Abstract.
In tropical and subtropical settings, the epidemiology of viral acute respiratory tract infections varies widely between countries. We determined the etiology, seasonality, and clinical presentation of viral acute respiratory tract infections among outpatients in southern Sri Lanka. From March 2013 to January 2015, we enrolled outpatients presenting with influenza-like illness (ILI). Nasal/nasopharyngeal samples were tested in duplicate using antigen-based rapid influenza testing and multiplex polymerase chain reaction (PCR) for respiratory viruses. Monthly proportion positive was calculated for each virus. Bivariable and multivariable logistic regression were used to identify associations between sociodemographic/clinical information and viral detection. Of 571 subjects, most (470, 82.3%) were ≥ 5 years of age and 53.1% were male. A respiratory virus was detected by PCR in 63.6% (N = 363). Common viral etiologies included influenza (223, 39%), human enterovirus/rhinovirus (HEV/HRV, 14.5%), respiratory syncytial virus (RSV, 4.2%), and human metapneumovirus (hMPV, 3.9%). Both ILI and influenza showed clear seasonal variation, with peaks from March to June each year. RSV and hMPV activity peaked from May to July, whereas HEV/HRV was seen year-round. Patients with respiratory viruses detected were more likely to report pain with breathing (odds ratio [OR] = 2.60, P = 0.003), anorexia (OR = 2.29, P < 0.001), and fatigue (OR = 2.00, P = 0.002) compared with patients with no respiratory viruses detected. ILI showed clear seasonal variation in southern Sri Lanka, with most activity during March to June; peak activity was largely due to influenza. Targeted infection prevention activities such as influenza vaccination in January–February may have a large public health impact in this region.
INTRODUCTION
Acute respiratory tract infections are a leading cause of morbidity and mortality worldwide and are most commonly caused by viruses.1 Influenza is the most extensively studied of the respiratory viruses, causing infections in 10–20% of the world’s population and accounting for substantial morbidity, mortality, and economic losses each year.2,3 In addition to influenza, viruses such as respiratory syncytial virus (RSV), human enterovirus/rhinovirus (HEV/HRV), adenovirus, and parainfluenza are also associated with a significant burden of disease in patients with acute respiratory tract infections.4–7
The epidemiology of viral acute respiratory tract infections in the tropics, especially in low- and middle-income countries, is not as well described as in temperate, more developed countries. Influenza remains a common cause of illness, but the burden of other viruses is not well defined.8,9 In addition, while influenza follows a clear seasonal pattern in temperate countries, in tropical and subtropical countries, seasonality appears to vary between countries and even within countries.8,9 Perhaps due to limited data, less than half of tropical and subtropical countries, comprising 20% of the world’s population, have a national vaccine policy for influenza.10 For tropical and subtropical countries, local data regarding epidemiology of influenza and other respiratory viruses are needed to guide preventative public health measures such as vaccination.11
We examined the etiology, seasonal variation, and clinical manifestations of viral acute respiratory tract infections among outpatients presenting to an acute care facility in Sri Lanka, a tropical, lower middle-income nation. Sri Lanka does not currently have a national influenza immunization policy and influenza vaccines are not routinely administered through the public sector.10
METHODS
Study population and surveillance procedures.
This was a cross-sectional study performed in the outpatient department (OPD) of the largest (1,500 bed) public, tertiary care hospital in the Southern Province of Sri Lanka. The OPD of this hospital serves over 1,000 patients daily between 8 am and 7 pm.
Consecutive adults and children ≥ 1 year presenting to the OPD with acute respiratory tract infections were screened for enrollment by trained study physicians from March 2013 to January 2015. Patients were eligible for enrollment if they met criteria for influenza-like illness (ILI), as defined by the World Health Organization (WHO): tympanic temperature ≥ 38°C/100.4°F and acute onset of cough in the past 7 days without alternative diagnosis.12 Consent was obtained from patients ≥ 18 years of age and the guardians of patients 1–17 years of age, and assent was obtained from patients 12–17 years of age. Enrolled patients were administered a standardized questionnaire in the local language of Sinhala and a physical examination was conducted. Two nasopharyngeal samples were collected from all patients for whom it was possible; patients unable to tolerate nasopharyngeal sample collection had a nasal sample collected instead. One sample was used immediately in rapid influenza testing, and the other was stored at −80°C for viral polymerase chain reaction (PCR) testing, as described below. Patients received standard clinical assessment and treatment including physical examination, additional diagnostic testing, and prescriptions from OPD physicians. Details regarding patients’ clinical diagnoses and management were recorded. The result from the rapid influenza test was only released to patients and clinicians during the latter half of the study (January 2014–January 2015), with the result being released prior to any clinical decision-making. Details from this pre-post study have been reported previously.13 Study physicians were not involved in clinical care or treatment, and OPD clinical personnel were not involved in study screening or enrollment procedures. Ethical approval for this study was obtained from the Ruhuna University Ethical Review Committee, Duke University Institutional Review Board, and Johns Hopkins Medicine Institutional Review Board.
Rapid influenza testing.
All patients were tested with the Veritor Flu A+B rapid influenza kit (Becton Dickinson, and Company, Franklin Lakes, NJ) using a nasal/nasopharyngeal sample collected and run at the point of care. This rapid chromatographic immunoassay detects influenza A and B viral nucleoprotein antigens from nasal and nasopharyngeal swabs using a single processed sample.14 The performance characteristics of the Veritor test were documented by Hassan and others, who showed that in pediatric patients, the sensitivity and specificity of the test when compared with PCR were 90.2% and 99.1%, respectively, for influenza A and 87.5% and 100%, respectively, for influenza B.15
Respiratory virus molecular testing.
The second nasal/nasopharyngeal sample from each patient was placed in viral transport media and frozen at −80°C. These samples were later tested by real-time reverse transcription PCR (RT-PCR) with the Luminex xTAG respiratory virus panel (Luminex Molecular Diagnostics, Toronto, Canada). The platform detects influenza A, influenza B, HEV/HRV, RSV, human metapneumovirus (hMPV), parainfluenza viruses 1–4, coronavirus 229E, coronavirus HKU1, coronavirus NL63, coronavirus OC43, bocavirus, and adenovirus.16,17
Statistical analysis.
Seasonality of ILI, influenza, and the three other most common respiratory viruses was assessed using a modified definition from the Program for Appropriate Technology (PATH) for determining influenza seasonality.11 The monthly proportion of positive cases for a specific virus out of all positive cases within a given calendar year was calculated. A “peak” in activity was defined as the monthly proportion being ≥ 10% during both years of the study.
Monthly weather data were obtained for the Galle region from the Sri Lanka Department of Meteorology (Colombo, Sri Lanka). Correlation between weather data (monthly rainfall, monthly minimum temperature, monthly maximum temperature, daily humidity, and nightly humidity) and the monthly proportion of subjects with ILI or influenza by PCR were determined using the Spearman correlation.18
Sociodemographic and clinical characteristics between patients with a positive versus negative respiratory viral test, and patients with a positive influenza versus positive other respiratory viral test, were compared using the Fisher’s exact test for categorical variables and the Kruskall–Wallis test for continuous variables. Bivariable and multivariable logistic regression were carried out to determine the association (odds ratios [ORs] with 95% confidence intervals) between patients’ sociodemographic characteristics/clinical symptoms and viral PCR test results.
For the multivariable analysis, adjusted models were constructed separately to determine features associated with respiratory virus positivity versus negativity and influenza positivity versus other virus positivity (all per PCR testing). Age ≥ 5 years (as a categorical variable) was included in all models. In addition, any sociodemographic characteristic or clinical symptom that was associated with the dependent variable in question at a P value < 0.05 on bivariable analysis was included in the individual multivariable model. Variables were checked for collinearity prior to inclusion in the models. To create more parsimonious models, variables were excluded in a stepwise fashion by decreasing P value until all P values were < 0.05 in the multivariable model. Each excluded variable was then added back sequentially to ensure that the variable was not significant in the final model.
Performance characteristics of the rapid test were calculated using the Luminex RT-PCR test result as a gold standard. Sensitivity, specificity, and positive and negative predictive values were determined. Patients with viral coinfections as identified by PCR were included in the analysis.
All analyses were performed using JMP PRO, Version 11 (SAS Institute Inc., Cary, NC) and STATA, version 11 (STATACorp, College Station, TX).
RESULTS
Sociodemographic and clinical description of study cohort.
We enrolled 571 outpatients during the study period, with approximately half (53.1%) being male. The majority (82.3%) of subjects were ≥ 5 years of age and median age was 13.2 years (range = 1.1–74.8). Median days of both fever and cough were 2 days (interquartile range = 2–3 days for both). No subjects reported a prior history of influenza vaccination. A total of 441 (77.2%) subjects were prescribed an antibiotic at their OPD visit and none were admitted to the hospital for treatment with antivirals (physicians are not allowed to prescribe oseltamivir through the OPD).
Respiratory virus detection.
Virus was detected by PCR in 63.6% (N = 363) of subjects, with 3.7% having more than one virus detected (Table 1). The most common viral etiology was influenza (223, 39.1%), with 163 (28.5%) influenza A and 60 (10.5%) influenza B. Other common viral etiologies included HEV/HRV (14.5%), RSV (4.2%), and hMPV (3.9%). The remainder of the viruses detected were parainfluenza virus (3.2%), coronavirus (1.4%), bocavirus (0.9%), and adenovirus (0.4%). Coinfections were present in 21 (3.7%), with the most common combination being influenza A and HEV/HRV (6, 1.0%). Influenza (16), parainfluenza (seven), and HEV/HRV (six) were the most commonly identified viruses in coinfections.
Table 1.
Distribution of respiratory viruses isolated from outpatients presenting with acute respiratory tract infections to a tertiary care hospital in southern Sri Lanka, 2013–2015
| Respiratory virus | Frequency (%) in all patients (N = 571) | Frequency (%) in patients < 5 years (N = 101) | Frequency (%) in patients ≥ 5 years (N = 470) | P value |
|---|---|---|---|---|
| Influenza | 223 (39.1) | 25 (24.8) | 198 (42.1) | 0.001 |
| Influenza A | 163 (28.5) | 23 (22.8) | 140 (29.8) | 0.18 |
| Influenza B | 60 (10.5) | 2 (2.0) | 58 (12.3) | 0.001 |
| HEV/HRV | 83 (14.5) | 19 (18.8) | 64 (13.6) | 0.21 |
| Respiratory syncytial virus | 24 (4.2) | 14 (13.9) | 10 (2.1) | < 0.001 |
| Human metapneumovirus | 22 (3.9) | 5 (5.0) | 17 (3.6) | 0.57 |
| Parainfluenza virus | 18 (3.2) | 9 (8.9) | 9 (1.9) | 0.002 |
| Parainfluenza 1 | 6 (1.1) | 3 (3.0) | 3 (0.6) | 0.07 |
| Parainfluenza 2 | 1 (0.2) | 0 (0) | 1 (0.2) | 1.00 |
| Parainfluenza 3 | 7 (1.2) | 4 (4.0) | 3 (0.6) | 0.02 |
| Parainfluenza 4 | 4 (0.7) | 2 (2.0) | 2 (0.4) | 0.15 |
| Coronovirus | 8 (1.4) | 2 (2.0) | 6 (1.3) | 0.63 |
| Coronavirus 229E | 0 (0) | 0 (0) | 0 (0) | N/A |
| Coronavirus HKU1 | 1 (0.2) | 0 (0) | 1 (0.2) | 1.00 |
| Coronavirus NL63 | 1 (0.2) | 0 (0) | 1 (0.2) | 1.00 |
| Coronavirus OC43 | 6 (1.1) | 2 (2.0) | 4 (0.9) | 0.29 |
| Bocavirus | 5 (0.9) | 4 (4.0) | 1 (0.2) | 0.004 |
| Adenovirus | 2 (0.4) | 0 (0) | 2 (0.4) | 1.00 |
| Negative | 208 (36.4) | 31 (30.7) | 177 (37.7) | 0.21 |
| Coinfections* | 21 (3.7) | 8 (7.9) | 13 (2.8) | 0.02 |
HEV/HRV = human enterovirus/rhinovirus.
Influenza (16), parainfluenza (seven), and HEV/HRV (six) were the most commonly identified viruses in coinfections.
When examined by age group, influenza was more common in patients ≥ 5 years of age than among those < 5 years of age (42.1% versus 24.8%, P = 0.001; Table 1). Among patients < 5 years of age, the following viruses were more common than among those ≥ 5 years of age: RSV (13.9% versus 2.1%, P < 0.0001), parainfluenza (8.9% versus 1.9%, P = 0.002), and bocavirus (4% versus 0.2%, P = 0.004). Coinfections were also more common is those less than 5 years of age (7.9% versus 2.8%, P = 0.02).
Seasonality of ILI and respiratory viruses.
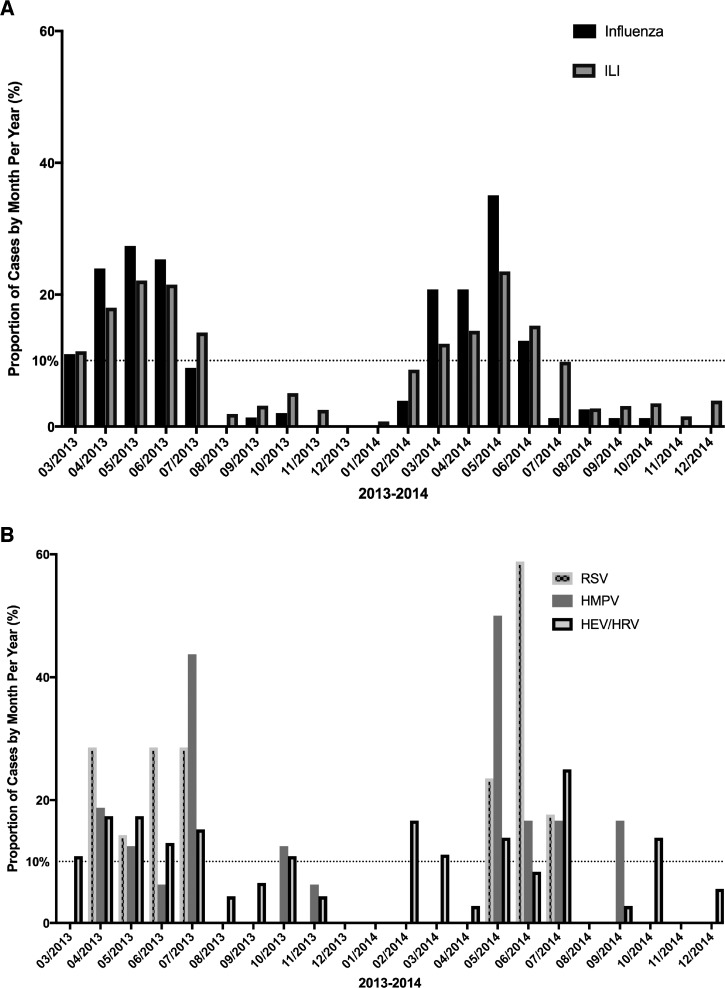
ILI peaked from March to June during both years of the study period, with May having the highest activity (Figure 1A). Influenza also peaked during March–June in both years, with May again having the highest activity. During each influenza peak month, influenza A activity was > 10% in both years, whereas influenza B did not consistently show activity > 10% in both years. Among other respiratory viruses, RSV peaked during May–July and hMPV peaked in May and in July in both years (Figure 1B). HEV/HRV showed sporadic peak activity throughout the year including in March, May, July, and October.
Figure 1.
Variation in influenza-like illness (ILI) and respiratory viruses over time, shown as a percent of monthly positives divided by positives for the year, March 2013–January 2015. A peak in virus activity was defined as monthly proportion of virus greater than 10%.11 (A) Variation in ILI and influenza (types A and B). (B) Variation in the three other most commonly isolated respiratory viruses: human enterovirus/rhinovirus (HEV/HRV), respiratory syncytial virus (RSV), and human metapneumovirus (hMPV).
Monthly rainfall, daily humidity, and nightly humidity did not show any correlation with ILI (P = 0.19, 0.63 and 0.54, respectively) or with influenza (P = 0.46, 0.96 and 0.17, respectively). ILI was positively associated with monthly maximum temperature (range = 28.5–32.5°C, P = 0.04). Influenza was positively associated with monthly minimum temperature (range = 23.5–26.3°C, P = 0.01).
Sociodemographic and clinical features associated with respiratory virus detection.
On bivariable analysis, subjects who were male were more likely to have a respiratory virus detected (57.6% versus 45.2%, P = 0.005; Table 2). Having a sick contact in the past month was associated with a positive respiratory virus test (46.2% versus 35.1%, P = 0.01), whereas having longer durations of fever or cough were associated with a negative respiratory virus test (P = 0.02 and P < 0.001, respectively). In addition, the following clinical symptoms were associated with respiratory virus detection: pain with breathing (12.4% versus 6.3%, P = 0.02), anorexia (68.0% versus 50.0%, P ≤ 0.001), fatigue/lethargy (73.3% versus 55.3%, P < 0.001), arthralgias (60.3% versus 47.6% P = 0.004), and myalgias (61.2% versus 48.6%, P = 0.004). Prior antibiotic use for the same illness was associated with having a negative respiratory viral test (5.2% versus 11.5%, P = 0.008). There was no association between respiratory virus positivity and receiving an antibiotic prescription at the OPD visit (P = 1.00).
Table 2.
Sociodemographic and clinical characteristics of outpatients with acute respiratory tract infections who had a respiratory virus detected, southern Sri Lanka, 2013–2015
| Characteristic | Frequency (%) or median (IQR) |
OR (95% CI) | P value | |
|---|---|---|---|---|
| Respiratory virus positive N = 363 (%) | Respiratory virus negative N = 208 (%) | |||
| Child (< 5 years) | 70 (19.3%) | 31 (14.9%) | 1.36 (0.84–2.24) | 0.21 |
| Male | 209 (57.6) | 94 (45.2) | 1.65 (1.16–2.32) | 0.005 |
| Sick contact in past month | 165 (46.2) | 73 (35.1) | 1.59 (1.12–2.26) | 0.01 |
| Prior medical visit for same illness | 95 (26.3) | 62 (30.7) | 0.81 (0.55–1.18) | 0.28 |
| Travel in past 4 weeks | 55 (15.2) | 32 (15.4) | 0.99 (0.61–1.58) | 1.00 |
| Smoking (passive or active) | 84 (23.1) | 37 (17.8) | 1.39 (0.90–2.14) | 0.14 |
| Prior antibiotic for same illness | 19 (5.2) | 24 (11.5) | 0.43 (0.23–0.81) | 0.008 |
| Days of fever | 2 (1–3) | 2 (2–4) | 0.02 | |
| Days of cough | 2 (2–3) | 3 (2–4) | < 0.001 | |
| Ear ache | 18 (5.0) | 11 (5.3) | 0.93 (0.43–2.02) | 0.85 |
| Rhinitis | 276 (76) | 149 (71.6) | 1.26 (0.85–1.85) | 0.27 |
| Sore throat | 175 (48.2) | 93 (44.7) | 1.15 (0.82–1.62) | 0.43 |
| Dyspnea | 64 (17.6) | 25 (12.0) | 1.57 (0.95–2.57) | 0.09 |
| Pain with breathing | 45 (12.4) | 13 (6.3) | 2.12 (1.12–4.04) | 0.02 |
| Anorexia | 247 (68.0) | 104 (50.0) | 2.13 (1.50–3.02) | < 0.001 |
| Vomiting | 67 (18.5) | 31 (14.9) | 1.29 (0.81–2.06) | 0.30 |
| Abdominal pain | 26 (7.2) | 11 (5.3) | 1.38 (0.67–2.86) | 0.48 |
| Headache | 269 (74.1) | 145 (69.7) | 1.24 (0.85–1.81) | 0.28 |
| Fatigue/lethargy | 266 (73.3) | 115 (55.3) | 2.22 (1.55–3.17) | < 0.001 |
| Arthralgias | 219 (60.3) | 99 (47.6) | 1.67 (1.18–2.36) | 0.004 |
| Myalgias | 222 (61.2) | 101 (48.6) | 1.67 (1.18–2.35) | 0.004 |
| Temperature (°F) | 101.0 (100.6–101.7) | 100.9 (100.5–101.7) | 0.02 | |
| Wheezing | 48 (13.3) | 23 (11.1) | 0.81 (0.48–1.37) | 0.51 |
| Antibiotic prescribed | 280 (77.1) | 161 (77.4) | 0.98 (0.66–1.48) | 1.00 |
| Diagnostic tests ordered | 60 (16.5) | 52 (25.0) | 0.59 (0.39–0.90) | 0.02 |
CI = confidence interval; IQR = interquartile range; OR = odds ratio. Bivariable analysis was performed using the Fisher’s exact test for categorical variables and the Kruskall–Wallis test for continuous variables.
On adjusted analysis, male sex (OR = 1.48, P = 0.04) and having a sick contact in the past month (OR = 1.86, P = 0.001) were positively associated with respiratory virus detection (Table 3). The symptoms of pain with breathing (OR = 2.60, P = 0.003), anorexia (OR = 2.29, P < 0.001), and fatigue (OR = 2.00, P = 0.002) were also associated with respiratory virus detection. Age < 5 years (OR = 0.45, P = 0.005) and greater days of cough (OR = 0.79, P < 0.001) were negatively associated with respiratory virus detection.
Table 3.
Multivariable analysis of sociodemographic and clinical features associated with respiratory virus positivity or influenza positivity, southern Sri Lanka, 2013–2015
| Characteristic | Respiratory virus positive vs. negative |
Influenza positive vs. other respiratory virus positive |
||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Male sex | 1.48 (1.02–2.17) | 0.04 | – | – |
| Child (< 5 years) | 0.45 (0.26–0.78) | 0.005 | 0.33 (0.17–0.62) | 0.001 |
| Sick contact | 1.86 (1.27–2.73) | 0.001 | 1.84 (1.14–2.97) | 0.01 |
| Number of days of cough | 0.79 (0.70–0.90) | < 0.001 | – | – |
| Pain with breathing | 2.60 (1.39–4.89) | 0.003 | 3.04 (1.12–8.24) | 0.03 |
| Anorexia | 2.29 (1.50–3.50) | < 0.001 | 2.20 (1.32–3.66) | 0.002 |
| Fatigue | 2.00 (1.28–3.13) | 0.002 | – | – |
| Arthralgias | – | – | 1.82 (1.09–3.05) | 0.02 |
CI = confidence interval; OR = odds ratio. In each model, age (categorical variable) and sociodemographic characteristics and clinical symptoms associated with the dependent variable at a P < 0.05 on bivariable analysis were included.
Features associated with detection of influenza versus other respiratory viruses by PCR.
On bivariable analysis, age < 5 years was associated with a positive test for another respiratory virus compared with influenza (32.1% versus 11.2%, P < 0.001; Table 4). The following clinical symptoms were associated with detection of influenza versus other respiratory viruses: sick contact in the past month (51.8% versus 37.2%, P = 0.009), pain with breathing (17.9% versus 3.6%, P < 0.001), anorexia (75.8% versus 55.7%, P < 0.001), headache (82.1% versus 61.4%, P ≤ 0.001), fatigue/lethargy (81.6% versus 60.0%, P < 0.001), arthralgias (71.3% versus 42.9%, P ≤ 0.001), and myalgias (71.7% versus 44.3%, P < 0.001). However, patients with influenza were less likely to report rhinitis (71.3% versus 83.6%, P = 0.008). Patients with influenza had a higher median temperature (101.2°F versus 100.9°F, P < 0.001) and were more likely to have an additional diagnostic test such as a complete blood count ordered, when compared with patients with other respiratory viruses detected (19.7% versus 11.4%, P = 0.04).
Table 4.
Sociodemographic and clinical characteristics of outpatients with influenza-like illness who had influenza vs. another respiratory virus isolated, southern Sri Lanka, 2013–2015
| Characteristic | Frequency (%) or median (IQR) |
OR (95% CI) | P value | |
|---|---|---|---|---|
| Influenza positive N = 223 | Other virus positive N = 140 | |||
| Child < 5 years | 25 (11.2) | 45 (32.1) | 0.27 (0.15–0.48) | < 0.001 |
| Male | 128 (57.4) | 81 (57.9) | 0.98 (0.64–1.50) | 1.00 |
| Sick contact in past month | 114 (51.8) | 51 (37.2) | 1.81 (1.17–2.79) | 0.009 |
| Prior medical visit for same illness | 60 (26.9) | 35 (25.4) | 1.08 (0.67–1.74) | 0.81 |
| Travel in past 4 weeks | 33 (14.9) | 22 (15.7) | 0.94 (0.53–1.65) | 0.88 |
| Smoking (passive or active) | 48 (21.5) | 36 (26.5) | 0.79 (0.48–1.30) | 0.37 |
| Prior antibiotic for same illness | 11 (4.9) | 8 (5.7) | 0.86 (0.34–2.13) | 0.81 |
| Days of fever/chills | 2 (1–3) | 2 (2–3) | 0.54 | |
| Days of cough | 2 (1–3) | 2 (2–3) | 0.11 | |
| Ear ache | 12 (5.4) | 6 (4.3) | 1.27 (0.48–3.39) | 0.81 |
| Rhinitis | 159 (71.3) | 117 (83.6) | 0.49 (0.29–0.82) | 0.008 |
| Sore throat | 108 (48.4) | 67 (47.9) | 1.02 (0.67–1.56) | 1.00 |
| Dyspnea | 46 (20.6) | 18 (12.9) | 1.76 (0.98–3.24) | 0.07 |
| Pain with breathing | 40 (17.9) | 5 (3.6) | 5.9 (2.43–14.1) | < 0.001 |
| Anorexia | 169 (75.8) | 78 (55.7) | 2.49 (1.59–3.95) | < 0.001 |
| Vomiting | 47 (21.1) | 20 (14.3) | 1.6 (0.92–2.81) | 0.13 |
| Abdominal pain | 16 (7.2) | 10 (7.1) | 1.0 (0.43–2.22) | 1.00 |
| Headache | 183 (82.1) | 86 (61.4) | 2.87 (1.78–4.58) | < 0.001 |
| Fatigue/lethargy | 182 (81.6) | 84 (60) | 2.96 (1.85–4.84) | < 0.001 |
| Athralgias | 159 (71.3) | 60 (42.9) | 3.31 (2.11–5.17) | < 0.001 |
| Myalgias | 160 (71.7) | 62 (44.3) | 3.2 (2.03–4.98) | < 0.001 |
| Temperature (°F) | 101.2 (100.8–102.0) | 100.9 (100.6–101.3) | < 0.001 | |
| Wheezing | 24 (10.9) | 24 (17.1) | 0.59 (0.33–1.09) | 0.11 |
| Antibiotic prescribed | 168 (75.3) | 112 (80) | 0.76 (0.46–1.26) | 0.37 |
| Diagnostic tests ordered | 44 (19.7) | 16 (11.4) | 1.91 (1.01–3.54) | 0.04 |
CI = confidence interval; IQR = interquartile range; OR = odds ratio. Bivariable analysis was performed using the Fisher’s exact test for categorical variables and the Kruskall–Wallis test for continuous variables. Coinfections with influenza were excluded.
On adjusted analysis, patients with influenza were more likely to report a sick contact within the past month (OR = 1.84, P = 0.01), pain with breathing (OR = 3.04, P = 0.03), anorexia (OR = 2.20, P = 0.002), and arthralgias (OR = 1.82, P = 0.02) compared with patients with other respiratory viruses detected (Table 3). Patients < 5 years of age were less likely to have influenza detected versus another respiratory virus detected (OR = 0.33, P = 0.001).
Rapid influenza test performance characteristics.
The sensitivity and specificity of the Veritor Flu A+B rapid influenza test were 88.2% and 95.1%, respectively, when compared with the Luminex PCR test (Table 5). The rapid test performed better at detecting influenza A than influenza B, with sensitivity of 89.7% versus 84.5% (P = 0.34) and specificity of 97.8% versus 98.4% (P = 0.47), respectively. The test was more sensitive at detecting influenza when patients were 5 years of age or older (88.8% versus 84.0%, respectively; P = 0.51) and when monthly influenza prevalence was greater than 20% (88.6% versus 80.0%, respectively; P = 0.13). Having a nasal versus nasopharyngeal sample did not impact test sensitivity greatly (86.7% versus 88.4%, respectively).
Table 5.
Performance characteristics of the Veritor Flu A+B rapid influenza kit (Becton Dickinson), when compared with the gold standard of polymerase chain reaction by the xTAG Respiratory Viral Panel (Luminex Corporation)
| Pathogen | Sensitivity (95% CI) | Specificity (95% CI) | Positive predictive value (95% CI) | Negative predictive value (95% CI) |
|---|---|---|---|---|
| Influenza A and B | 88.2% (83.2–92.2) | 95.1% (92.3–97.1) | 92.0% (87.5–95.3) | 92.7% (89.5–95.2) |
| Influenza A | 89.6% (83.8–93.8) | 97.8% (95.8–99.0) | 94.2% (89.3–97.3) | 95.9% (93.5–97.6) |
| Influenza B | 84.5% (72.6–92.7) | 98.4% (96.9–99.3) | 86.0% (74.2–93.7) | 98.2% (96.7–99.2) |
| Age < 5 years | 84.0% (65.4–93.6) | 97.4% (91.0–99.5) | 91.3% (73.2–98.5) | 94.9% (87.5–98.0) |
| Age ≥ 5 years | 88.8% (83.6–92.5) | 94.4% (91.0–96.6) | 92.1% (87.3–95.1) | 92.1% (88.3–94.7) |
| Prevalence ≤ 20%* | 80.0% (49.0–96.5) | 98.3% (93.9–99.7) | 80.0% (49.0–96.4) | 98.3% (93.9–99.7) |
| Prevalence > 20%† | 88.6% (83.6–92.2) | 93.5% (89.6–96.0) | 92.6% (88.1–95.5) | 90.0% (85.6–93.2) |
| Nasal sample | 86.7% (62.1–97.6) | 97.4% (86.8–99.9) | 92.9% (68.5–99.6) | 95.0% (83.5–99.1) |
| Nasopharyngeal sample | 88.4% (83.3–93.0) | 94.8% (91.7–96.8) | 91.9% (87.3–95.0) | 92.4% (88.9–94.8) |
CI = confidence interval. Overall performance characteristics for influenza A and B, as well as for specific subsets, are listed in the table.
All months during study in which influenza prevalence was ≤ 20%.
All months during study in which influenza prevalence was > 20%.
DISCUSSION
There are few studies that examine the viral etiology of acute respiratory tract infections in tropical, low- or middle-income countries. In our study in southern Sri Lanka, over 60% of outpatient subjects presenting with ILI were found to have a viral pathogen; influenza was the most common etiology, but other viruses such as HEV/HRV and RSV also contributed to disease burden. Distinct seasonality in ILI activity was noted from March to June during each year of the study period, which also corresponded to the months with highest influenza activity. However, no patients reported a prior history of influenza vaccination. Clinical symptoms such as anorexia and arthralgia were most frequently described with influenza, but were also common in patients with other respiratory viruses or no respiratory viruses detected. The Veritor Flu A+B rapid influenza test performed well in this setting, with an overall sensitivity and specificity of 88.2% and 95.1%, respectively.
In this study in southern Sri Lanka, ILI and influenza both had a clear seasonal pattern, with peak activity occurring from March to June of each year. RSV and hMPV accounted for a lower though important burden of disease during peak ILI period, whereas HEV/HRV showed sporadic activity throughout the year. The seasonality of respiratory viruses in tropical regions is not well understood, unlike in higher-income countries in temperate climates, where peak activity typically coincides with the winter months.9,19,20 In tropical regions, RSV has been found to have different peaks such as March–August (Singapore) and April–September (Hong Kong), and in some countries has been associated with the rainy season (Papua New Guinea, Colombia, Kenya, and Gambia).9,19 Other viruses such as HEV/HRV and parainfluenza do not appear to have a clear seasonal association, and may be endemic throughout the year, sporadic, or associated with epidemics.9,19 In Sri Lanka, to our knowledge, only one other study has described the epidemiology of respiratory viruses other than influenza. Among hospitalized children ≤ 5 years of age in two regions of Sri Lanka, RSV accounted for > 50% of disease, with peak activity during May–July in the dry zone and during December–January and in April in the wet zone.21 Our study was conducted in a wet region in the country, but peak RSV activity was during May–July. The reason for variation in seasonality within the country is not clear, but underscores the need for further studies on respiratory virus epidemiology in tropical and subtropical settings.
Influenza accounted for the largest burden of disease in our study, with peak activity between March and June each year. As with other respiratory viruses, influenza seasonality in the tropics is not well defined. Variable seasonality as well as year-round circulation have been observed, although influenza activity is not believed to be random.20,22 In one study examining several countries in tropical and subtropical areas of southern and south-eastern Asia, peak influenza activity occurred during June–July or October–November. Countries closest to the equator had year-round circulation.23 In Sri Lanka, the seasonality of influenza is debated. Two analyses based on FluNet data from the WHO have proposed peak influenza activity in October–December or in December.11,24 One study examined influenza A activity among hospitalized patients in the Western Province of Sri Lanka and found peak activity from May to July, with a minor peak from October to December.25 The Sri Lanka Ministry of Health identifies two peaks in influenza activity, with the first occurring during May-July and the second occurring during November–January.26 The results from our study overlap with the first peak period defined by the Ministry of Health. The reasons for variation in influenza peak activity between studies is unclear, but may be related to the specific region in Sri Lanka that was studied.27,28 The lack of clear correlation between influenza and weather parameters in our study suggests that influenza seasonality is complex and likely affected by multiple factors, and again highlights the need for local epidemiological data.
The WHO’s recommendations regarding the timing and composition of influenza vaccination are based on a country’s location in the Northern or Southern hemisphere. However, studies suggest that such recommendations may not be applicable for more than half of countries in the tropical belt, given variable influenza activity.24 Our data from 2013 to 2015 suggest that in southern Sri Lanka, influenza vaccination may need to be targeted to January–February, preceding peak influenza activity in March–June. Although Sri Lanka does not currently have a national influenza vaccine policy and the coverage rate with influenza vaccine is < 1%, the high burden of influenza in our study suggests that vaccination may provide benefit in this region.10 We did not assess cost effectiveness of vaccination in our study, but studies from other low- or middle-income countries indicate that influenza vaccine is cost effective in the elderly, infants, and children and adults with high-risk conditions.29–31 Influenza and ILI in low- or middle-income countries have a larger impact on indirect costs and productivity when compared with higher-income countries.32 Knowledge of local epidemiology is a key component in developing and implementing public health measures such as vaccination. In Sri Lanka, further country and region-specific surveillance for respiratory viruses is needed.20,23,24,33
We identified several features such as having a sick contact within the last month, pain with breathing, anorexia, and fatigue as being associated with having a respiratory virus detected. These features are not dissimilar to clinical characteristics identified in other studies.34–38 We also found clinical symptoms such as anorexia and arthralgias to be more common in patients with influenza versus other respiratory viruses. However, many of the symptoms associated with influenza were also commonly found in patients with other respiratory viruses or with no respiratory viruses, which speaks to the difficulty of clinical diagnosis in identifying patients with influenza.34,38 While symptomatic treatment and public health measures in most cases are similar regardless of the type of respiratory virus, the implementation of vaccination and antiviral treatment of influenza makes accurate diagnosis an important consideration in this setting with substantial influenza disease burden.
The Veritor Flu A+B rapid influenza test performed well as a rapid point-of-care test in our study environment. Studies from higher-income settings have reported similar results with the Veritor test and there are data to suggest that the Veritor test has the ability to detect influenza at lower limits of detection when compared with other rapid tests.15,39,40 The use of a rapid test with good performance characteristics in settings with limited laboratory infrastructure has the potential to individualize patient treatment and to add to local data on influenza epidemiology, which could influence public health decisions such as vaccination policy.41 While the cost of rapid influenza testing (approximately $10–30 per test) may still prove prohibitive for resource-limited settings, the targeting of testing to patients with certain clinical characteristics such as anorexia and arthralgias, as found in our study, may prove to be more cost efficient.42 In addition, over 70% of participants with ILI received an antibiotic prescription in our study. The use of rapid viral testing such as with the Veritor Flu A+B test may help reduce unnecessary antibiotic use in this setting.13
Some limitations to this study must be noted. ILI surveillance for the months of February and December was only performed during 1 year. However, the monthly proportion for ILI and the examined respiratory viruses was < 10% for each of these months, with the exception of HEV/HRV (> 10% in February 2014). By using a previously published definition by PATH and examining ILI epidemiology over a 2-year period, we attempted to define respiratory virus seasonality in a rigorous fashion. Our surveillance was only performed at one hospital in the Southern Province. However, this hospital serves as a tertiary care and referral center for the region, and our findings likely reflect broader disease patterns within the region. Finally, our surveillance activities were conducted over the period of two calendar years, and may not reflect seasonal patterns in other years. Further longitudinal studies on respiratory viral epidemiology are needed in this region.
To our knowledge, this is the first published description of respiratory virus epidemiology in southern Sri Lanka or among Sri Lankan outpatients. Influenza was the largest contributor to respiratory infections, with peak activity during March–June, although other viruses such as HEV/HRV and RSV also contributed to disease burden. The Veritor rapid influenza test performed well in our study population and may be a useful tool for describing local influenza epidemiology in resource-limited settings. Our study based on 2 years of surveillance data suggests that in southern Sri Lanka, targeting infection control and influenza vaccination efforts in January–February may have a large public health impact.
Acknowledgments:
We would like to thank Becton, Dickinson, and Company for providing the Veritor rapid influenza test kits.
REFERENCES
- 1.Ferkol T, Schraufnagel D, 2014. The global burden of respiratory disease. Ann Am Thorac Soc 11: 404–406. [DOI] [PubMed] [Google Scholar]
- 2.Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB, 2007. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 25: 5086–5096. [DOI] [PubMed] [Google Scholar]
- 3.Peasah SK, Azziz-Baumgartner E, Breese J, Meltzer MI, Widdowson M-A, 2013. Influenza cost and cost-effectiveness studies globally: a review. Vaccine 31: 5339–5348. [DOI] [PubMed] [Google Scholar]
- 4.Puzelli S, Valdarchi C, Ciotti M, Dorrucci M, Farchi F, Babakir-Mina M, Perno CF, Donatelli I, Rezza G, 2009. Viral causes of influenza-like illness: insight from a study during the winters 2004–2007. J Med Virol 81: 2066–2071. [DOI] [PubMed] [Google Scholar]
- 5.Huo X, Qin Y, Qi X, Zu R, Tang F, Li L, Hu Z, Zhu F, 2012. Surveillance of 16 respiratory viruses in patients with influenza-like illness in Nanjing, China. J Med Virol 84: 1980–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thiberville SD, Ninove L, Vu Hai V, Botelho-Nevers E, Gazin C, Thirion L, Salez N, de Lamballerie X, Charrel R, Brouqui P, 2012. The viral etiology of an influenza-like illness during the 2009 pandemic. J Med Virol 84: 1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellei N, Carraro E, Perosa A, Watanabe A, Arruda E, Granato C, 2008. Acute respiratory infection and influenza-like illness viral etiologies in Brazilian adults. J Med Virol 80: 1824–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breiman RF, Van Beneden CA, Farnon EC, 2013. Surveillance for respiratory infections in low- and middle-income countries: experience from the Centers for Disease Control and Prevention’s Global Disease Detection International Emerging Infections Program. J Infect Dis 208 (Suppl 3): S167–S172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shek LP, Lee BW, 2003. Epidemiology and seasonality of respiratory tract virus infections in the tropics. Paediatr Respir Rev 4: 105–111. [DOI] [PubMed] [Google Scholar]
- 10.Hirve S, Lambach P, Paget J, Vandemaele K, Fitzner J, Zhang W, 2016. Seasonal influenza vaccine policy, use and effectiveness in the tropics and subtropics: a systematic literature review. Influenza Other Respir Viruses 10: 254–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirve S, Newman LP, Paget J, Azziz-Baumgartner E, Fitzner J, Bhat N, Vandemaele K, Zhang W, 2016. Influenza seasonality in the tropics and subtropics: when to vaccinate? PLoS One 11: e0153003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization , 2012. WHO Interim Global Epidemiological Surveillance Standards for Influenza. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 13.Tillekeratne LG, Bodinayake CK, Nagahawatte A, Vidanagama D, Devasiri V, Arachchi WK, Kurukulasooriya R, De Silva AD, Ostbye T, Reller ME, Woods CW, 2015. Use of rapid influenza testing to reduce antibiotic prescriptions among outpatients with influenza-like illness in southern Sri Lanka. Am J Trop Med Hyg 93: 1031–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickinson Becton, 2014. BD Veritor System: CLIA-Waived for Rapid Detection of Flu A+B. Available at: http://www.bd.com/ds/productCenter/256045.asp. Accessed May 31, 2014.
- 15.Hassan F, Nguyen A, Formanek A, Bell JJ, Selvarangan R, 2014. Comparison of the BD Veritor System for Flu A+B with the Alere BinaxNOW influenza A and B card for detection of influenza A and B viruses in respiratory specimens from pediatric patients. J Clin Microbiol 52: 906–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pabbaraju K, Wong S, Tokaryk KL, Fonseca K, Drews SJ, 2011. Comparison of the Luminex xTAG respiratory viral panel with xTAG respiratory viral panel fast for diagnosis of respiratory virus infections. J Clin Microbiol 49: 1738–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esposito S, Daleno C, Prunotto G, Scala A, Tagliabue C, Borzani I, Fossali E, Pelucchi C, Principi N, 2013. Impact of viral infections in children with community-acquired pneumonia: results of a study of 17 respiratory viruses. Influenza Other Respir Viruses 7: 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minh An DT, Ngoc NT, Nilsson M, 2014. Influenza-like illness in a Vietnamese province: epidemiology in correlation with weather factors and determinants from the surveillance system. Glob Health Action 7: 23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haynes AK, Manangan AP, Iwane MK, Sturm-Ramirez K, Homaira N, Brooks WA, Luby S, Rahman M, Klena JD, Zhang Y, Yu H, Zhan F, Dueger E, Mansour AM, Azazzy N, McCracken JP, Bryan JP, Lopez MR, Burton DC, Bigogo G, Breiman RF, Feikin DR, Njenga K, Montgomery J, Cohen AL, Moyes J, Pretorius M, Cohen C, Venter M, Chittaganpitch M, Thamthitiwat S, Sawatwong P, Baggett HC, Luber G, Gerber SI, 2013. Respiratory syncytial virus circulation in seven countries with Global Disease Detection Regional Centers. J Infect Dis 208 (Suppl 3): S246–S254. [DOI] [PubMed] [Google Scholar]
- 20.Azziz Baumgartner E, Dao CN, Nasreen S, Bhuiyan MU, Mah-E-Muneer S, Al Mamun A, Sharker MAY, Zaman RU, Cheng P-Y, Klimov AI, Widdowson M-A, Uyeki TM, Luby SP, Mounts A, Bresee J, 2012. Seasonality, timing, and climate drivers of influenza activity worldwide. J Infect Dis 206: 838–846. [DOI] [PubMed] [Google Scholar]
- 21.Jayaweera JAAS, Noordeen F, Morel A, Pitchai N, Kothalawala S, Abeykoon AMSB, Peiris JSM, 2016. Viral burden in acute respiratory tract infections in hospitalized children in the wet and dry zones of Sri Lanka. Int J Infect Dis 45: 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng S, Gordon A, 2015. Influenza burden and transmission in the tropics. Curr Epidemiol Rep 2: 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saha S, Chadha M, Al Mamun A, Rahman M, Sturm-Ramirez K, Chittaganpitch M, Pattamadilok S, Olsen SJ, Sampurno OD, Setiawaty V, Pangesti KNA, Samaan G, Archkhawongs S, Vongphrachanh P, Phonekeo D, Corwin A, Touch S, Buchy P, Chea N, Kitsutani P, Mai LQ, Thiem VD, Lin R, Low C, Kheong CC, Ismail N, Yusof MA, Tandoc A, Roque V, Mishra A, Moen AC, Widdowson M-A, Partridge J, Lal RB, 2014. Influenza seasonality and vaccination timing in tropical and subtropical areas of southern and south-eastern Asia. Bull World Health Organ 92: 318–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alonso WJ, Yu C, Viboud C, Richard SA, Schuck-Paim C, Simonsen L, Mello WA, Miller MA, 2015. A global map of hemispheric influenza vaccine recommendations based on local patterns of viral circulation. Sci Rep 5: 17214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perera KVHKK, Chan KH, Ma E, Peiris JSM, 2010. Influenza virus infections among a sample of hospital attendees in Ragama, Sri Lanka. Ceylon Med J 55: 40–44. [DOI] [PubMed] [Google Scholar]
- 26.Sri Lanka Ministry of Health EU , 2015. Health Education Presentation on Seasonal Influenza 2015 Available at: internal-pdf://3829176326/Influenza update from Sri Lanka Ministry of He.pdf. Accessed June 1, 2016. [Google Scholar]
- 27.Alonso WJ, Viboud C, Simonsen L, Hirano EW, Daufenbach LZ, Miller MA, 2007. Seasonality of influenza in Brazil: a traveling wave from the Amazon to the subtropics. Am J Epidemiol 165: 1434–1442. [DOI] [PubMed] [Google Scholar]
- 28.Green HK, Zhao H, Boddington NL, Andrews N, Durnall H, Elliot AJ, Smith G, Gorton R, Donati M, Ellis J, Zambon M, Pebody R, 2015. Detection of varying influenza circulation within England in 2012/13: informing antiviral prescription and public health response. J Public Health (Oxf) 37: 295–304. [DOI] [PubMed] [Google Scholar]
- 29.Ott JJ, Klein Breteler J, Tam JS, Hutubessy RCW, Jit M, de Boer MR, 2013. Influenza vaccines in low and middle income countries: a systematic review of economic evaluations. Hum Vaccin Immunother 9: 1500–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lall D, Cason E, Pasquel FJ, Ali MK, Narayan KMV, 2016. Effectiveness of influenza vaccination for individuals with chronic obstructive pulmonary disease (COPD) in low- and middle-income countries. COPD 13: 93–99. [DOI] [PubMed] [Google Scholar]
- 31.Breteler JK, Tam JS, Jit M, Ket JCF, De Boer MR, 2013. Efficacy and effectiveness of seasonal and pandemic A (H1N1) 2009 influenza vaccines in low and middle income countries: a systematic review and meta-analysis. Vaccine 31: 5168–5177. [DOI] [PubMed] [Google Scholar]
- 32.de Francisco Shapovalova N, Donadel M, Jit M, Hutubessy R, 2015. A systematic review of the social and economic burden of influenza in low- and middle-income countries. Vaccine 33: 6537–6544. [DOI] [PubMed] [Google Scholar]
- 33.Caini S, Andrade W, Badur S, Balmaseda A, Barakat A, Bella A, Bimohuen A, Brammer L, Bresee J, Bruno A, Castillo L, Ciblak MA, Clara AW, Cohen C, Cutter J, Daouda C, de Lozano C, De Mora D, Dorji K, Emukule GO, Fasce RA, Feng L, Ferreira de Almeida WA, Guiomar R, Heraud J-M, Holubka O, Huang QS, Kadjo HA, Kiyanbekova L, Kosasih H, Kusznierz G, Lara J, Li M, Lopez L, Mai Hoang PV, Pessanha Henriques CM, Matute ML, Mironenko A, Moreno B, Mott JA, Njouom R, Ospanova A, Owen R, Pebody R, Pennington K, Puzelli S, Quynh Le MT, Razanajatovo NH, Rodrigues A, Rudi JM, Tzer Pin Lin R, Venter M, Vernet M-A, Wangchuk S, Yang J, Yu H, Zambon M, Schellevis F, Paget J, Study GIB, 2016. Temporal patterns of influenza A and B in tropical and temperate countries: what are the lessons for influenza vaccination? PLoS One 11: e0152310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fischer JB, Prasad PA, Coffin SE, Alpern ER, Mistry RD, 2014. Canadian Acute Respiratory Illness and Flu Scale (CARIFS) for clinical detection of influenza in children. Clin Pediatr (Phila) 53: 1174–1180. [DOI] [PubMed] [Google Scholar]
- 35.Howard PF, McCaw JM, Richmond PC, Nissen M, Sloots T, Lambert SB, Lai M, Greenberg M, Nolan T, McVernon J, 2013. Virus detection and its association with symptoms during influenza-like illness in a sample of healthy adults enrolled in a randomised controlled vaccine trial. Influenza Other Respi Viruses 7: 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schnepf N, Resche-Rigon M, Chaillon A, Scemla A, Gras G, Semoun O, Taboulet P, Molina JM, Simon F, Goudeau A, LeGoff J, 2011. High burden of non-influenza viruses in influenza-like illness in the early weeks of H1N1v epidemic in France. PLoS One 6: e23514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang X, Yao Y, Chen M, Yang X, Xie Y, Liu Y, Zhao X, Gao Y, Wei L, 2012. Etiology and clinical characteristics of influenza-like illness (ILI) in outpatients in Beijing, June 2010 to May 2011. PLoS One 7: e28786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimmerman RK, Rinaldo CR, Nowalk MP, Gk B, Thompson MG, Moehling KK, Bullotta A, Wisniewski S, 2014. Influenza and other respiratory virus infections in outpatients with medically attended acute respiratory infection during the 2011–12 influenza season. Influenza Other Respi Viruses 8: 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peters TR, Blakeney E, Vannoy L, Poehling KA, 2013. Evaluation of the limit of detection of the BD Veritor™ system flu A+B test and two rapid influenza detection tests for influenza virus. Diagn Microbiol Infect Dis 75: 200–202. [DOI] [PubMed] [Google Scholar]
- 40.Dunn J, Obuekwe J, Baun T, Rogers J, Patel T, Snow L, 2014. Prompt detection of influenza A and B viruses using the BD Veritor™ System Flu A+B, Quidel® Sofia® Influenza A+B FIA, and Alere BinaxNOW® Influenza A and B compared to real-time reverse transcription-polymerase chain reaction (RT-PCR). Diagn Microbiol Infect Dis 79: 10–13. [DOI] [PubMed] [Google Scholar]
- 41.Center for Disease Control and Prevention , 2015. Guidance for Clinicians on the Use of Rapid Influenza Diagnostic Tests. Available at: https://www.cdc.gov/flu/professionals/diagnosis/rapidclin.htm. Accessed June 1, 2016. [Google Scholar]
- 42.Tillekeratne LG, Bodinayake CK, Nagahawatte A, Vidanagama D, Devasiri V, Arachchi WK, Kurukulasooriya R, De Silva AD, Ostybe T, Reller ME, Woods CW, 2015. An under-recognized influenza epidemic identified by rapid influenza testing, southern Sri Lanka, 2013. Am J Trop Med Hyg 92: 1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]