Abstract.
Understanding the epidemiology of asymptomatic Plasmodium falciparum infections is critical for countries to move toward malaria elimination. Using different methods for parasite detection, we evaluated how seasonality, spatial location, and other factors affect the age-specific epidemiology of asymptomatic malaria in Bongo District, Ghana. Asymptomatic prevalence by microscopy decreased significantly from 42.5% at the end of the wet to 27.5% at the end of the dry season (P < 0.001). Using the 18S rRNA polymerase chain reactions (PCRs), all microscopy-negative samples were screened and prevalence of submicroscopic infections also decreased significantly from the wet (55.4%) to the dry (20.7%) season (P < 0.001). Combining detection methods, 74.4% and 42.5% of the population in the wet and dry seasons, respectively, had evidence of a P. falciparum infection. Interestingly in those > 20 years of age, we found evidence of infection in 64.3% of the population in the wet and 27.0% in the dry season. Using both microscopy and PCR, the asymptomatic P. falciparum reservoir peaks at the end of the wet season and infections in all age groups constitute the reservoir of malaria infection. At the end of the wet season, spatial heterogeneity in the prevalence and density of P. falciparum infections was observed between the two catchment areas surveyed in Bongo District. These results indicate that if elimination is to succeed, interventions will need to target not just P. falciparum infections in children but also in adults, and be implemented toward the end of the dry season in this area of West Africa.
INTRODUCTION
Although the coverage of malaria interventions across endemic regions has increased since 2000,1 evidence suggests that combining vector control strategies, including insecticide-treated bed nets (ITNs) and indoor residual spraying (IRS), with targeted chemotherapy programs such as intermittent preventative treatment in pregnancy and infants (IPTp and IPTi) and seasonal malaria chemoprevention (SMC) will not be sufficient to interrupt transmission and eliminate malaria.2–5 Part of the challenge of implementing chemotherapy programs to target the reservoir of Plasmodium falciparum has been the limitation of current diagnostics to accurately identify and measure the prevalence of chronic asymptomatic infections in the population, often associated with low and/or submicroscopic parasite densities.6–8 To augment and plan future malaria control programs across sub-Saharan Africa (SSA), reliable surveillance data that accounts for the size and persistence of the reservoir of asymptomatic P. falciparum infections will be necessary to fully understand the impacts of interventions on disease estimates in different epidemiological settings.
Several different diagnostic methods with varying accuracy are available to estimate parasite prevalence in the peripheral blood, including rapid diagnostic tests (RDTs), microscopy, and sensitive molecular methods (e.g., polymerase chain reaction [PCR]). RDTs are a point-of-care diagnostic originally developed in the 1990s to inform clinical treatment by measuring the presence of the P. falciparum antigen, histidine-rich protein 2 (PfHRP-2). Despite RDTs' ease of use for population-based prevalence surveys, RDTs lack specificity as the PfHRP-2 antigen can circulate for several weeks after malaria treatment leading to false-positive results due to prior infections.9 Since RDTs have limited sensitivity at parasite densities less than 100 parasites per μL of blood, they can fail to identify asymptomatic P. falciparum infections and therefore are not adequate tools for elimination.10–15 Historically microscopy has been used as the gold standard in clinical and epidemiological studies for measuring parasite prevalence. Longitudinal monitoring has revealed that although asymptomatic P. falciparum infections can have densities that are detectable by microscopy (> 10 parasites per μL of blood), more sensitive molecular methods such as PCR, which can detect infections as low as 0.05 parasites per μL of blood, are necessary to further identify submicroscopic asymptomatic infections that are frequent in endemic countries.3,6,16,17 Previous reviews in varying transmission settings have found that when microscopy is compared with PCR it fails to identify nearly half of all P. falciparum infections.16 Consequently the significance of these low-density asymptomatic infections as a reservoir for sustaining transmission has been underestimated.
Over the last 10 years, there have been various studies that have assessed the prevalence and risk factors associated with asymptomatic P. falciparum infections among children in SSA, but very few have simultaneously measured the reservoir in the general adult population.5,18,19 This study evaluated how age, seasonality, spatial location, and other relevant factors affect the epidemiology of asymptomatic P. falciparum infections in Bongo District (BD), Ghana. Using microscopy combined with sensitive molecular diagnostic methods, we found that the asymptomatic P. falciparum reservoir persists between seasons and across all age groups in BD. This finding was more widespread in this region than previously realized from studies conducted with different objectives in the neighboring district, Kassena-Nankana District (KND).20–23 Our findings are interpreted and discussed in the context of the design of future malaria interventions, which are necessary to shift endemic countries from malaria control toward elimination.
METHODS
Human subjects ethical approval.
The study was reviewed and approved by the ethics committees at the Navrongo Health Research Center, Ghana; Noguchi Memorial Institute for Medical Research, Ghana; New York University, United States; The University of Melbourne, Australia; University of Michigan, United States; and the University of Chicago, United States. Individual informed consent was obtained in the local language from each enrolled participant by signature/thumbprint accompanied by the signature of an independent witness. A parent or guardian provided consent for children under the age of 18 years, and all children between the ages of 12 and 17 years also provided assent. Pregnant women, individuals with disabilities, and individuals presenting with a serious or acute disease (including symptomatic/clinical malaria) on the day the survey was conducted were not eligible for enrollment and were excluded. All individuals requiring treatment were referred to the Vea or Soe Health Center and/or the Bongo District Hospital for appropriate care.
Study area and population.
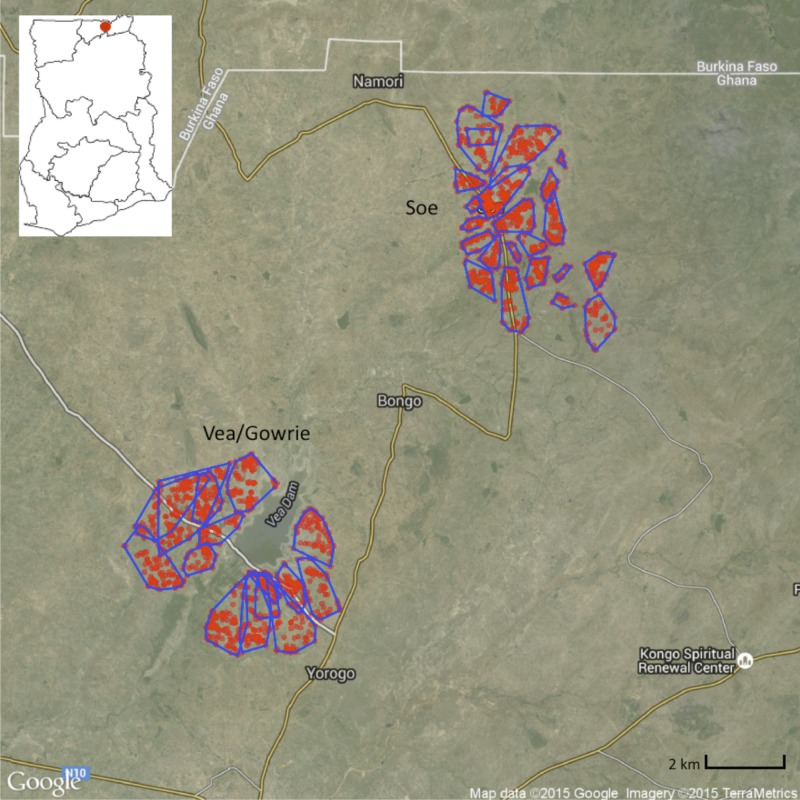
This study was conducted in BD (latitude 10°48′-10°56′N; longitude 0°44′-0°55′W) (Figure 1), which is one of 13 municipalities and districts in the Upper East Region (UER) of Ghana. The district shares boundaries with Burkina Faso to the north, KND to the west, and Bolgatanga Municipality to the south. The area is relatively flat (altitude ∼200 m) and covers 459 km2, with limited vegetation and occasional outcrops of rocks. The district borders the Sudan Savannah Zone, although it is considered part of the Guinea Savannah Zone. The area experiences approximately 70 rain days per year, with an average annual rainfall between 600 and 1,400 mm that peaks in August when more than 50% of total rainfall occurs.24,25 BD is characterized by a short rainy season (June–October) and a prolonged dry season (November–May). Monthly mean temperatures range from 20°C to 40°C, with the lowest annual temperatures in December.
Figure 1.
The distribution of the compounds in Bongo District (BD) included in this study (red points) and the sections (blue lines) within each catchment area: Vea/Gowrie (lower left) and Soe (upper right). The local topography, major centers, road network (yellow and white lines) and the Ghanaian boarder with Burkina Faso (solid white line) are also indicated. A dam and irrigation area is proximal to the Vea/Gowrie catchment area. The location of the study site within Ghana is presented in the insert map (upper left). Note: The population of BD resides in rural communities made up of small farm settlements scattered throughout the district. For the purposes of this study not all compounds/households in BD were geolocated and therefore are not included on the map. This figure appears in color at www.ajtmh.org.
Apart from the Bongo Town the district capital, 94% of the population reside in rural communities made up of small scattered farm settlements characterized by large households, high population density, and high birth rates.25 The 2010 census estimated the population of the district to be 84,545 with a population density of 186 persons/km2 and no significant in-migration.25,26 The average household size is 5.5 persons, with females constituting 53% of the population and with 50% of the population being less than 18 years of age.25 The main ethnic group in the district is the Frafra, which constitute about 94.2% of the population. In BD, the Vea Dam and irrigation area, built between 1965 and 1980, is one of the major dams built in the UER for agricultural purposes and for supporting dry season farming.27 The economy of BD is dominated by subsistence agriculture of self-used farmers, which is greatly determined by climate. Crops grown include millet, maize, rice, groundnuts, sweet potatoes, tomatoes, peppers, and other vegetables.
Malaria has remained the number one public health problem in BD, as in most parts of the region and the country. Malaria in BD is hyperendemic (stable malaria) and is characterized by marked seasonal transmission of P. falciparum (minority: Plasmodium malariae and Plasmodium ovale). The main vector is Anopheles gambiae s.l with limited transmission from Anopheles funestus (S. Dadzie, M. Appawu, personal communication). Annual P. falciparum entomological inoculation rate (EIR) in BD has been estimated to be 25.3 infectious bites per person per year, based on current data from monthly collections in 2013 between February and September (S. Dadzie, M. Appawu, personal communication). Through a “Hang-Up” Campaign in 2012, supported by the United Nations Children's Emergency Fund and the Ministry of Health/Ghana Health Services (MOH/GHS), long-lasting insecticide-treated nets were mass distributed in BD to increase household ownership and usage of bed nets, particularly in children under 5 years of age.28
Study design.
This age-stratified serial cross-sectional study was designed to evaluate the reservoir of asymptomatic Plasmodium spp. in BD using microscopy and molecular diagnostic methods. The cross-sectional study was carried out over two sequential seasons, with each survey lasting approximately 4 weeks in duration. The first survey (Survey 1) was completed at the end of the wet season in October 2012, followed by the second survey (Survey 2) at the end of the dry season between mid-May and June 2013. These two time points were selected to assess the impact of seasonal changes in transmission on the burden of malaria. In June 2012, an enumeration/demographic survey was completed for the two broad “catchment areas” in BD that were included for this study: Vea/Gowrie and Soe (Figure 1). The Vea/Gowrie and Soe catchment areas were selected as they were considered to be similar in population size, age structure, and ethnic composition; however, it was hypothesized that they could potentially differ with respect to malaria transmission as Vea/Gowrie is proximal to the Vea Dam and irrigation area.27 In contrast, Soe is not located near any large bodies of water, although smaller dams for irrigation are located throughout the catchment area. The enumeration/demographic data was used to establish the villages, sections, and compounds in each catchment area and to ensure that the study participants enrolled represented the characteristics of the general underlying population using proportional age-stratified sampling.
Based on the average enumeration data of 5.6 persons per compound, a total of 500 compounds were selected at random for the survey from each catchment area (Vea/Gowrie and Soe) allowing for 10% nonrespondents. Index compounds were selected at random from the catchment areas (Vea/Gowrie and Soe) for participant enrollment. From the index compounds, an equal number of participants in each of the age-stratified categories (1–5, 6–10, 11–20, 21–39, ≥ 40 years) were selected. Equal numbers of male and female participants were recruited sequentially into each age category until the required enrollment number for each catchment area was reached. Where necessary, participants were recruited from nearby compounds in order of proximity to the index compounds to reach the enrollment numbers required for each catchment area. At the time the study was designed, malaria parasite prevalence data in BD were limited. Based on malaria prevalence data between irrigated and nonirrigated areas from neighboring KND, an estimate risk ratio of 3.0 during the dry season for malaria prevalence between the catchments areas (Vea/Gowrie and Soe) was used. Therefore at a 95% confidence level, 80% power and sample ratio of 1:1 between the irrigated (Vea/Gowrie) and nonirrigated (Soe) catchment areas the estimated sample size per area was 865, allowing for a 15% nonresponse rate. Based on these numbers, 1,000 participants per catchment area were recruited.
Sample collection.
During Survey 1 a total of 1,961 healthy participants between the ages of 1–92 years were enrolled. Data from 61 participants were excluded as they either failed to meet the study's case definition (provided below) for an asymptomatic P. falciparum infection or had Plasmodium ssp. infections that were positive for P. malariae and/or P. ovale but not P. falciparum. Samples from 1,900 participants were included for the Survey 1 analyses. All participants enrolled during Survey 1 were also invited for Survey 2 with an 83% retention rate between the two surveys. During Survey 2 additional participants were recruited to maintain an equal number of subjects per catchment area and age group for analysis. For Survey 2 there were 1,910 participants enrolled between the ages of 1–92 years. Data from 42 participants were excluded as they either failed to meet the studies case definition for an asymptomatic P. falciparum infection or had Plasmodium ssp. infections that were positive for P. malariae and/or P. ovale but not P. falciparum. Samples from 1,868 participants were included for the Survey 2 analyses. For both surveys there were no statistical differences between the participants included for analysis and those that were excluded with respect to any of the study variables including age group, gender, catchment area, etc. (P > 0.05) (see Table 1).
Table 1.
Demographics of the study population collected at the end of the wet (Survey 1) and dry (Survey 2) seasons
| Characteristic | Survey 1: End of wet season (October 2012) |
Survey 2: End of dry season (June 2013) |
||||
|---|---|---|---|---|---|---|
| Total | Vea/Gowrie | Soe | Total | Vea/Gowrie | Soe | |
| Age groups* | ||||||
| All | 1,900 | 901 (47.4) | 999 (52.6) | 1,868 | 910 (48.7) | 958 (51.3) |
| 1–5 years | 354 (18.6) | 160 (17.8) | 194 (19.4) | 347 (18.6) | 162 (17.8) | 185 (19.3) |
| 6–10 years | 391 (20.6) | 183 (20.3) | 208 (20.8) | 397 (21.3) | 193 (21.2) | 204 (21.3) |
| 11–20 years | 405 (21.3) | 203 (22.5) | 202 (20.2) | 395 (21.1) | 207 (22.7) | 188 (19.6) |
| 21–39 years | 321 (16.9) | 151 (16.8) | 170 (17.0) | 310 (16.6) | 146 (16.0) | 164 (17.1) |
| ≥ 40 years | 429 (22.6) | 204 (22.6) | 225 (22.5) | 419 (22.4) | 202 (22.2) | 217 (22.7) |
| Gender* | ||||||
| Female | 1,016 (53.5) | 469 (52.1) | 547 (54.8) | 1,034 (55.4) | 494 (54.3) | 540 (56.4) |
| Male | 884 (46.5) | 432 (47.9) | 452 (45.2) | 834 (44.6) | 416 (45.7) | 418 (43.6) |
| Bed net usage previous night* | ||||||
| No | 210 (11.1) | 77 (8.5) | 133 (13.3) | 274 (14.7) | 98 (10.8) | 176 (18.4) |
| Yes | 1,690 (88.9) | 824 (91.5) | 866 (86.7) | 1,594 (85.3) | 812 (89.2) | 782 (81.6) |
| Antimalarial treatment (previous 2 weeks)* | ||||||
| No treatment | 1,109 (58.4) | 601 (66.7) | 508 (50.9) | 1,718 (92.0) | 866 (95.2) | 852 (88.9) |
| Treatment | 791 (41.6) | 300 (33.3) | 491 (49.1) | 150 (8.0) | 44 (4.8) | 106 (11.1) |
| Anemia status*† | ||||||
| Severe | 28 (1.5) | 17 (1.9) | 11 (1.1) | 9 (0.5) | 4 (0.4) | 5 (0.5) |
| Moderate | 439 (23.1) | 178 (19.8) | 261 (26.1) | 209 (11.3) | 93 (10.2) | 116 (12.3) |
| Mild | 420 (22.1) | 214 (23.8) | 206 (20.6) | 371 (20.1) | 179 (19.7) | 192 (20.5) |
| Nonanemic | 1,013 (53.3) | 492 (54.6) | 521 (52.2) | 1,257 (68.1) | 634 (69.7) | 623 (66.6) |
Data reflect no. (% [n/N]) of subjects.
For anemia status for Survey 2, there were 1,846 participants included in the analysis: exclusions were participants in Soe whose hemoglobin was not measured (N = 22) on the day the survey was conducted.
During recruitment in Survey 1 and 2, each participant enrolled was assigned a unique identifier code to label all study materials and biological samples. All participants enrolled completed a study questionnaire to collect information on their demographics, socioeconomic characteristics, malaria history, control behaviors, recent clinical symptoms, and recent malaria drug use; in addition, body weight, blood pressure and auxiliary temperature were measured. Five drops of blood were collected for thick/thin blood films and hemoglobin determination using an automated HemoCue® Hb 301 (Hemocue, Angelholm, Sweden) and anemia was defined according to the World Health Organization (WHO) guidelines for age and gender.29,30 The remaining blood sample was collected as a dried blood spot (DBS) onto filter paper (3MM Whatman, Maidston, United Kingdom), allowed to air dry for several hours and stored in individual self-sealing plastic bags with a desiccant for subsequent laboratory molecular analyses. The DBS were maintained away from sunlight and were stored under dry conditions at 4°C in the laboratory until they were processed for DNA extraction. Additionally a blood sample was further screened using a First Response® (HRP-2) RDT to determine the presence of a P. falciparum infection. Participants who had a positive RDT and were febrile (temperature ≥ 37.5°C) on the day the survey was conducted were considered to be symptomatic for malaria. During Survey 1 and Survey 2, 27 and 6 participants, respectively, met the criteria for symptomatic malaria and were provided with treatment by clinical personnel from the MOH/GHS. All treatments given followed the Ghana MOH/GHS Anti-Malarial Drug Policy for uncomplicated malaria.31 Sample collection, laboratory procedures and analysis were performed by trained phlebotomists/laboratory technicians and standardized between both catchment areas and surveys.
Parasitological measurements.
Parasite densities were counted against white blood cells (WBC) on 10% Giemsa-stained thick film blood smears and examined under oil immersion of a 100-fold magnification. Two experienced technicians each independently read all the study slides and the results were reported into separate structured laboratory books. Parasite densities were counted per 200 WBC according to the WHO standards, and were calculated by averaging the two independent readings. When the slide readings were nonconcordant for either Plasmodium species identified and/or the counts recorded by more than 50%, the slide was reread by a third independent technician whose readings were final, and used as the tiebreaker. The parasite densities were further quantified as parasites per microliter of blood assuming an average WBC count of 8,000 per μL of blood.32 Parasite species were also identified using a 100-fold magnification of the thin film smears and categorized based on morphology. Additionally, gametocytes were also counted and characterized when present.
Case definitions.
Currently there is no standard definition or a diagnostic criterion for defining a “case” of malaria as asymptomatic since it often varies between studies. Generally the most widely accepted criteria for diagnosing asymptomatic malaria includes the detection of asexual and/or sexual parasites in peripheral blood combined with the absence of fever and/or other relevant clinical symptoms associated with malaria during a specified time frame.3,33–37 For this study individuals who tested positive for P. falciparum (including mixed P. falciparum infections) by either microscopy or 18S rRNA PCR, were afebrile (temperature < 37.5°C) on the day the survey was conducted and did not report a history of fever in the 24 hours prior to being surveyed, were defined as having an “asymptomatic P. falciparum infection.” Analyses for Survey 1 and Survey 2 were completed on all asymptomatic infections that were positive for P. falciparum (including mixed P. falciparum infections) by microscopy or 18S rRNA PCR. Individuals who tested positive for P. falciparum (including mixed P. falciparum infections) by microscopy were defined as having an “asymptomatic microscopic infection.” Individuals with an “asymptomatic submicroscopic infection” were further defined as those who tested negative for P. falciparum by microscopy, but were positive for P. falciparum (including mixed P. falciparum infections) by the 18S rRNA PCR. Since the data from both cross-sectional surveys were collected at a single time point, no follow up of the enrolled participants was completed.
DNA extraction.
From each DBS sample two 5 mm × 5 mm sections were cut and placed in appropriatly labeled 1.5 mL centrifuge tubes. Genomic DNA was extracted from the DBS using the QIAamp™ DNA blood mini kit extraction procedure as described by the manufacturer (Qiagen, Valencia, California, United States). The DNA samples were eluted into 50 μL of buffer AE and stored at −20°C for short-term use and −80°C for long-term storage.
Molecular estimation of submicroscopic parasite prevalence.
If a sample was found to be negative for P. falciparum by microscopy a polymerase chain reaction (PCR) was performed to further detect the presence of submicroscopic Plasmodium spp. Using previously published protocols with modifications, a nested PCR targeting the 18S ribosomal RNA gene (18S rRNA) of P. falciparum, P. malariae, P. ovale, and P. vivax was used to detect parasite DNA for all microscopy negative samples.38,39 All PCR reactions were carried out in a total volume of 20 μL consisting of 1× buffer, 2 mM MgCl2, 0.125 mM dNTPs, 0.125 μM of each primer and 0.4 units of GoTaq G2 Flexi DNA polymerase (Promega), 2 μL of purified DNA template was used for the first round of the nested PCR using the rPLU5 and rPLU6 primer pair.38 The second round of the nested PCR was carried out in four separate tubes, each containing a single species-specific primer pair (rFAL1/rFAL2, rMAL1/rMAL2, rOVA1/rOVA2, rVIV1/rVIV2) as described in the literature,38 with 2 μL aliquot of the PCR product from the first PCR round. Modifications were made to the cycling conditions for both the first and second rounds as follows: 95°C for 2 min, 25 cycles of 58°C for 2 min, 72°C for 5 min and 94°C for 1 min, followed by 58°C for 2 min ad 72°C for 2 min. For each PCR reaction, positive (3D7) and negative controls were included for quality control. PCRs were carried out on an Eppendorf Mastercycler nexus and visualized on a 2% agarose gel stained with EZ Vision™ DNA Dye/Loading Buffer (Amresco) to assign samples as parasite positive by species. A positive result was noted for P. falciparum, P. malariae, P. ovale, and P. vivax at 205 bp, 144 bp, 800 bp, and 120 bp, respectively. For each survey and catchment area, a subset of 25 asymptomatic microscopic P. falciparum-positive samples were randomly selected and further evaluated using the 18S rRNA PCR to look for the reliability between microscopy and PCR. All microscopy-positive samples (N = 100) examined produced the expected band at 205 bp for P. falciparum by gel electrophoresis.
Molecular estimation of multiplicity of infection.
For a subset of children (≤ 12 years) from each survey with a positive microscopy result (Survey 1 N = 200; Survey 2 N = 200), a nested PCR was performed to determine the number of P. falciparum merozoite surface protein 2 (msp2) (IC/3D7 and FC27) gene clones. The PCR was completed as described by Snounou and others with modifications.40 All PCR reactions were carried out in a total volume of 20 μL consisting of 1× buffer, 2 mM MgCl2, 0.125 mM dNTPs, 0.2 μM of each primer, and 0.4 units of GoTaq G2 Flexi DNA polymerase (Promega), 2 μL of purified DNA template was used in the first round of the nested PCR using the OF and OR primer pair. The second round of the nested PCR was carried out in two separate tubes, each containing a single msp2 primer pair (IC1/IC2 or FC1/FC2) as previously described in the literature with 2 μL aliquot of the PCR product from the first PCR round. Modifications were made to the cycling conditions for both the first and second rounds: Round 1 (95°C for 2 min, 30 cycles of 94°C for 1 min, 56°C for 2 min and 72°C for 2 min, followed by 72°C for 10 min) and Round 2 (95°C for 2 min, 30 cycles of 94°C for 1 min, 57°C for 2 min and 72°C for 2 min, followed by 72°C for 10 min). For each PCR reaction, positive (3D7) and negative controls were included for quality control and all PCRs were carried out on an Eppendorf Mastercycler nexus and visualized on a 2% agarose gel stained with EZ Vision™ DNA Dye/Loading Buffer (Amresco) to determine the number of infecting IC/3D7 and FC27 clones, and to calculate the multiplicity of infection (MOI).
Statistical analysis.
Statistical analyses were carried out using IBM SPSS Statistics (Version 22) software. A test was deemed to be statistically significant if the P value was less than 0.05. For all analyses study participants were categorized into defined age groups (1–5, 6–10, 11–20, 21–39, ≥ 40 years) and catchment areas (Vea/Gowrie and Soe) as described in the study design. Continuous variables are presented as medians with interquartile ranges (IQRs) and discrete variables are presented using the calculated/observed prevalence values with 95% confidence intervals (CIs). Fisher exact tests or χ2 were used for univariate analyses of discrete variables to compare proportions; nonparametric Mann–Whitney U (comparing distributions across two groups) and Kruskal-Wallis (comparing distributions across k groups) tests were used for comparing distributions across groups of continuous variables. Logistic regression was used to assess the factors associated with having an asymptomatic P. falciparum infection (microscopic and submicroscopic), with a univariate analysis being performed first. Independent variables examined as predictors included year/season of survey, age group, gender, catchment area, bed net usage the previous night, and antimalarial drug treatment in the previous 2 weeks. The final adjusted multivariate logistic regression was performed by retaining all independent variables with a P value less than 0.20 in a backward fashion. A Homer-Lemeshow goodness of fit test was used to verify the how well the data fit the logistic regression model. If a P value less than 0.05 was obtained the model was not considered to be a good fit for the data.
RESULTS
Study population demographics.
At the end of the wet (Survey 1) and dry (Survey 2) seasons there were no significant differences observed in the proportion of participants sampled across all age categories for both the total study population and within/between the catchment areas (Table 1). During both surveys there was a similar proportion of female and male participants surveyed in the 1–5, 6–10, and 11–20 years age categories, with significantly more females than male participants being sampled in the 21–39 and ≥ 40 years age groups at the end of the wet (P = 0.006 and P < 0.001) and the end of the dry (P = 0.001 and P < 0.001) season.
At the end of the wet season, 88.9% (95% CI: 88.2–89.7%) individuals reported sleeping under a bed net the previous night, with participants ages 1–5 years being significantly more likely to have reported using a bed net than the older age groups surveyed (P < 0.001). The same trend was observed at the end of the dry season, with 85.3% (95% CI: 84.5–86.2%) of the population sleeping under a bed net the previous night with participants between the ages of 11–20 and ≥ 40 years being significantly less likely to have reported using a bed net (P < 0.021). When the seasonal surveys were compared, it was found that at the end of the wet season (odds ratio [OR]: 1.38 [95% CI: 1.14–1.68]; P = 0.001) participants were more likely to have slept under a bed net the previous night than at the end of the dry season. During the wet and dry seasons female participants were significantly more likely to have reported sleeping under a bed net the previous night than the male participants surveyed (OR: 1.65 [95% CI: 1.22–2.21]; P = 0.001 and OR: 1.53 [95% CI: 1.18–1.98]; P = 0.001, respectively, in Survey 1 and 2). When bed net usage was compared by catchment area it was found that participants in Soe during the wet season (OR: 0.61 [95% CI: 0.45–0.82]; P = 0.001) and the dry season (OR: 0.54 [95% CI: 0.41–0.70]; P < 0.001) were less likely to have slept under a bed net the previous night compared with participants in Vea/Gowrie.
Overall 41.6% (95% CI: 40.5–42.8%) and 8.0% (95% CI: 7.4–8.7%) of participants at the end of the wet and dry seasons, respectively, reported taking an antimalarial medication in the 2 weeks prior to being surveyed. In both surveys, 1- to 5-year-olds were significantly more likely to have reported taking an antimalarial medication in the previous 2 weeks than the other age groups surveyed (P < 0.001 and P < 0.022, respectively, Survey 1 and Survey 2). When the surveys were compared it was found that at the end of the wet season participants were more likely to have taken an antimalarial in the previous 2 weeks (OR: 8.169 [95% CI: 6.754–9.880]; P < 0.001) than at the end of the dry season. At the end of the wet season, women were more likely to have reported taking an antimalarial treatment than the male participants surveyed (OR: 1.23 [95% CI: 1.03–1.48]; P = 0.025). When previous antimalarial treatment was compared between catchment areas, participants in Soe were more likely to have taken an antimalarial in the previous 2 weeks compared with participants in Vea/Gowrie during both the wet season survey (OR: 1.94 [95% CI: 1.61–2.33]; P < 0.001) and the dry season survey (OR: 2.45 [95% CI: 1.70–3.52]; P < 0.001). Of those participants who had reported receiving an antimalarial treatment, 47.0% (95% CI: 45.3–48.8%) at the end of the wet and 99.3% (95% CI: 98.7–100%) at the end of the dry season identified they had received treatments that followed the MOH/GHS Anti-Malarial Drug Policy on ACTs for uncomplicated malaria.31
During the wet season survey, 46.7% (95% CI: 45.4–47.8%) of individuals were categorized as anemic. In the adjusted model, participants between the ages of 1–5 years compared with the other age categories were significantly more likely to be anemic (P < 0.001). In addition at the end of the wet season participants with asymptomatic microscopic P. falciparum infections were significantly more likely to be anemic [52.6% (95% CI: 50.8–54.4%) versus 42.9% (95% CI: 40.7–45.2%), P = 0.028]. In the dry season, the number of participants classified as anemic declined significantly from the end of wet season to 31.9% (95% CI: 30.8–33.0%, P < 0.001). In the adjusted model for the dry season survey, participants between the ages of 1–5 years compared with the older age groups (> 20 years) were significantly more likely to be anemic (P < 0.006). It was also found that at the end of the dry season asymptomatic (combined microscopic and submicroscopic) P. falciparum infected participants were significantly more likely to be categorized as anemic [40.1% (95% CI: 38.4–41.9%) versus 25.9% (95% CI: 24.5–27.2%), P < 0.001].
Plasmodium falciparum prevalence.
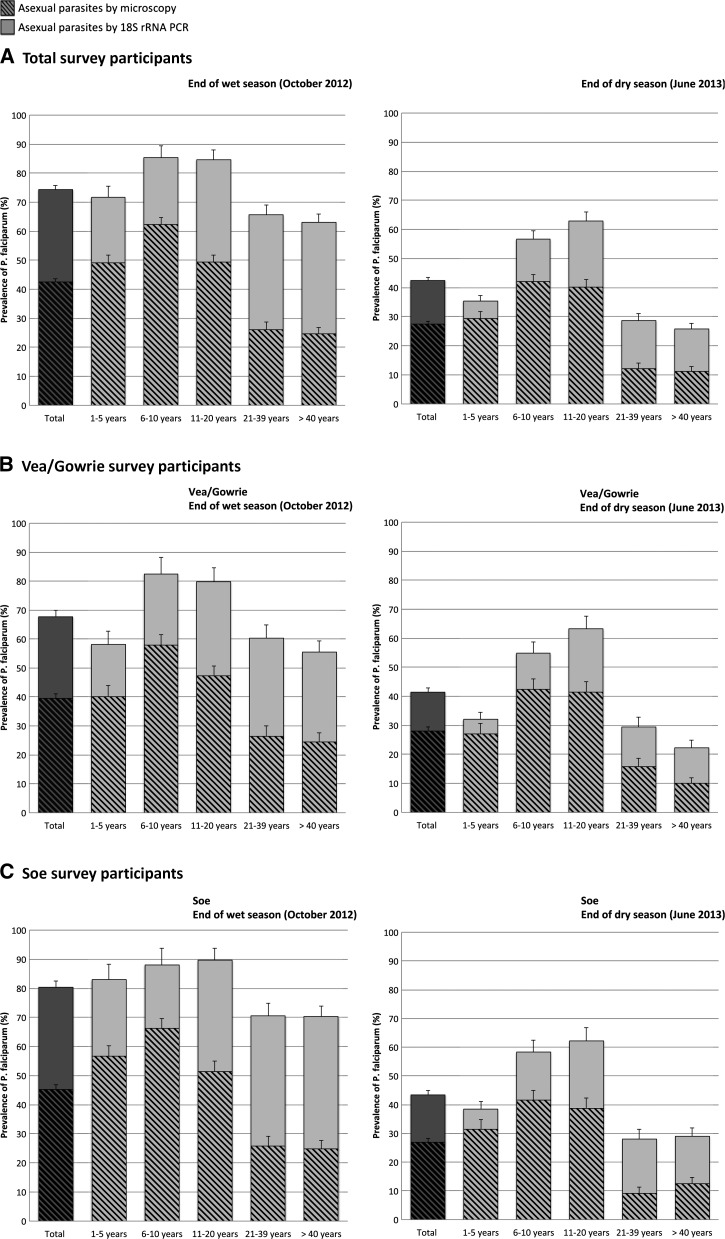
Table 2 summarizes the parasitological characteristics of asymptomatic P. falciparum infections stratified by method of parasite detection (microscopy and 18S rRNA PCR), year of survey, participants' age, sex, and catchment area. The prevalence of asymptomatic microscopic P. falciparum infections decreased significantly from 42.5% (95% CI: 41.4–43.6%) at the end of wet season) to 27.5% (95% CI: 26.5–28.5%) at the end of the dry season (P < 0.001). The prevalence of asymptomatic submicroscopic infections at the end of the wet season was found to be significantly higher than at the end of the dry [55.4% (95% CI: 53.9–56.9%) versus 20.7% (95% CI: 19.6–21.8%), respectively, in Survey 1 and Survey 2; P < 0.001] (Table 2). The combined prevalence of asymptomatic P. falciparum infections was significantly higher in the wet season survey compared with the dry season survey [74.4% (95% CI: 73.4–75.4%) versus 42.5% (95% CI: 41.4–43.6%), respectively, in Survey 1 and 2; P < 0.001] with this trend being observed within all age groups and across both catchment areas (Table 2, Figure 2). Using microscopy the prevalence of P. falciparum gametocytes was 1.9% (95% CI: 1.6–2.2%) at the end of the wet season and 0.3% (95% CI: 0.2–0.5%) at the end of the dry season.
Table 2.
Parasitological characteristics of the asymptomatic Plasmodium falciparum infections collected at the end of the wet (Survey 1) and dry (Survey 2) seasons
| Characteristic | Survey 1: End of wet season (October 2012) |
Survey 2: End of dry season (June 2013) |
||||
|---|---|---|---|---|---|---|
| Total | Vea/Gowrie | Soe | Total | Vea/Gowrie | Soe | |
| Microscopic P. falciparum prevalence | ||||||
| Number of participants* | 1,900 (100) | 901 (100) | 999 (100) | 1,868 (100) | 910 (100) | 958 (100) |
| Age groups† | ||||||
| All | 808 (42.5) | 356 (39.5) | 452 (45.2) | 513 (27.5) | 255 (28.0) | 258 (26.9) |
| 1–5 years | 174 (49.2) | 64 (40.0) | 110 (56.7) | 102 (29.4) | 44 (27.2) | 58 (31.4) |
| 6–10 years | 244 (62.4) | 106 (57.9) | 138 (66.3) | 167 (42.1) | 82 (42.5) | 85 (41.7) |
| 11–20 years | 200 (49.4) | 96 (47.3) | 104 (51.5) | 159 (40.3) | 86 (41.5) | 73 (38.8) |
| 21–39 years | 84 (26.2) | 40 (26.5) | 44 (25.9) | 38 (12.3) | 23 (15.8) | 15 (9.1) |
| ≥ 40 years | 106 (24.7) | 50 (24.5) | 56 (24.9) | 47 (11.2) | 20 (9.9) | 27 (12.4) |
| Gender† | ||||||
| Female | 380 (37.4) | 159 (33.9) | 221 (45.2) | 240 (23.2) | 120 (24.3) | 120 (22.2) |
| Male | 428 (48.4) | 197 (45.6) | 231 (51.1) | 273 (32.7) | 135 (32.5) | 138 (33.0) |
| P. falciparum median density‡ | ||||||
| Age groups | ||||||
| All | 520 [160–1,640] | 360 [160–1,200] | 680 [200–2,190] | 160 [80–560] | 160 [80–520] | 200 [80–600] |
| 1–5 years | 1,600 [360–9,860] | 1,040 [240–3,320] | 1,820 [580–13,510] | 440 [120–1,270] | 460 [130–1,180] | 420 [120–1,380] |
| 6–10 years | 760 [240–1,900] | 520 [200–1,370] | 900 [280–3,120] | 240 [120–680] | 280 [120–680] | 240 [120–780] |
| 11–20 years | 320 [130–760] | 280 [160–720] | 360 [120–820] | 120 [80–400] | 120 [80–240] | 160 [80–500] |
| 21–39 years | 200 [120–720] | 200 [120–540] | 200 [130–880] | 120 [40–170] | 120 [80–280] | 80 [40–160] |
| ≥ 40 years | 200 [120–680] | 200 [120–560] | 260 [120–260] | 80 [40–160] | 80 [40–150] | 80 [40–160] |
| Gender | ||||||
| Female | 520 [160–1,630] | 400 [160–1,400] | 640 [200–1,840] | 160 [80–480] | 160 [80–500] | 160 [50–480] |
| Male | 500 [160–1,670] | 320 [160–1,000] | 680 [160–2,760] | 200 [100–600] | 160 [80–520] | 220 [120–740] |
| Submicroscopic P. falciparum prevalence | ||||||
| Number of participants§ | 1,092 (100) | 545 (100) | 547 (100) | 1,355 (100) | 655 (100) | 700 (100) |
| Age groups‖ | ||||||
| All | 605 (55.4) | 254 (46.6) | 351 (64.2) | 280 (20.7) | 122 (18.6) | 158 (22.6) |
| 1–5 years | 80 (44.4) | 29 (30.2) | 51 (60.7) | 21 (8.6) | 8 (6.8) | 13 (10.2) |
| 6–10 years | 90 (61.2) | 45 (58.4) | 45 (64.3) | 58 (25.2) | 24 (21.6) | 34 (28.6) |
| 11–20 years | 143 (69.8) | 66 (61.7) | 77 (78.6) | 89 (37.7) | 45 (37.2) | 44 (38.3) |
| 21–39 years | 127 (53.6) | 51 (45.9) | 76 (60.3) | 51 (18.8) | 20 (16.3) | 31 (20.8) |
| ≥ 40 years | 165 (51.1) | 63 (40.9) | 102 (60.4) | 61 (16.4) | 25 (13.7) | 36 (18.9) |
| Gender‖ | ||||||
| Female | 335 (52.7) | 136 (43.9) | 199 (61.0) | 139 (17.5) | 63 (16.8) | 76 (18.1) |
| Male | 270 (59.2) | 118 (50.2) | 152 (68.8) | 141 (25.1) | 59 (21.0) | 82 (29.3) |
Number of participants sampled that were analyzed by microscopy.
Data reflect no. (% [n/N]) of participants sampled that were microscopically positive for P. falciparum (including mixed P. falciparum infections).
Median parasite density for microscopically positive P. falciparum (including mixed P. falciparum infections) (value/μL, interquartile range [IQR]) samples.
Number of microscopically negative samples that were analyzed by 18S rRNA polymerase chain reaction (PCR).
Data reflect no. (% [n/N]) of microscopically negative samples that were 18S rRNA PCR positive for P. falciparum (including mixed P. falciparum infections).
Figure 2.
Asymptomatic Plasmodium falciparum prevalence as determined by microscopy and 18S rRNA polymerase chain reactions (PCR) in relation to age group and survey/season. Hatched bars indicate individuals with microscopically detectable P. falciparum parasites; and solid bars, submicroscopic P. falciparum parasites detected by 18S rRNA PCR. The darker hatched and solid bars represent the data for the total number of participants surveyed.(A) Prevalence among all participants surveyed in Bongo District. (B) Prevalence among participants surveyed in Vea/Gowrie. (C) Prevalence among participants surveyed in Soe. Error bars represent the upper limits of the 95% confidence interval (CI).
Age-specific Plasmodium falciparum prevalence.
Asymptomatic microscopic and submicroscopic P. falciparum infections occurred across all age groups. The highest prevalence of a microscopic infection occurred in children between 6 and 10 years of age in both Survey 1 and Survey 2, with prevalence decreasing as expected among older age groups (Table 2, Figure 2); 62.4% (95% CI: 60.0–64.8%) of 6- to 10-year-olds in the wet and 42.1% (95% CI: 39.6–44.6%) of 6- to 10-year-olds in the dry season had microscopic P. falciparum infections (Table 2). Among the participants that were microscopy negative in Survey 1 and Survey 2, the highest prevalence of submicroscopic infections was detected in adolescents between 11 and 20 years; 69.8% (95% CI: 66.6–73.0%) of 11- to 20-year-olds at the end of the wet season and 37.7% (95% CI: 34.5–40.9%) of 11- to 20-year-olds at the end of the dry season that were microscopy negative had submicroscopic P. falciparum infections. During both cross-sectional surveys, parasite prevalence declined in older age groups (Figure 2), yet surprisingly among participants > 20 years of age, submicroscopic infections made up a larger proportion (more than half of the total number) of the total number of asymptomatic P. falciparum infections identified using both detection methods (microscopy and 18S rRNA PCR) compared with the younger age groups. At the end of the wet season (Survey 1) 64.3% (95% CI: 62.6–66.0%) of adults (> 20 years) had an asymptomatic P. falciparum infection, with 60.6% of these infections being identified as submicroscopic (Figure 2A). At the end of the dry season (Survey 2) asymptomatic P. falciparum prevalence decreased in adults (> 20 years) to 27.0% (95% CI: 25.4–28.6%), however 56.9% of these infections were submicroscopic (Figure 2A). This pattern of the older groups (21–39 and ≥ 40 years) carrying a greater percentage of submicroscopic P. falciparum infections was also observed across both catchment areas during Survey 1 and Survey 2 (Figure 2B and C).
Gender-specific Plasmodium falciparum prevalence.
In both Survey 1 and Survey 2, men compared with women showed a significantly higher prevalence of asymptomatic microscopic P. falciparum infections [48.4% (95% CI: 46.7–50.1%) versus 37.4% (95% CI: 35.9–38.9%) and 32.7% (95% CI: 31.1–34.4%) versus 23.2% (95% CI: 21.9–24.5), respectively, in Surveys 1 and 2; P < 0.001 and P < 0.001] (Table 2). The same trend was observed for asymptomatic submicroscopic P. falciparum infections, with the prevalence of infection being significantly higher in men compared with women during both Survey 1 [59.2% (95% CI: 56.9–61.5%) versus 52.7% (95% CI: 50.7–54.7); P = 0.032] and Survey 2 [25.1% (95% CI: 23.3–27.0) versus 17.5% (95% CI: 16.2–18.9%); P = 0.001] (Table 2). These differences in the prevalence of asymptomatic infections by gender were observed in both catchment areas and were only significant within the adult age categories (> 20 years).
Comparison of Plasmodium falciparum prevalence between catchment areas.
At the end of the wet season (Survey 1) the prevalence of asymptomatic P. falciparum infections in Vea/Gowrie was significantly lower compared with Soe. This trend was observed for both asymptomatic microscopic [39.5% (95% CI: 37.9–41.1%) versus 45.2% (95% CI: 43.7–46.8%), respectively, Vea/Gowrie and Soe; P = 0.012] and asymptomatic submicroscopic [46.6% (95% CI: 44.5–48.7%) versus 64.2% (95% CI: 62.1–66.2%), respectively, Vea/Gowrie and Soe; P < 0.001] P. falciparum infections (Table 2, Figure 2). In contrast at the end of the dry season (Survey 2), there were no significant differences in asymptomatic microscopic or submicroscopic P. falciparum infection prevalences between Vea/Gowrie and Soe (Table 2, Figure 2).
Plasmodium falciparum MOI.
During Survey 1, both younger (1–5 years) and older (6–12 years) children had a median MOI of 3 and did not differ significantly [3 (IQR: 2–4) versus 3 (IQR: 2–4), respectively, 1–5 years and 6–12 years; P = 0.624]. When comparisons were made between catchment areas in Survey 1, children between 1 and 5 years in Soe had a significantly greater median MOI compared with 1- to 5-year-olds in Vea/Gowrie [3 (IQR: 2–4) versus 2 (IQR: 1–3), respectively, in Soe and Vea/Gowrie; P = 0.048]. During Survey 2, younger (1–5 years) and older (6–12 years) children had a median MOI of 3 and did not differ significantly [3 (IQR: 2–4) versus 3 (IQR: 2–4), respectively, 1–5 years and 6–12 years; P = 0.936]. During Survey 2, children between 6 and 12 years in Soe had a significantly greater median MOI compared with 6- to 12-year-olds in Vea/ Gowrie [3 (IQR: 2–4) versus 2 (IQR: 2–3), respectively, in Soe and Vea/Gowrie; P = 0.022]. When median MOI for all children was compared between Surveys 1 and 2 it was found that the median MOI between the surveys did not differ significantly [3 (IQR: 2–4) versus 3 (IQR: 2–4), respectively, Survey 1 and Survey 2; P = 0.473].
Plasmodium falciparum density.
This study was focused on diagnosing asymptomatic P. falciparum infections and as anticipated participants of all ages were found to have low to moderate densities (40–9,999 parasites/μL) with the proportion having densities ≥ 10,000 parasites/μL being 8.7% in Survey 1 and 1.0% in Survey 2. Median P. falciparum density was significantly greater in Survey 1 compared with that found during Survey 2 (520 parasites/μL versus 160 parasites/μL, respectively, Survey 1 and Survey 2; P < 0.001) (Table 2). With respect to age, the median P. falciparum density was highest in the 1- to 5-year-olds compared with the older age groups for both Surveys 1 and 2 (Table 2). Median density for 1–5, 6–10, and 11–20 year olds was significantly higher in Survey 1 compared with Survey 2 (P < 0.001) but did not change appreciably between surveys for the 21–39 and ≥ 40 years age categories (P > 0.235) (Table 2).
During Survey 1 the median P. falciparum densities were statistically higher in 1–5 and 6–10 year olds in Soe compared with the same age categories in Vea/Gowrie (P < 0.031). However during Survey 2 there were no significant differences in the median P. falciparum densities between Vea/Gowrie and Soe based on age categories surveyed. In Survey 1 there were no significant differences in median P. falciparum densities between genders (Table 2). Conversely during Survey 2 parasitemia was significantly higher for men compared with women (200 parasites/μL versus 160 parasites/μL, respectively, P = 0.031). With regard to catchment area, in Survey 1 the median P. falciparum density in Vea/Gowrie was significantly lower compared with Soe (360 parasites/μL versus 680 parasites/μL, respectively, P < 0.001). However at the end of the dry season (Survey 2), there were no significant differences in median density between Vea/Gowrie and Soe (Table 2). These data further support previous analyses that at the end of the wet season participants in Soe not only had a higher prevalence of asymptomatic infection, but that these infections also had greater median P. falciparum densities.
Risk factors associated with asymptomatic microscopic infections.
To evaluate the factors associated with having an asymptomatic microscopic infection compared with being microscopically negative for malaria, all individuals who were positive for P. falciparum (including mixed P. falciparum infections) by microscopy were included (Supplemental Table 1). In the adjusted model, participants from Soe in Survey 1 had 1.36 (95% CI: 1.12–1.65, P = 0.002) times the odds of having an asymptomatic microscopic infection compared with participants in Vea/Gowrie. In Survey 2 there was no difference in the odds of an asymptomatic microscopic infection between the catchment areas (Supplemental Table 1). Participants between the ages of 6–10 years had 1.70 (95% CI: 1.28–2.30, P < 0.001) and 1.71 (95% CI: 1.26–2.33, P = 0.001) times the chance of having an asymptomatic microscopic infection compared with children ≤ 5 years in Survey 1 and Survey 2, respectively. Participants between the ages of 6–10 years were significantly more likely to have an asymptomatic microscopic infection compared with children ≤ 5 years in Survey 1 (P < 0.001) and Survey 2 (P = 0.001) (Supplemental Table 1). Adults (> 20 years) in Survey 1 and Survey 2 were less than half as likely to have an asymptomatic infection as detected by microscopy compared with children ≤ 5 years (P < 0.001, Supplemental Table 1). Men had 1.39 (95% CI: 1.14–1.68, P = 0.001) and 1.36 (95% CI: 1.10–1.68, P = 0.005) times the odds of having an asymptomatic microscopic infection compared with women in Survey 1 and Survey 2, respectively. Those who reported taking an antimalarial drug in the previous 2 weeks were less likely to have an asymptomatic microscopic infection in Survey 1 (OR = 0.79 [95% CI: 0.64–0.97], P = 0.021) and Survey 2 (OR = 0.56 [95% CI: 0.36–0.87], P = 0.010) compared with participants who reported not having taken an antimalarial medication. A Hosmer-Lemeshow test was performed to verify the quality of the model fit. Survey 1: Microscopic adjusted logistic regression (χ2 = 4.56, P = 0.80) and Survey 2: Microscopic adjusted logistic regression (χ2 = 6.34, P = 0.50). The tests confirm the good fit of the models.
Risk factors associated with asymptomatic submicroscopic infections compared with asymptomatic microscopic infections.
To evaluate the factors associated with an asymptomatic submicroscopic infection compared with an asymptomatic microscopic infection, all individuals who were positive for P. falciparum (including mixed P. falciparum infections) by either 18S rRNA PCR or microscopy were included (Supplemental Table 2). In the adjusted models there were no significant differences in the odds of a submicroscopic compared with a microscopic infection between the catchment areas during both Survey 1 and Survey 2. Participants > 20 years of age were significantly more likely to have an asymptomatic submicroscopic infection than a microscopic infection compared with children ≤ 5 years in both Survey 1 and Survey 2 (P < 0.001, Supplemental Table 2). During Survey 1, men were significantly less likely to have a submicroscopic infection than a microscopic infection (OR = 0.77 [95% CI: 0.62–0.96], P = 0.019) compared with women (Supplemental Table 3). However during Survey 2, there were no differences in the odds of a submicroscopic versus a microscopic infection between genders. A Hosmer-Lemeshow test was performed to verify the quality of the model fit. Survey 1: Submicroscopic adjusted logistic regression (χ2 = 3.41, P = 0.91) and Survey 2: Submicroscopic adjusted logistic regression (χ2 = 1.46, P = 0.98). The tests confirm the good fit of the models.
Risk factors associated with asymptomatic submicroscopic infections.
To evaluate the factors associated with an asymptomatic submicroscopic infection, all individuals who were positive for P. falciparum by 18S rRNA PCR compared with being negative (by both microscopy and PCR) for P. falciparum were included (Supplemental Table 3). In the adjusted models, participants from Soe in Survey 1 had 2.26 (95% CI: 1.75–2.91, P < 0.001) times the odds of having an asymptomatic submicroscopic infection compared with participants in Vea/Gowrie. In Survey 2 the same trend was observed and participants in Soe had 1.39 (95% CI: 1.05–1.83, P = 0001) times the odds of also having an asymptomatic infection compared Vea/Gowrie. This finding was in contrast to the results for P. falciparum infection by microscopy in Survey 2, where no difference in the odds was found. These results suggest that although the catchment areas appear similar at the end of the dry season with respect to the prevalence of microscopic infections, Soe has a higher prevalence of low-density asymptomatic infections at the end of the dry. This difference could partially explain the significant difference in the burden of asymptomatic malaria between Soe and Vea/Gowrie observed at the end of the wet season. Participants between the ages of 11–20 years were at increased odds of having an asymptomatic submicroscopic infection compared with children ≤ 5 years in Survey 1 (P < 0.001) and Survey 2 (P < 0.001) (Supplemental Table 3). Men had 1.32 (95% CI: 1.02–1.70, P = 0.035) and 1.60 (95% CI: 1.22–2.11, P = 0.001) times the odds of having an asymptomatic submicroscopic infection compared with women in Survey 1 and Survey 2, respectively. These results support the previous differences in the burden of asymptomatic microscopic malaria between genders. Those who reported taking an antimalarial drug in the previous 2 weeks were less likely to have an asymptomatic submicroscopic infection in Survey 1 (P = 0.017) compared with participants who reported not having taken an antimalarial (Supplemental Table 3). A Hosmer-Lemeshow test was performed to verify the quality of the model fit. Survey 1: Submicroscopic adjusted logistic regression (χ2 = 8.09, P = 0.43) and Survey 2: Submicroscopic adjusted logistic regression (χ2 = 1.87, P = 0.98). The tests confirm the good fit of the models.
Risk factors associated with asymptomatic (combined microscopic and submicroscopic) infections.
To evaluate the factors associated with an asymptomatic infection compared with being negative (by both microscopy and PCR) for P. falciparum, all participants who were found to be positive for P. falciparum (including mixed P. falciparum infections) by microscopy and 18S rRNA PCR were combined for the analyses (Table 3). In the adjusted models, participants at the end of the dry season (Survey 2) were significantly less likely to have an asymptomatic infection compared with participants at the end of the wet season (Survey 1) (P < 0.001, Table 3). Overall it was found that participants in Soe had 1.57 (95% CI: 1.36–1.82, P < 0.001) times the odds of having an asymptomatic infection compared with participants in Vea/Gowrie. Participants between the ages of 6–10 years and 11–20 were at increased odds of having an asymptomatic infection compared with children ≤ 5 years, with the adult categories (21–39 and ≥ 40 years) being significantly less likely to have an asymptomatic infection compared with children ≤ 5 years (Table 3). It was also observed that gender influenced the prevalence of asymptomatic infections, with men being 1.50 (95% CI: 1.30–1.73, P < 0.001) times more likely to have an asymptomatic P. falciparum infection compared with women. In addition those who reported taking an antimalarial drug in the previous 2 weeks were less than half as likely to have an asymptomatic infection compared with participants who reported not taking an antimalarial (P < 0.001, Table 3). A Hosmer-Lemeshow test was performed to verify the quality of the model fit. The results of the test (χ2 = 10.89, P = 0.21) confirm that the model is a good fit.
Table 3.
Unadjusted and adjusted logistic regression for the risk factors associated with having an asymptomatic Plasmodium falciparum infection compared with being negative (microscopy and PCR) for a P. falciparum infection
| Factor | Asymptomatic P. falciparum prevalence (N = 3,768)* |
|||
|---|---|---|---|---|
| Unadjusted |
Adjusted |
|||
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Survey | ||||
| Survey 1 (October 2012) | 1.00 | – | 1.00 | – |
| Survey 2 (June 2013) | 0.25 (0.22–0.29) | < 0.001 | 0.19 (0.16–0.23) | < 0.001 |
| Age groups | ||||
| 1–5 years | 1.00 | – | 1.00 | – |
| 6–10 years | 2.10 (1.69–2.60) | < 0.001 | 2.33 (1.85–2.93) | < 0.001 |
| 11–20 years | 2.43 (1.96–3.02) | < 0.001 | 2.67 (2.11–3.38) | < 0.001 |
| 21–39 years | 0.78 (0.63–0.97) | 0.023 | 0.73 (0.58–0.93) | 0.009 |
| ≥ 40 years | 0.69 (0.57–0.85) | < 0.001 | 0.68 (0.55–0.85) | 0.001 |
| Gender | ||||
| Female | 1.00 | – | 1.00 | – |
| Male | 1.60 (1.41–1.83) | < 0.001 | 1.50 (1.30–1.73) | < 0.001 |
| Catchment area | ||||
| Vea/Gowrie | 1.00 | – | 1.00 | – |
| Soe | 1.38 (1.21–1.57) | < 0.001 | 1.57 (1.36–1.82) | < 0.001 |
| Bed net usage previous night | ||||
| No | 1.00 | – | † | |
| Yes | 0.95 (0.78–1.15) | 0.577 | ||
| Antimalarial treatment (previous 2 weeks) | ||||
| No treatment | 1.00 | – | 1.00 | – |
| Treatment | 0.72 (0.62–0.84) | < 0.001 | 0.67 (0.56–0.82) | < 0.001 |
CI = confidence interval; OR = odds ratio; PCR = polymerase chain reaction.
Asymptomatic data reflect the combined prevalence of both microscopic and 18S rRNA PCR-positive samples for P. falciparum (includes P. falciparum mixed infections).
Variables not introduced in the final adjusted model.
DISCUSSION
The majority of malaria interventions deployed across SSA are targeted to improve the survival of children ≤ 5 years of age, as they are the population that bears the largest burden of clinical disease in areas with intense transmission.41 Reducing the burden of disease in children is important, but asymptomatic infections among older age groups, which are less likely to proceed to a clinical infection requiring treatment, also need to be prioritized since they contribute to the reservoir of P. falciparum infection and fuel continued transmission. This study from BD, in the northeastern part of Ghana, clearly shows that if we are to move toward malaria elimination the reservoir of asymptomatic P. falciparum infections that exists in both children and adults will have to be targeted through the deployment of appropriate intervention strategies. In addition, this study revealed considerable complexities and heterogeneities of malaria transmission at a local level in BD, which are critical to understand and monitor if we are to move beyond malaria control toward elimination in this region of West Africa.
This study was designed to characterize the age-specific patterns and risk factors for asymptomatic P. falciparum infections in BD by comparing microscopy and sensitive molecular methods to develop a more accurate description of the reservoir of P. falciparum. The prevalence of asymptomatic P. falciparum infections by microscopy differed between the age groups surveyed, gradually increasing during childhood and then declining from late adolescences into adulthood. This age-dependent trend in microscopic prevalence was in agreement with previous studies42–44 and was observed at the end of the wet and dry seasons across both catchment areas in BD. These results indicate the importance of understanding the burden of disease and consequences of asymptomatic malaria in the adolescent population, which have traditionally been neglected and rarely targeted for malaria control.45 The attitudes and behaviors of adolescents are different from those of children and adults, and hence influence their risk of developing and transmitting malaria.44,46 Although ITNs, ITPp, ITPi, and SMC reduce the morbidity and mortality associated with malaria they are typically targeted toward children ≤ 5 years of age and therefore do not adequately account for the whole reservoir of P. falciparum infections in the population. Reducing the burden of disease among both adolescents and older age groups needs to be made a priority in regions with stable malaria transmission.
By using PCR, a sensitive molecular method to identify low-density submicroscopic infections, a more comprehensive pattern of the prevalence of asymptomatic P. falciparum was observed in BD. It was found that at the end of both the wet and dry seasons, older age groups were at greater odds of carrying asymptomatic submicroscopic P. falciparum infections. The P. falciparum infections detected in the adult population (> 20 years) were significantly more likely to be submicroscopic than microscopic, and therefore will not be effectively targeted through community screening and treatment programs such as mass screening and treatment (MSAT) and active case detection if they were adopted. Consistent with other studies, these results show an age shift in the prevalence patterns for asymptomatic submicroscopic P. falciparum infections, with adults harboring higher proportions of low density submicroscopic infections,47,48 likely the result of acquired partial immunity.49,50 These molecular data clearly show that submicroscopic infections, which are difficult to detect with conventional diagnostic methods such as RDT and/or microscopy, can contribute upwards of an additional 30–60% of asymptomatic P. falciparum infections to the reservoir of chronic infections depending on age, spatial location and seasonality. Thus, these results make a strong case for including molecular diagnostics (e.g., PCR), or equally sensitive tests (e.g., loop-mediated isothermal amplification), when monitoring the epidemiology of the P. falciparum reservoir across all ages during malaria control and elimination programs.
Another important finding of this study was that the prevalence of asymptomatic (combined microscopic and submicroscopic) P. falciparum infections peaked at the end of the wet season (74.4%), and that despite a prolonged dry period asymptomatic infections still persisted at the end of the dry season survey (42.5%) across all age groups and catchment areas. Since BD is characterized by marked seasonal transmission patterns,51 it is not surprising that the prevalence of asymptomatic P. falciparum infections decreased significantly from the wet to dry season compared with other studies where prevalence shows less seasonality.52 Secondly it was observed that despite asymptomatic P. falciparum infections being most prevalent among children and adolescents, 64.2% of participants > 20 years of age at the end of the wet season and 27.0% at the end of the dry, harbored asymptomatic P. falciparum infections. In BD the end of the dry season represents a bottleneck in the number of asymptomatic infections across all age groups, and as a result this seasonal time point could be further exploited for elimination by interventions that target antimalarial treatments at the population level.
Despite the presence of a year-round irrigation dam being proximal to the Vea/Gowrie catchment area, at the end of the wet season the prevalence of asymptomatic P. falciparum infections was significantly higher in Soe. These differences suggest micro-heterogeneities between the two catchment areas in relation to seasonal transmission patterns, vector dispersion, differential use of malaria control tools, host genetics and/or parasite diversity that need to be further investigated. Moreover at end of the wet season it was observed that median asymptomatic P. falciparum densities were significantly higher in Soe compared with Vea/Gowrie, specifically for the 1–5 and 6–10 year age groups. These spatial differences observed at the end of the wet season suggest that in Soe transmission may be more seasonally intense compared with Vea/Gowrie with consequences on the rate at which immunity is acquired. These patterns may also indicate that participants residing in the irrigated Vea/Gowrie area acquire partial immunity to P. falciparum clones earlier in life due to year-round vector exposure, or perhaps are more likely to use protective tools such as bed nets to prevent mosquito bites, therefore limiting the onward transmission of the parasite.
The findings from this study have important implications for understanding the contribution of asymptomatic infections as potential reservoirs for gametocytes that could be relevant for initiating transmission at the start of the wet season.4,6 Research has hypothesized that the failure of previous MSAT programs to interrupt transmission in Burkina Faso and Zanzibar was a result of not adequately addressing asymptomatic P. falciparum carriers, which act as reservoirs for gametocytes and thus are capable of infecting mosquitoes.14,33,53 Recent findings from an area with intense seasonal malaria in Burkina Faso suggests that submicroscopic parasite carriers are an important source of onward transmission, particularly at the end of the dry season, and are responsible for about half of the infectious P. falciparum reservoir.4 Although our results are similar to previous studies and demonstrate that asymptomatic infections made up a substantial portion of the P. falciparum reservoir across all ages,42,44,48,54 their ability to produce mature gametocytes and transmit remains vague based on the microscopy results. Further work to evaluate the potential contribution of these asymptomatic infections for onward transmission needs to be assessed and monitored using more sensitive methods if we are to implement appropriate elimination strategies in BD.
Additional factors, beyond age, seasonality and spatial location that are known to be associated with malaria, were included and evaluated for their influence on the prevalence of asymptomatic infections. Bed nets are known to reduce the risk of malaria infection and in this study bed net usage was included as a factor that can influence parasite prevalence. Bed net usage was reported to be 88.9% at the end of the wet season and 85.3% at the end of the dry seasons across both catchment areas. When these rates were compared with data collected in 2008 by the USAID NetMark Project that found that 71% of households across six regions in Ghana had bed nets, our reported estimates were consistent with the national average.55,56 During both surveys it was found that there was no significant difference in the prevalence of asymptomatic P. falciparum infections between those who reported using a bed net the previous night and those who did not. This finding is likely the result of high bed net coverage and reported usage in BD, which has indirect protective effects on nonusers of bed nets in the area.
Another intervention evaluated was the use of antimalarial treatments on malaria infection. During both surveys participants who reported receiving an antimalarial treatment in the 2 weeks prior to the survey were significantly less likely to harbor an asymptomatic P. falciparum infection. At the end of the wet season, approximately four out of ten (41.6%) participants in BD reported taking an antimalarial medication for varied reasons including the treatment of acute malaria symptoms. On the other hand, at the end of the dry season significantly fewer participants (8.0%) reported taking an antimalarial treatment as expected. This difference may account for the high prevalence of asymptomatic P. falciparum infections among all ages at the end of the dry seasons. In addition during both surveys, children ≤ 5 years of age were significantly more likely to have reported receiving an antimalarial treatment.
At the end of the wet and dry seasons it was found that male participants in both catchment areas showed significantly higher prevalence of both microscopic and submicroscopic infections, and therefore gender was shown to be a risk factor for harboring asymptomatic infections in BD. These gender differences for infection prevalence were only significant for the adult age groups (> 20 years). Upon further examination this association was not the result of differences in bed net or antimalarial usage and perhaps is a result of underlying occupational patterns, health seeking behaviors, physiological differences and/or immunity status.57–61 It is important to note that pregnant women were excluded from this study, and that perhaps by not surveying this known malaria risk group we have not appropriately evaluated the impact of asymptomatic P. falciparum infections in adult women.62 To improve the effectiveness of malaria treatment and prevention interventions, understanding how gender contributes to the transmission and reservoir of asymptomatic infections needs to be incorporated into malaria surveillance programs.
Furthermore as expected children ≤ 5 years were most likely to be anemic and this was observed at the end of both the wet and dry seasons. This finding was expected since median P. falciparum densities were significantly higher in 1–5 year olds compared with the older age groups during both surveys. However what was particularly interesting to note was that at the end of the dry season, across both catchments areas, participants with either asymptomatic microscopic or submicroscopic P. falciparum infections were at greater odds of being anemic. During the dry season, when transmission was interrupted, being anemic is most likely the result of harboring chronic P. falciparum infections throughout the prolonged dry season. Although the continued circulation of chronic low-density P. falciparum infections during the dry season is important for the maintenance of immunity and provides protection against infection and/or clinical disease when transmission rebounds, these infections can also cause chronic anemia that has detrimental effects on health particularly in children.49,63
As endemic countries such as Ghana continue to roll out targeted chemotherapy programs, National Malaria Control Progammes need to consider the fact that their utilization does not adequately address the asymptomatic infections that contribute to maintaining the reservoir of P. falciparum. Although controversial, integrating mass drug administration (MDA) concurrently with, or after vector control strategies (ITN, IRS, etc.) have been carried out, needs to be revisited as a valuable approach that can eliminate the vast number of chronic asymptomatic infections that exist across all ages groups. Over the last 60 years, MDA clinical trial data combined more recently with modeling studies have provided the evidence that is necessary to properly guide the design and implementation of MDA programs. The research suggests that multiple rounds of MDA, carried out over several consecutive seasons prior to the rise in EIR would be the most suitable strategy to eliminate the P. falciparum reservoir.7,53,64–68 Based on the study results, both the density and prevalence of asymptomatic P. falciparum infections in BD are at their lowest point at the end of the dry season, making this an ideal time frame to consider targeting an MDA intervention for malaria elimination in this area of West Africa.
We require a fundamental shift in how we monitor the P. falciparum reservoir and evaluate the success of an intervention. The extent of the asymptomatic P. falciparum as a potential reservoir for fueling transmission among all ages in this population from the UER of Ghana demonstrates that if we are to move beyond malaria control toward elimination it is essential that both children and adults be targeted by interventions and surveillance. Indeed, the size and persistence of the asymptomatic P. falciparum reservoir in those greater than 10 years of age supports the exploration of MDA as a potential intervention tool if we are to achieve malaria elimination in regions with intense seasonal malaria transmission.
Supplementary Material
Acknowledgments:
We thank the participants, communities, and the Ghana Health Service in Bongo District Ghana for their willingness to participate in this study. We would like to thank the field teams for their technical assistance in the field and the laboratory personnel at the Navrongo Health Research Centre for sample collection and parasitological assessment/expertise. Additionally we would like to thank laboratory personnel at New York University and The University of Melbourne for their assistance with laboratory experiments. Finally we would like to acknowledge Oscar Bangre, Lucas Amenga-Etego, Michael Duffy, Shazia Ruybal, Charles Narh, Aprielle Wills, and Samantha Deed for their helpful input related to this work. Mercedes Pascual is an Investigator at the Howard Hughes Medical Institute.
Note: Supplemental tables appear at www.ajtmh.org.
REFERENCES
- 1.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, Battle KE, Moyes CL, Henry A, Eckhoff PA, Wenger EA, Briet O, Penny MA, Smith TA, Bennett A, Yukich J, Eisele TP, Griffin JT, Fergus CA, Lynch M, Lindgren F, Cohen JM, Murray CLJ, Smith DL, Hay SI, 2015. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526: 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffin JT, Hollingsworth TD, Okell LC, Churcher TS, White M, Hinsley W, Bousema T, Drakeley CJ, Ferguson NM, Basáñez MG, Ghani AC, 2010. Reducing Plasmodium falciparum malaria transmission in Africa: a model-based evaluation of intervention strategies. PLoS Med 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindblade K, Steinhardt L, Samuels A, Kachur SP, Slutsker L, 2013. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther 11: 623–639. [DOI] [PubMed] [Google Scholar]
- 4.Ouédraogo AL, Gonçalves BP, Gnémé A, Wenger EA, Guelbeogo MW, Ouédraogo A, Gerardin J, Bever CA, Lyons H, Pitroipa X, Verhave JP, Eckhoff PA, Drakeley C, Sauerwein R, Luty AJ, Kouyaté B, Bousema T, 2015. Dynamics of the human infectious reservoir for malaria determined by mosquito feeding assays and ultrasensitive malaria diagnosis in Burkina Faso. J Infect Dis 213: 90–99. [DOI] [PubMed] [Google Scholar]
- 5.Gerardin J, Ouédraogo AL, McCarthy K, Eckhoff P, Wenger E, 2015. Characterization of the infectious reservoir of malaria with an agent-based model calibrated to age-stratified parasite densities and infectiousness. Malar J 14: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bousema T, Okell L, Felger I, Drakeley C, 2014. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol 12: 833–840. [DOI] [PubMed] [Google Scholar]
- 7.Newby G, Hwang J, Koita K, Chen I, Greenwood B, von Seidlein L, Shanks GD, Slutsker LM, Kachur SP, Wegbreit J, Ippolito MM, Poirot E, Gosling R, 2015. Review of mass drug administration for malaria and its operational challenges. Am J Trop Med Hyg 93: 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bottius E, Guanzirolli A, Trape J, Rogier C, Konate L, Druilhe P, 1996. Malaria: even more chronic in nature than previously thought; evidence for subpatent parasitaemia detectable by the polymerase chain reaction. Trans R Soc Trop Med Hyg 90: 15–19. [DOI] [PubMed] [Google Scholar]
- 9.Kyabayinze DJ, Tibenderana JK, Odong GW, Rwakimari JB, Counihan H, 2008. Operational accuracy and comparative persistent antigenicity of HRP2 rapid diagnostic tests for Plasmodium falciparum malaria in a hyperendemic region of Uganda. Malar J 7: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ochola LB, Vounatsou P, Smith TA, Mabaso MLH, Newton CRJC, 2006. The reliability of diagnostic techniques in the diagnosis and management of malaria in the absence of a gold standard. Lancet Infect Dis 6: 582–588. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization, 2014. Malaria Rapid Diagnostics Test Performance: Results of WHO Product Testing of Malaria RDTs (Round 5). Vol 1. Available at: http://www.finddiagnostics.org/export/sites/default/resource-centre/reports_brochures/docs/malaria_rdt_results_Round5_eng.pdf. [Google Scholar]
- 12.Tiono AB, Guelbeogo MW, Sagnon N, Nébié I, Sirima SB, Mukhopadhyay A, Hamed K, 2013. Dynamics of malaria transmission and susceptibility to clinical malaria episodes following treatment of Plasmodium falciparum asymptomatic carriers: results of a cluster-randomized study of community-wide screening and treatment and a parallel entomology. BMC Infect Dis 13: 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell D, Wongsrichanalai C, Barnwell JW, 2006. Ensuring quality and access for malaria diagnosis: how can it be achieved? Nat Rev Microbiol 4: 682–695. [DOI] [PubMed] [Google Scholar]
- 14.Cook J, Xu X, Msellem MI, Vonk M, Bergström B, Gosling R, Al-Mafazy AW, McElroy P, Molteni F, Abass AK, Garimo I, Ramsan M, Ali A, Mårtensson A, Björkman A, 2015. Mass screening and treatment on the basis of results of a Plasmodium falciparum-specific rapid diagnostic test did not reduce malaria incidence in Zanzibar. J Infect Dis 211: 1476–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu L, van den Hoogen LL, Slater H, Walker PGT, Ghani AC, Drakeley CJ, Okell LC, 2015. Comparison of diagnostics for the detection of asymptomatic Plasmodium falciparum infections to inform control and elimination strategies. Nature 528: S86–S93. [DOI] [PubMed] [Google Scholar]
- 16.Okell LC, Ghani AC, Lyons E, Drakeley CJ, 2009. Submicroscopic infection in Plasmodium falciparum-endemic populations: a systematic review and meta-analysis. J Infect Dis 200: 1509–1517. [DOI] [PubMed] [Google Scholar]
- 17.Okell LC, Bousema T, Griffin JT, Ouédraogo AL, Ghani AC, Drakeley CJ, 2012. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Commun 3: 1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkins R, Omollo R, Ongecha M, Sifuna P, Othieno C, Ongeri L, Kingora J, Ogutu B, 2015. Prevalence of malaria parasites in adults and its determinants in malaria endemic area of Kisumu County, Kenya. Malar J 14: 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geiger C, Agustar HK, Compaoré G, Coulibaly B, Sié A, Becher H, Lanzer M, Jänisch T, 2013. Declining malaria parasite prevalence and trends of asymptomatic parasitaemia in a seasonal transmission setting in north-western Burkina Faso between 2000 and 2009–2012. Malar J 12: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owusu-Agyei S, Smith T, Beck HP, Amenga-Etego L, Felger I, 2002. Molecular epidemiology of Plasmodium falciparum infections among asymptomatic inhabitants of a holoendemic malarious area in northern Ghana. Trop Med Int Health 7: 421–428. [DOI] [PubMed] [Google Scholar]
- 21.Koram KA, Owusu-Agyei S, Fryauff DJ, Anto F, Atuguba F, Hodgson A, Holfman SL, Nkrumah FK, 2003. Seasonal profiles of malaria infection, anaemia, and bednet use among age groups and communities in northern Ghana. Trop Med Int Health 8: 793–802. [DOI] [PubMed] [Google Scholar]
- 22.Sama W, Owusu-Agyei S, Felger I, Dietz K, Smith T, 2006. Age and seasonal variation in the transition rates and detectability of Plasmodium falciparum malaria. Parasitology 132: 13–21. [DOI] [PubMed] [Google Scholar]
- 23.Felger I, Maire M, Bretscher MT, Falk N, Tiaden A, Sama W, Beck HP, Owusu-Agyei S, Smith TA, 2012. The dynamics of natural Plasmodium falciparum infections. PLoS One 7: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bongo District Assembly, 2014. District Medium Term Development Plan (2010–2013). Ghana: Bongo District. Available at: https://s3.amazonaws.com/ndpc-static/CACHES/PUBLICATIONS/2016/04/16/UE_Bongo_2010-2013+DMTDP.pdf.
- 25.Ghana Statistical Service, 2014. 2010 Population and Housing Census: Bongo District. Ghana: Ghana Statistical Service. Available at: http://www.statsghana.gov.gh/docfiles/2010_District_Report/Upper%20East/Bongo.pdf.
- 26.Sakeah E, Doctor HV, McCloskey L, Bernstein J, Yeboah-Antwi K, Mills S, 2014. Using the community-based health planning and services program to promote skilled delivery in rural Ghana: socio-demographic factors that influence women utilization of skilled attendants at birth in northern Ghana. BMC Public Health 14: 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akurugu BA, Zango MS, Abanyie SK, Ampofo S, Region UE, 2015. Assessing the impact of a dam on the livelihood of surrounding communities : a case study of Vea Dam in the Upper East Region of Ghana. J Environ Earth Sci 5: 20–26. [Google Scholar]
- 28.United Nations Children's Emergency Fund (UNICEF), 2012. UNICEF Annual Report 2012 for Ghana, WCARO; New York, NY: UNICEF; Available at: https://www.unicef.org/about/annualreport/files/Ghana_COAR_2012.pdf. [Google Scholar]
- 29.WHO/UNICEF/UNU, 2001. Iron Deficiency Anaemia: Assessment, Prevention and Control, a Guide for Programme Managers. Available at: http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/WHO_NHD_01.3/en/index.html.
- 30.WHO/NMH/NHD/MNM, 2011. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Geneva, Switzerland: World Health Organization; Available at: http://apps.who.int/iris/bitstream/10665/85839/3/WHO_NMH_NHD_MNM_11.1_eng.pdf7. [Google Scholar]
- 31.GHS/MOH, 2013. Final Report: National Malaria Control Programme Ghana (Ministry of Health, Ghana Health Service). Ghana: MOH/GHS; Availabl at: https://www.ghanahealthservice.org/downloads/ghana_malaria_programme_review_final_report_june_2013.pdf. [Google Scholar]
- 32.World Health Organization, 2010. Basic Malaria Microscopy: Part I. Learner's Guide, 2nd edition Geneva, Switzerland: World Health Organization. [Google Scholar]
- 33.Laishram DD, Sutton PL, Nanda N, Sharma VL, Sobti RC, Carlton JM, Joshi H, 2012. The complexities of malaria disease manifestations with a focus on asymptomatic malaria. Malar J 11: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Mast Q, Syafruddin D, Keijmel S, Olde Riekerink T, Deky O, Asih P, Swinkels D, van der Ven A, 2010. Increased serum hepcidin and alterations in blood iron parameters associated with asymptomatic P. falciparum and P. vivax malaria. Haematologica 95: 1068–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leoratti F, Durlacher R, Lacerda M, Alecrim M, Ferreira A, Sanchez M, Moraes S, 2008. Pattern of humoral immune response to Plasmodium falciparum blood stages in individuals presenting different clinical expressions of malaria. Malar J 7: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Males S, Gaye O, Garcia A, 2008. Long-term asymptomatic carriage of Plasmodium falciparum protects from malaria attacks: a prospective study among Senegalese children. Clin Infect Dis 46: 516–522. [DOI] [PubMed] [Google Scholar]
- 37.Duarte J, Deshpande P, Guiyedi V, Mecheri S, Fesel C, Cazenave P, Mishra G, Kombila M, Pied S, 2007. Total and functional parasite specific IgE responses in Plasmodium falciparum-infected patients exhibiting different clinical status. Malar J 6: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snounou G, Viriyakosol S, Xin PZ, Jarra W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN, Zhu X, 1993. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol 61: 315–320. [DOI] [PubMed] [Google Scholar]
- 39.Boonma P, Christensen PR, Suwanarusk R, Price RN, Russell B, Lek-Uthai U, 2007. Comparison of three molecular methods for the detection and speciation of Plasmodium vivax and Plasmodium falciparum . Malar J 6: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snounou G, Zhu X, Siripoon N, Jarra W, Thaithong S, Brown KN, Viriyakosol S, 1999. Biased distribution of msp1 and msp2 allelic variants in Plasmodium falciparum populations in Thailand. Trans R Soc Trop Med Hyg 93: 369–374. [DOI] [PubMed] [Google Scholar]
- 41.Snow RW, Marsh K, 1998. New insights into the epidemiology of malaria relevant for disease control. Br Med Bull 54: 293–309. [DOI] [PubMed] [Google Scholar]
- 42.Owusu EDA, Buabeng V, Dadzie S, Brown CA, Grobusch MP, Mens P, 2016. Characteristics of asymptomatic Plasmodium spp. parasitaemia in Kwahu-Mpraeso, a malaria endemic mountainous district in Ghana, west Africa. Malar J 15: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith DL, Guerra CA, Snow RW, Hay SI, 2007. Standardizing estimates of the Plasmodium falciparum parasite rate. Malar J 6: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pegha Moukandja I, Biteghe Bi Essone JC, Sagara I, Kassa Kassa RF, Ondzaga J, Lékana Douki J-B, Bouyou Akotet M, Nkoghe Mba D, Touré Ndouo FS, 2016. Marked rise in the prevalence of asymptomatic Plasmodium falciparum infection in rural Gabon. PLoS One 11: e0153899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lalloo DG, Olukoya P, Olliaro P, 2006. Malaria in adolescence: burden of disease, consequences, and opportunities for intervention. Lancet Infect Dis 6: 780–793. [DOI] [PubMed] [Google Scholar]
- 46.Yimer F, Animut A, Erko B, Mamo H, 2015. Past five-year trend, current prevalence and household knowledge, attitude and practice of malaria in Abeshge, south-central Ethiopia. Malar J 14: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stresman GH, Stevenson JC, Ngwu N, Marube E, Owaga C, Drakeley C, Bousema T, Cox J, 2014. High levels of asymptomatic and subpatent Plasmodium falciparum parasite carriage at health facilities in an area of heterogeneous malaria transmission intensity in the Kenyan highlands. Am J Trop Med Hyg 91: 1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rek J, Katrak S, Obasi H, Nayebare P, Katureebe A, Kakande E, Arinaitwe E, Nankabirwa JI, Jagannathan P, Drakeley C, Staedke SG, Smith DL, Bousema T, Kamya M, Rosenthal PJ, Dorsey G, Greenhouse B, 2016. Characterizing microscopic and submicroscopic malaria parasitaemia at three sites with varied transmission intensity in Uganda. Malar J 15: 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doolan DL, Dobaño C, Baird JK, 2009. Acquired immunity to malaria. Clin Microbiol Rev 22: 13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Langhorne J, Ndungu FMM, Sponaas A-M, Marsh K, 2008. Immunity to malaria: more questions than answers. Nat Immunol 9: 725–732. [DOI] [PubMed] [Google Scholar]
- 51.Roca-Feltrer A, Schellenberg JRMA, Smith L, Carneiro I, 2009. A simple method for defining malaria seasonality. Malar J 8: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith T, Charlwood JD, Kihonda J, Mwankusye S, Billingsley P, Meuwissen J, Lyimo E, Takken W, Teuscher T, Tanner M, 1993. Absence of seasonal variation in malaria parasitaemia in an area of intense seasonal transmission. Acta Trop 54: 55–72. [DOI] [PubMed] [Google Scholar]
- 53.Tiono AB, Ouédraogo A, Ogutu B, Diarra A, Coulibaly S, Gansané A, Sirima SB, O'Neil G, Mukhopadhyay A, Hamed K, 2013. A controlled, parallel, cluster-randomized trial of community-wide screening and treatment of asymptomatic carriers of Plasmodium falciparum in Burkina Faso. Malar J 12: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dal-Bianco MP, Köster KB, Kombila UD, Kun JFJ, Grobusch MP, Ngoma GM, Matsiegui PB, Supan C, Ospina Salazar CL, Missinou MA, Issifou S, Lell B, Kremsner P, 2007. High prevalence of asymptomatic Plasmodium falciparum infection in Gabonese adults. Am J Trop Med Hyg 77: 939–942. [PubMed] [Google Scholar]
- 55.Baume CA, Franca-Koh AC, 2011. Predictors of mosquito net use in Ghana. Malar J 10: 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baume CA, Marin C, Franca-Koh AC, 2009. NetMark 2008 Household Survey on Insecticide-Treated Nets in Ghana. AED/USAID. Available at: http://pdf.usaid.gov/pdf_docs/Pnado570.pdf. [Google Scholar]
- 57.Steketee RW, Nahlen BL, Parise ME, Menendez C, 2001. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg 64: 28–35. [DOI] [PubMed] [Google Scholar]
- 58.Duffy PE, Fried M, 2005. Malaria in the pregnant woman. Curr Top Microbiol Immunol 295: 169–200. [DOI] [PubMed] [Google Scholar]
- 59.Arahman SH, Mohamedani AA, Mirgani EM, Ibrahim AM, 1996. Gender aspects and women's participation in the control and management of malaria in central Sudan. Soc Sci Med 42: 1433–1446. [DOI] [PubMed] [Google Scholar]
- 60.Müller I, Smith T, Mellor S, Rare L, Genton B, 1998. The effect of distance from home on attendance at a small rural health centre in Papua New Guinea. Int J Epidemiol 27: 878–884. [DOI] [PubMed] [Google Scholar]
- 61.Reuben R, 1993. Women and malaria-special risks and appropriate control strategy. Soc Sci Med 37: 473–480. [DOI] [PubMed] [Google Scholar]
- 62.van Eijk AM, Hill J, Noor AM, Snow RW, ter Kuile FO, 2015. Prevalence of malaria infection in pregnant women compared with children for tracking malaria transmission in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health 3: e617–e628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen I, Clarke SE, Gosling R, Hamainza B, Killeen G, Magill A, O'Meara W, Price RN, Riley EM, 2016. “Asymptomatic” malaria: a chronic and debilitating infection that should be treated. PLoS Med 13: e1001942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okell LC, Griffin JT, Kleinschmidt I, Hollingsworth TD, Churcher TS, White MJ, Bousema T, Drakeley CJ, Ghani AC, 2011. The potential contribution of mass treatment to the control of Plasmodium falciparum malaria. PLoS One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.von Seidlein L, Walraven G, Milligan PJM, Alexander N, Manneh F, Deen JL, Coleman R, Jawara M, Lindsay SW, Drakeley C, De Martin S, Olliaro P, Bennett S, van der Loeff MS, Okunoye K, Targett GA, McAdam KP, Doherty JF, Greenwood BM, Pinder M, 2003. The effect of mass administration of sulfadoxine-pyrimethamine combined with artesunate on malaria incidence: a double-blind, community-randomized, placebo-controlled trial in the Gambia. Trans R Soc Trop Med Hyg 97: 217–225. [DOI] [PubMed] [Google Scholar]
- 66.Shekalaghe SA, Drakeley C, van den Bosch S, ter Braak R, van den Bijllaardt W, Mwanziva C, Semvua S, Masokoto A, Mosha F, Teelen K, Hermsen R, Okell L, Gosling R, Sauerwein R, Bousema T, 2011. A cluster-randomized trial of mass drug administration with a gametocytocidal drug combination to interrupt malaria transmission in a low endemic area in Tanzania. Malar J 10: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poirot E, Skarbinski J, Sinclair D, Kachur SP, Slutsker L, Hwang J, 2013. Mass drug administration for malaria. Cochrane Database Syst Rev 12: CD008846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kern SE, Tiono AB, Makanga M, Gbadoé AD, Premji Z, Gaye O, Sagara I, Ubben D, Cousin M, Oladiran F, Sander O, Ogutu B, 2011. Community screening and treatment of asymptomatic carriers of Plasmodium falciparum with artemether-lumefantrine to reduce malaria disease burden: a modelling and simulation analysis. Malar J 10: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.