health threat that needs to be addressed.
EPIDEMIOLOGY AND ECOLOGY OF VECTORS
The distribution and public health impact of rickettsial disease in Australia is poorly defined. A diagnosis of any rickettsial infection does not require compulsory reporting in Australia, relying on voluntary disease surveillance systems.7 Acute infection can be misdiagnosed or treated on an empiric basis. Seropositivity studies are difficult to interpret due to cross-reactivity of antibodies among rickettsial species and nonrickettsial infectious diseases, especially in tropical regions.1,8,9
Like many tropical infectious diseases in Queensland, SFG rickettsial infections are known to correlate with latitude, altitude, and rainfall.10 Rickettsia australis is endemic along the east coast of Australia, extending from the Torres Strait Islands (and nearby Papua New Guinea8,11) to Gippsland, Victoria.2 Regions of high human population densities including southeast and far north Queensland12; Sydney, New South Wales and Gippsland, Victoria, are identified as endemic areas.2 Of the reported 33 cases of SFG infections in Australia in 2011, 15 (45%) occurred in Queensland, the majority of which were in the southeast.7 Population growth, changing demographics, climate change, and changing vector distribution are likely to result in new endemic foci of QTT.1,12 The emergence of adventure tourism within tropical Oceania could also contribute to a higher disease burden.1
Like many vector-associated zoonoses, infection risk is derived principally from human interaction with the invertebrate vector and only rarely from its normal vertebrate hosts.13,14 Living conditions, rural residence, and tropical climate are predictors of disease. These conditions relate to vector abundance and their proximity to humans.1 Many cases reported in the literature are preceded by exposure to bush environments during activities such as gardening,15 living near bushland,11 military training exercises,16 fishing,1 and fieldwork.15 Occupational (e.g., farming, commercial harvesting) and recreational (e.g., bushwalking) activities have been identified as strong risk factors for R. australis infection with up to 50% of infections acquired in this way.15 Year round infection from R. australis in immunocompetent individuals of all ages and ethnicities occurs,17 although seasonal variation is described with 80% of cases documented as occurring in the winter and spring (June to November), coinciding with increased tick densities in these months.15,18
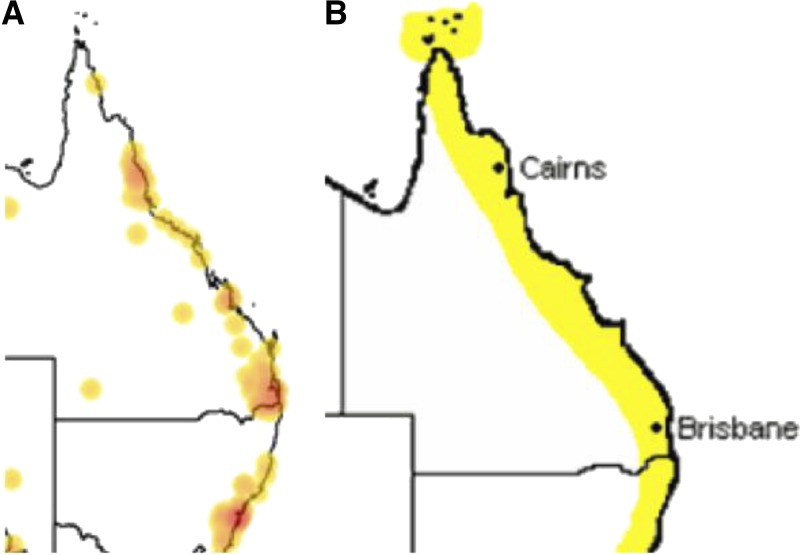
The epidemiology of QTT is largely determined by the distribution of its main arthropod vectors, with R. australis being transmitted by the bite of certain Ixodes spp. Ticks,18,19 which are present predominantly along the eastern coast of Australia.2,18 Rickettsia australis has been isolated from both Ixodes holocyclus (Australian paralysis, or scrub, tick) and Ixodes tasmania,15,18 with the adult female I. holocyclus the predominant vector. Ixodes holocyclus has a predilection for forested areas with annual rainfall of more than 1,000 mm and requires the presence of appropriate vertebrate hosts for its survival.18,20 The first focus of Ixodes ticks infected with R. australis were identified in Canungra, Queensland, after soldiers training in bushland became ill with acute fever and rash.16,21,22 The main distribution of the I. holocyclus tick is within 20 km of the coast18,23 but it has been isolated in areas more than 100-km inland including the Bunya Mountains, Barcaldine, and Thargomindah in Queensland and the Lower Blue Mountains in New South Wales. These areas could be future sites of emerging infection2,18 (Figure 1), although it is unknown whether these ticks harbor SFG Rickettsia.20,23 Ixodes holocyclus is not known to be distributed north of Cooktown, Queensland,23 yet a case of QTT has been documented in the Torres Strait.4 This observation requires explanation as Cape York Peninsula and the Torres Strait islands are suitable environments for this tick species. Ixodes tasmania is distributed throughout eastern and southeastern Australia but rarely bites humans. It has been found as far inland as Emerald and Roma, Queensland.18 It is likely to maintain transmission of R. australis among small mammals.25
Figure 1.
(A) Known Queensland distribution of Ixodes ticks24; (B) proposed distribution of Rickettsia australis infection.2 This figure appears in color at www.ajtmh.org.
Rickettsia honei subsp. marmionii is a newly recognized SFG Rickettsia, phylogenetically related to R. australis and has been identified in a Haemaphysalis novaeguineae tick on Cape York, Queensland.26 This was the first documented SFG rickettsial infection in this region, occurring in an adult male in 2002.27 Haemaphysalis spp. ticks could prove to be an important vector for SFG Rickettsia in Queensland.
A transmission cycle of rickettsiae exists between ticks and species of vertebrates; humans are an accidental host.3 Large numbers of mammalian species are bitten by ticks harboring SFG Rickettsia including bandicoots, rodents, cattle, wombats, and companion animals.1,15 Of the recorded, 34 species of mammals and seven species of birds from which Ixodes spp. ticks have been detected, the bandicoot species Isoodon macrourus and Perameles nasuta carry the highest densities of ticks and are necessary for Ixodes tick populations to persist through the seasons in Queensland.18
Domestic dogs from southeastern coastal Australia were found to have a SFG rickettsial seropositive rate of 11.2% in one study.28 Native reptiles are documented hosts for R. honei, playing a role in the transmission cycle of this species. Difficulty in isolating Rickettsia spp. from hosts and ticks, increases the difficulty of accurately defining the distribution and transmission of R. australis.29 Serologic studies have been the predominate method of investigation. Molecular techniques may better characterize our understanding of R. australis epidemiology.
CLINICAL FEATURES AND COURSE OF INFECTION
All four northeastern Queensland rickettsioses, including scrub typhus (O. tsutsugamushi), murine typhus (Rickettsia typhi), Australian spotted fever (R. honei subsp. marmionii), and QTT (R. australis), have similar core clinical manifestations, with a range of other symptoms observed.2 Rickettsia australis shares many clinical features with rickettsialpox, caused by a closely related organism, Rickettsia akari.30 Early clinical features are often nonspecific, making clinical diagnosis challenging.31 Infection can progress to severe sepsis with multiorgan failure. Rickettsia spp. usually produce symptoms between 1 and 2 weeks after inoculation by an Ixodes tick15,32 with R. australis having a shorter incubation period averaging 5 days (range 3–6 days).15,31,33 Acute R. australis infection often begins with fever, headache, malaise, myalgia, and a rash.31 High-grade fever of up to 41°C15 is observed but in acute uncomplicated QTT resolves within 48 hours after initiation of treatment with doxycycline.15,31 Prolonged fever is associated with rickettsaemia, end-organ dysfunction, and intensive care admissions.1
Rash morphology is variable, with a macular or maculopapular rash in 90% of patients in one retrospective study.15 Vesicular or pustular rashes also occur and can be confused with acute varicella.34 The rash usually lasts for 10–12 days and can appear as early as 24 hours after a tick bite. The rash typically follows a widespread, global eruption involving both trunk and limbs. Evolution to a petechial or purpuric rash toward the end of the clinical course is described in hospitalized patients with severe illness5,15 (Figure 2). Erythema migrans at and around the Ixodes attachment site is not uncommon in QTT, as is observed in some other tick borne illnesses (e.g., other Rickettsia and Borrelia spp. including Lyme Disease).18 Infrequently the rash is pruritic.35
Figure 2.
Putative clinical course for uncomplicated acute Queensland tick typhus in treated hospitalized patients. This is a descriptive, composite data graph based on average length of time to commencement and resolution of clinical and laboratory features of illness as obtained from retrospective studies and case series. This figure appears in color at www.ajtmh.org.
Tender lymphadenopathy occurs in approximately 70% of patients and is usually localized to the region draining the tick bite or eschar.15 An eschar is seen in approximately 50–65% of R. australis infections and results from rapid focal replication of Rickettsia organisms and localized skin necrosis.36 The detection of an eschar is diagnostically valuable, but this lesion is often difficult to find as it can occur in sites that can be missed on examination such as in the axilla or groin. Compared with other rickettsial spotted fever infections in Australia, R. australis is associated with higher rates of lymphadenopathy and eschar.15 Less common manifestations of disease including arthralgia, splenomegaly, abdominal pain, dry cough, sore throat, conjunctivitis, and photophobia.6 A forestry worker who was bitten on the scalp developed a pansinusitis with bilateral antral opacities.33
Over the past three decades, there were numerous cases of severe R. australis infection reported.5,6,37 There was one reported fatality from QTT since R. australis was first isolated in 1946.21 Complications from severe disease are usually vascular in origin, signifying endothelial dysfunction and vasculitis that can lead to end-organ dysfunction.38 There are no known risk factors identified for developing severe disease or complications of QTT. It is uncertain if the risk factors for Rocky Mountain spotted fever (RMSF) can be ascribed to QTT.39 Rarer severe manifestations are described. Pneumonitis can be severe enough to require mechanical ventilation, as described in a case report of a man presenting with acute respiratory distress syndome.6 Myocarditis and small-volume pericardial effusions have been described in several rickettsial infections, including R. australis.17,40 Rarely, severe vasculitis-driven skin manifestations complicate severe infection causing skin necrosis, bullae, and purpura mimicking Stevens–Johnson syndrome.6 Purpura fulminans was reported in one case series.17 Renal failure as a direct result of R. australis infection has not been described, in contrast to other rickettsial species.6,8,41 Necrotic phenomena are rare but have been reported sporadically in case reports including widespread digital necrosis and splenic infarction.5,40 Although QTT is not known to directly affect the central nervous system, some reports have documented confusion, seizures, and hallucinations as a prominent feature of this disease.15
Laboratory investigations include mild-to-moderate thrombocytopenia (platelets 50–150 × 109/L), commonly early in the disease course, transforming into a reactive thrombocytosis during recovery from severe disease.15 A transient and mild elevation of hepatic transaminases is seen in early disease.24 Leukopenia is observed in mild cases. Patients presenting with severe infection usually have neutrophilia and toxic changes on blood film.17,19 C-reactive protein measurements are usually raised significantly in systemic rickettsial infection, in contrast to uncomplicated viral infections.24
RICKETTSIA AUSTRALIS: MECHANISMS OF DISEASE
Rickettsia are small gram-negative, obligate intracellular bacteria belonging to the group alphaproteobacteria.2,25 There are 19 known pathogenic Rickettsia spp. worldwide.1 Rickettsia australis is endogenous to Australia and phylogenetically distinct from rickettsiae in other parts of the world.24,42 The R. australis genome has recently been sequenced and is 1.29 MB. Rickettsia akari (rickettsialpox) is, genetically, the most closely related known Rickettsia to R. australis.43,44 Use of new molecular-based identification techniques has resulted in the discovery of many new Rickettsia species, many of unclear pathogenicity.45 It is postulated that both bacterial and human factors play important roles in human disease,19 but further studies are required to characterize this further.
Mechanisms for invasive infection have been elucidated using guinea pig and mouse models.33,36 Postarthropod bite, at the site of R. australis inoculation, tissue damage including cutaneous vasculitis and vessel thrombosis, which may result in eschar formation.45 Rickettsia multiplication within target cells including endothelial cells and macrophages occurs at the inoculation site. Suppression of T cell immunity allows intracellular rickettsial survival.38 Direct invasion of vascular endothelial cells likely represents initiation of clinical infection. A direct relationship between pathogen infectivity and temperature has been reported, where Rickettsia spp. from ticks kept at 37 degrees-infected guinea pigs, but failed to do so at lower temperatures.46 The eschar may represent an intense local inflammatory and immune response that functions in an attempt to prevent blood-borne spread of the organism. It is not known whether the presence of an eschar is associated with disease severity,36 but it may be associated with inoculum size and an increased probability of disseminated disease. Vascular injury in the form of intramural and perivascular infiltration of lymphocytes and macrophages occurs.38 Rickettsia australis (along with Rickettsia africae and Rickettsia japonica) has been identified as causing a neutrophil-rich infiltrate in eschars.36 Severe vasculitis and vessel thrombosis can then cause isolated end-organ infarction. Phospholipase A and/or intrinsic protease activity is hypothesized to initiate invasion through blood vessel walls and endothelial cell infection.39 To date, there has been no direct identification of an endo- or exotoxin in any Rickettsia species.36
DIAGNOSIS OF QTT
Diagnosing R. australis infection can be challenging. Epidemiologic data and knowledge of high-risk exposure activities can be invaluable in considering the disease in patients presenting with a fever and rash. A high degree of suspicion is required as nonspecific symptoms in early QTT can lead to a delay in diagnosis.
Serological assays remain the main diagnostic modality for diagnosing rickettsial infections.47 The Weil–Felix Proteus OX19 agglutination assay provided a means of QTT diagnosis for most of the twentieth century.48 Currently, the indirect microimmunofluoresence assay (IFA) identifies antibodies using SFG rickettsiae antigens, including R. australis and is considered to be the gold-standard assay for diagnosing QTT.2 Acute and convalescent serum samples are taken 10–14 days apart. A 4-fold rise in SFG antibody titer or a single positive titer of 1:256 is used to indicate acute or recent infection.19 Complicating the diagnosis, there is substantial cross-reactivity of antibodies between all the SFG rickettsiae, between the SFG rickettsiae and the Typhus Group (TG) rickettsiae and with other bacterial species such as Proteus and Legionella.1 In one study, cross-reaction between SFG and TG Rickettsia occurred in 8.3% of patients.49 Concomitant illness such as rheumatologic- and immune-mediated disorders can yield false-positive rickettsial serological tests.1,47
Occasionally, patients infected with R. australis or other SFG Rickettsia do not seroconvert.47 A study of 24 confirmed cases of QTT revealed seroconversion in only 20 patients, with the remaining four having an initial specimen titer of more than 1:128.15 It was unlikely that participants’ serum samples were all taken at the same point in time during their course of infection and low titer measurements may have represented prior seroconversion. Serology results can be difficult to interpret in acute illness, with low-level titers being associated with previous SFG Rickettsia exposure and not to a patient’s current, nonrickettsial infection.2
Direct detection of Rickettsia through cell culture or DNA detection and amplification by polymerase chain reaction (PCR) carries more diagnostic specificity,49–51 but culture can be technically difficult. Culture and PCR done on blood specimens have lower sensitivity than SFG rickettsial serology. PCR swabs for detection of SFG rickettsial nucleic acid in skin lesions have been performed with varied success.49 PCR and culture on eschar lesions can be performed, with potential increased yield due to higher density of rickettsiae.50,51 Tissue cell culture using patient blood specimens has a low yield, although the isolation rate of Rickettsia is improved with specimens taken earlier in the illness course (during early rickettsaemia). Rickettsiae that subsequently grow can be detected by Gimenez stain or IFA or molecular techniques.49 Antibiotic administration can impair diagnosis based on culture and molecular techniques.50
PRINCIPLES OF TREATMENT AND OUTCOMES
Doxycycline demonstrates good activity against Rickettsia spp. and is the drug of choice for QTT (Box 1).18,28 A delay in correct antimicrobial therapy can be associated with a protracted and complicated course.1 Doxycycline should be considered in pediatric patients, despite concerns over possible dental staining. The risk of withholding therapy likely outweighs this concern. Patients usually show marked clinical improvement after 48 hours of starting treatment.41 Doxycycline should be administered orally in mild-to-moderate infection and intravenously in severe infection. The importance of antibiotics in mild R. australis infection is unclear as there are no published data on this, although early administration probably prevents hospitalization and morbidity.1
There are no data to suggest superior efficacy of any particular intracellularly active antibiotic agent for the treatment of Australian SFG rickettsial infection. In severe R. australis infection, the therapeutic approach is extrapolated from data for RMSF (R. rickettsii) studies.52 Key differences between the two diseases, such as disease severity and identification of individuals at risk of severe disease in R. australis limit the value of this extrapolation. Delay in appropriate antimicrobial therapy is associated with increased likelihood of progression to severe disease and complications.1,5 Some individuals progress to severe disease and sepsis despite early doxycycline therapy for reasons that remain uncertain. Suggested factors include concurrent comorbidities, Rickettsia inoculum size, and inherent virulence differences in rickettsial strains.1 Azithromycin has been suggested as second-line therapy based on widespread availability, but direct evidence of efficacy is limited.52 Other options include intravenous tigecycline1,40 and chloramphenicol.4 Infections with several rickettsial diseases have been shown to relapse if antimicrobial therapy is ceased as soon as patients become afebrile.33,34
Rarely will ticks remain on patients until presentation. They tend to reside in moist, hidden areas on the body such as the groin, axillae, and scalp.18 Ticks should be removed by grasping the tick either side of its mouthparts, pressing well down into the skin, while avoiding pressure on the main body of the tick. The tick can then be levered straight out with steady upward tension. This method avoids expulsion of the gut contents of the tick into the wound.18
Currently, there is no effective vaccine against any Rickettsia species, including R. australis.1 Difficulties exist in creating a universal SFG rickettsial vaccine due to variation in the chemical configuration of the immunologically important outer membrane proteins, as demonstrated in murine models.53,54 Emergence of rickettsial diseases in Oceania as a public health threat may promote research into vaccine development.
Little systematic evidence is available regarding the outcomes of acute R. australis infection, particularly for nonhospitalized patients. In the documented severe hospitalized cases with complications, a full recovery following acute illness is expected. There is no evidence of chronic infection, although a postinfective syndrome of lethargy, malaise, and muscle pains persisting for several months or more after acute infection is described.55–57
UNCERTAINTY AND FUTURE DIRECTION
There remains a limited understanding of many features of R. australis infection.16,21 Currently, there is no up-to-date epidemiological data demonstrating the true burden of rickettsial disease in Australia.7 As a potential public health threat, rickettisal infections should be a notifiable disease in Australia, with active surveillance systems in place to determine longitudinal trends. This will help in the determination of risk factors for severe disease, complications, and outcomes. A thorough understanding of the risk factors for, and outcome of, severe disease caused by R. australis is critical for optimal management to prevent morbidity and mortality.39,58 Increased understanding of microbial pathogenesis and the immune response in disseminated rickettsial infection may assist in identifying potential vaccine targets.
CONCLUSION
QTT can cause serious illness and death.46 A strong index of suspicion by Australian clinicians and early initiation of doxycycline is critical. It is a growing public health threat in coastal, eastern Australia with significant gaps in our knowledge that need to be addressed. Human and animal serological surveillance and notification systems will be essential for providing up to date and accurate data, facilitating improved public health strategies. Prospective cohort studies are necessary to establish the effect of different and time-dependent treatment strategies, as well as to define patient outcomes following mild and severe infection.
Box 1.
Recommendations for antimicrobial treatment of suspected Rickettsia australis infection52
| 1. Doxycycline 100 mg (child: 2 mg/kg up to 100 mg) orally, 12 hourly for 7 days |
| OR |
| 2. Azithromycin 500 mg (child: 10 mg/kg up to 500 mg) orally on the first day, then 250 mg (child: 5 mg/kg up to 250 mg) orally, daily for a further 4 days |
| Rifampicin may be an alternative to azithromycin in pregnancy |
Acknowledgment:
We would like to thank John Stenos from the Australian Rickettsial Reference Laboratory (ARRL) for his contribution.
REFERENCES
- 1.Derne B, Weinstein P, Musso D, Lau C, 2015. Distribution of rickettsioses in Oceania: past patterns and implications for the future. Acta Trop 143: 121–133. [DOI] [PubMed] [Google Scholar]
- 2.Laboratory Australian Rickettsial Reference, 2015. Australian Rickettsial Reference Laboratory: Disease Description and/or Epidemiology. Australian Rickettsial Reference Laboratory. Available at: http://www.rickettsialab.org.au. Accessed July 22, 2015. [Google Scholar]
- 3.Baird RW, Lloyd M, Stenos J, Ross BC, Stewart RS, Dwyer B, 1992. Characterization and comparison of Australian human spotted fever group rickettsiae. J Clin Microbiol 30: 2896–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unsworth NB, Stenos J, Faa AG, Graves SR, 2007. Three rickettsioses, Darnley Island, Australia. Emerg Infect Dis 13: 1105–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birch TF, Muller M, 2009. Severe Queensland tick typhus complicated by diabetes in south-eastern Queensland. Med J Aust 191: 290–291. [DOI] [PubMed] [Google Scholar]
- 6.McBride WJ, Hanson JP, Miller R, Wenck D, 2007. Severe spotted fever group rickettsiosis, Australia. Emerg Infect Dis 13: 1742–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NNDSS, 2013. Australia’s Notifiable Disease Status: Annual Report of the National Notifiable Diseases Surveillance System. Department of Health and Aging. Available at: http://www.commcarelink.health.gov.au/internet/main/publishing.nsf/Content/cda-pubs-annlrpt-nndssar.htm. Accessed July 22, 2015. [Google Scholar]
- 8.Faa AG, Graves SR, Nguyen C, Stenos J, 2006. A serological survey of rickettsial infections in the Gazelle Peninsula. East New Britain and a review of the literature. P N G Med J 49: 43–46. [PubMed] [Google Scholar]
- 9.Graves SR, Stewart L, Stenos J, Stewart RS, Schmidt E, Hudson S, Banks J, Huang Z, Dwyer B, 1993. Spotted fever group rickettsial infection in south-eastern Australia: isolation of rickettsiae. Comp Immunol Microbiol Infect Dis 16: 223–233. [DOI] [PubMed] [Google Scholar]
- 10.Derrick EH, 1957. The challenge of north Queensland fevers. Australas Ann Med 6: 173–188. [DOI] [PubMed] [Google Scholar]
- 11.Kende M, Graves S, 2003. Survey of rickettsial antibodies at two local sites and review of rickettsiosis in Papua New Guinea. P N G Med J 46: 53–62. [PubMed] [Google Scholar]
- 12.Pope JH, 1955. The isolation of a Rickettsia resembling Rickettsia australis in south-east Queensland. Med J Aust 42: 761–763. [PubMed] [Google Scholar]
- 13.Campbell R, Domrow R, 1974. Rickettsioses in Australia: isolation of Rickettsia tsutsugamushi and Rickettsia australis from naturally infected anthropods. Trans R Soc Trop Med Hyg 68: 397–402. [DOI] [PubMed] [Google Scholar]
- 14.Dwyer BW, Graves SR, McDonald MI, Yung AP, Doherty RR, McDonald JK, 1991. Spotted fever in East Gippsland, Victoria: a previously unrecognized focus of rickettsial infection. Med J Aust 154: 121–125. [DOI] [PubMed] [Google Scholar]
- 15.Sexton DJ, Dwyer B, Kemp R, Graves S, 1991. Spotted fever group rickettsial infections in Australia. Rev Infect Dis 13: 876–886. [DOI] [PubMed] [Google Scholar]
- 16.Andrew R, Bonnin J, Williams S, 1946. Tick typhus in north Queensland. Med J Aust 2: 253–258. [PubMed] [Google Scholar]
- 17.Graves S, Stenos J, 2009. Rickettsioses in Australia. Hechemy KE, Brouqui P, Samuel JE, Raoult DA, eds. Annals of New York Academy of Sciences, Wiley Online Library. Rickettsiology and Rickettsial Diseases, Vol. 1166, 151–155. [DOI] [PubMed] [Google Scholar]
- 18.Barker SC, Walker AR, 2014. Ticks of Australia. The species that infect domestic animals and humans. Zootaxa 3816: 1–144. [DOI] [PubMed] [Google Scholar]
- 19.Graves S, Stenos J, 2003. Rickettsioses in Australia. Raoult D, Brouqui P, eds. Rickettsiae and Rickettsial Diseases at the Turn of the Third Millenium. Marseille, France: Elsevier, 244–246. [Google Scholar]
- 20.Domrow R, Derrick EH, 1964. Ixodes holocyclus, the man-biting tick in S.E. Queensland. Aust J Sci 27: 234–236. [Google Scholar]
- 21.Brody J, 1946. A case of tick typhus in north Queensland. Med J Aust 13: 511–512. [DOI] [PubMed] [Google Scholar]
- 22.Fenner F, 1946. The epidemiology of north Queensland tick typhus: natural mammalian hosts. Med J Aust 2: 666–668. [PubMed] [Google Scholar]
- 23.Hardy MC, Cochrane J, Allavena RE, 2014. Venomous and poisonous Australian animals of veterinary importance: a rich source of novel therapeutics. BioMed Res Int 2014: 671041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graves S, 2013. Management of rickettsial diseases and Q Fever. Med Today 14: 65–69. [Google Scholar]
- 25.Azad AF, Beard CB, 1998. Rickettsial pathogens and their arthropod vectors. Emerg Infect Dis 4: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lane AM, Shaw MD, McGraw EA, O’Niell SL, 2005. Evidence of a spotted fever-like Rickettsia and a potential new vector from northeastern Australia. J Med Entomol 42: 918–921. [DOI] [PubMed] [Google Scholar]
- 27.Unsworth NB, Stenos J, Graves SR, Faa AG, Cox GE, Dyer JR, Boutlis CS, Lane AM, Shaw MD, Robson J, Nissen MD, 2007. Flinders Island spotted fever rickettsioses caused by “marmionii” strain of Rickettsia honei, eastern Australia. Emerg Infect Dis 13: 566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sexton DJ, Banks J, Graves S, Hughes K, Dwyer B, 1991. Prevalence of antibodies to spotted fever group Rickettsiae in dogs from south-eastern Australia. Am J Trop Med Hyg 45: 243–248. [DOI] [PubMed] [Google Scholar]
- 29.Huang Z, Graves S, Stewart L, Banks J, Dwyer B, 1990. Enzyme-linked immunosorbent assays for detecting antibody to Rickettsia australis in sera of various animal species. Comp Immunol Microbiol Infect Dis 13: 119–125. [DOI] [PubMed] [Google Scholar]
- 30.Knyvett AF, Sandars DF, 1964. North Queensland tick typhus: a case report defining a new endemic area. Med J Aust 2: 592–594. [DOI] [PubMed] [Google Scholar]
- 31.Streeten G, Cohens RS, Gutteridge NM, Wilmer NB, Brown HE, Smith DJW, Derrick EH, 1948. Tick typhus in south Queensland: report of three cases. Med J Aust 1: 372–373. [PubMed] [Google Scholar]
- 32.Pinn TG, Sowden D, 1998. Queensland tick typhus. Aust N Z J Med 28: 824–826. [DOI] [PubMed] [Google Scholar]
- 33.Ash M, Smithurst BA, 1995. A case of Queensland tick typhus. Med J Aust 163: 167. [DOI] [PubMed] [Google Scholar]
- 34.Hudson BJ, McPetrie RA, Kitchener-Smith J, Eccles JE, 1994. Vesicular rash due to Rickettsia australis . Clin Infect Dis 18: 118–119. [DOI] [PubMed] [Google Scholar]
- 35.Hudson BJ, McPetrie R, Ravich RB, Chambers I, Cross D, 1993. Queensland tick typhus in Sydney Harbour. Med J Aust 159: 356–357. [DOI] [PubMed] [Google Scholar]
- 36.La Scola B, Bechah Y, Lepidi H, Raoult D, 2009. Prediction of rickettsial skin eschars in humans using an experimental guinea pig model. Microb Pathog 47: 128–133. [DOI] [PubMed] [Google Scholar]
- 37.Sexton DJ, King G, Dwyer B, 1990. Fatal Queensland tick typhus. J Infect Dis 162: 779–780. [DOI] [PubMed] [Google Scholar]
- 38.Feng HM, Wen J, Walker DH, 1993. Rickettsia australis infection: a murine model of a highly invasive vasculopathic rickettsiosis. Am J Pathol 142: 1471–1482. [PMC free article] [PubMed] [Google Scholar]
- 39.Holman RC, Paddock CD, Curns AT, Krebs JW, McQuiston JH, Childs JE, 2010. Analysis of risk factors for fatal Rocky Mountain spotted fever: evidence for superiority of tetracyclines for therapy. J Infect Dis 184: 1437–1444. [DOI] [PubMed] [Google Scholar]
- 40.Wilson PA, Tierney L, Lai K, Graves S, 2013. Queensland tick typhus: three cases with unusual clinical features. Intern Med J 43: 823–825. [DOI] [PubMed] [Google Scholar]
- 41.Walker DH, Mattern WD, 1979. Acute renal failure in Rocky Mountain spotted fever. Arch Intern Med 139: 443–448. [PubMed] [Google Scholar]
- 42.Baird RW, Stenos J, Stewart R, Hudson B, Lloyd M, Aiuto S, Dwyer B, 1996. Genetic variation in Australian spotted fever group rickettsiae. J Clin Microbiol 34: 1526–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong X, El Karkouri K, Robert C, Raoult D, Fournier PE, 2012. Genome sequence of Rickettsia australis, the agent of Queensland tick typhus. J Bacteriol 194: 5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stenos J, Graves S, Dwyer B, 1992. Quantification of Rickettsia australis. Am J Trop Med Hyg 47: 141–146. [DOI] [PubMed] [Google Scholar]
- 45.Eremeeva M, Yu X, Raoult D, 1994. Differentiation among spotted fever group rickettsiae species by analysis of restriction fragment length polymorphism of PCR-amplified DNA. J Clin Microbiol 32: 803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Policastro PF, Munderloh UG, Fischer ER, Hackstadt T, 1997. Rickettsia rickettsii growth and temperature-inducible protein expression in embryonic tick cell lines. J Med Microbiol 46: 839–845. [DOI] [PubMed] [Google Scholar]
- 47.Heehemy KE, Raoult D, Fox J, Han Y, Elliott LB, Rawlings J, 1989. Cross-reaction of immune sera from patients with rickettsial diseases. J Med Microbiol 29: 199–202. [DOI] [PubMed] [Google Scholar]
- 48.Neilson GH, 1955. A case of Queensland tick typhus. Med J Aust 42: 763–764. [DOI] [PubMed] [Google Scholar]
- 49.La Scola B, Raoult D, 1997. Laboratory diagnosis of rickettsioses: current approaches to diagnosis of old and new rickettsial diseases. J Clin Microbiol 35: 2715–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Renvoisé A, Rolain JM, Socolovschi C, Raoult D, 2012. Widespread use of real-time PCR for rickettsial diagnosis. FEMS Immunol Med Microbiol 64: 126–129. [DOI] [PubMed] [Google Scholar]
- 51.Wang JM, Hudson BJ, Watts MR, Karagiannis T, Fisher NJ, Anderson C, Roffey P, 2009. Diagnosis of Queensland tick typhus and African tick bite fever by PCR of lesion swabs. Emerg Infect Dis 15: 963–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antibiotic Expert Groups, 2014. Rickettsial infections. In Therapeutic Guidelines: Antibiotic. Version 15. Melbourne, Australia: Therapeutic Guidelines Limited, 536. [Google Scholar]
- 53.Stenos J, Walker DH, 2000. The rickettsial outer-membrane protein A and B genes of Rickettsia australis, the most divergent Rickettsia of the spotted fever group. Int J Syst Evol Microbiol 50: 1775–1779. [DOI] [PubMed] [Google Scholar]
- 54.Feng HM, Walker DH, 2003. Cross-protection between distantly related spotted fever group rickettsiae. Vaccine 21: 3901–3905. [DOI] [PubMed] [Google Scholar]
- 55.Watts MR, Benn RA, Hudson BJ, Graves SR, 2008. A case of prolonged fatigue following an acute rickettsial infection. QJM 101: 591–593. [DOI] [PubMed] [Google Scholar]
- 56.Unsworth N, Graves S, Nguyen C, Kemp G, Graham J, Stenos J, 2008. Markers of exposure to spotted fever rickettsiae in patients with chronic illness, including fatigue, in two Australian populations. QJM 101: 269–274. [DOI] [PubMed] [Google Scholar]
- 57.Knyvett AF, Sandars DF, 1964. North Queensland tick typhus: a case report defining a new endemic area. Med J Aust 2: 592–594. [DOI] [PubMed] [Google Scholar]
- 58.Kirkland KB, Wilkinson WE, Sexton DJ, 1995. Therapeutic delay and mortality in cases of Rocky Mountain spotted fever. Clin Infect Dis 20: 1118–1121. [DOI] [PubMed] [Google Scholar]