Abstract.
Few data are available on the burden of malaria hospitalization in the United States. Study of malaria using hospital-based data can better define the impact of malaria and help inform prevention efforts. U.S. malaria cases identified from hospitalization discharge records in the 2000–2014 Nationwide Inpatient Sample were examined. Frequencies and population rates were reported by demographics, infecting species, clinical, financial, institutional, geographic, and seasonal characteristics, and disparities were identified. Time trends in malaria cases were assessed using negative binomial regression. From 2000 to 2014, there were an estimated 22,029 malaria-related hospitalizations (4.88 per 1 million population) in the United States, including 182 in-hospital deaths and 4,823 severe malaria cases. The rate of malaria-related hospitalizations did not change significantly over the study period. The largest number of malaria-related hospitalizations occurred in August. Malaria-related hospitalizations occurred disproportionately among patients who were male, black, or 25–44 years of age. Plasmodium falciparum accounted for the majority of malaria-related hospitalizations. On average, malaria patients were hospitalized for 4.36 days with charges of $25,789. Patients with a malaria diagnosis were more often hospitalized in the Middle Atlantic and South Atlantic census divisions, urban teaching, private not-for-profit, and large-bed-size hospitals. Malaria imposes a substantial disease burden in the United States. Enhanced primary and secondary prevention measures, including strategies to increase the use of pretravel consultations and prompt diagnosis and treatment are needed.
INTRODUCTION
Malaria is the leading cause of death by parasitic disease in the world and remains one of the most important and intractable global public health problems. An estimated 212 million cases of malaria, and 429,000 deaths due to malaria occurred in 2015.1 Malaria is caused by infection with the protozoan agents of the genus Plasmodium. Several species of Plasmodium (Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, Plasmodium malariae, and Plasmodium knowlesi) are known to affect humans, with P. falciparum causing the most morbidity and the vast majority of the mortality. Transmission typically occurs through the bite from an infected female Anopheles mosquito, which has widespread distribution throughout the world, including the United States. Malaria transmission can also occur by blood transfusion, organ transplant, percutaneous exposure, and vertically (from mother to fetus), though these forms of transmission are uncommon.2
If untreated, malaria can cause severe and fatal disease. Clinical manifestations can range from fever, shaking chills, muscle pains, and other nonspecific symptoms in uncomplicated malaria, to jaundice, acute renal failure, severe anemia, cerebral malaria, acute respiratory distress syndrome (ARDS), and other serious complications in severe malaria,2 which can be rapidly fatal. Malaria during pregnancy is associated with many adverse outcomes, including maternal mortality, maternal anemia, low birth weight, intrauterine growth retardation, and fetal loss.3
In recent years, despite increasing antimalarial drug and vector resistance, the scale-up of interventions in the endemic areas of the world has encouragingly reduced the estimated global malaria incidence by 41% and mortality by 62% from 2000 to 2015.1,4 However, the number of imported malaria cases has steadily increased in the United States,5 where previously endemic malaria was eliminated in the 1950s. Similar to other countries6 that eliminated malaria, this increase has mostly occurred among returned travelers, as well as among foreign visitors and immigrants from malaria-endemic countries.7 Imported cases pose the potential threat for reintroduction of malaria into the naturally present Anopheles population in the United States.8 Many documented outbreaks of autochthonous malaria fueled by returned travelers, migrant workers, and immigrants have occurred in the United States since malaria was declared eliminated.9
Malaria is a nationally reportable disease in the United States, and the Centers for Disease Control and Prevention (CDC) maintains a highly regarded surveillance system of confirmed (positive microscopy or polymerase chain reaction [PCR]) or suspected (positive rapid diagnostic test without microscopy or PCR confirmation) malaria cases. The surveillance data are used to provide annual general malaria status updates, to detect local transmission, to monitor the patterns of resistance to antimalarial drugs, and to guide malaria prevention recommendations for international travelers.5 However, with possible underreporting of malaria in the current passive surveillance systems,10,11 the complete domestic burden of malaria remains unknown.
Epidemiological study of malaria using hospital data can complement the findings from current nationwide surveillance system data to elucidate the malaria disease burden and case characteristics, and provide insight into the health‐care utilization and outcomes for malaria in the United States. We report the analysis of malaria-related hospitalizations in the United States from 2000 to 2014.
MATERIALS AND METHODS
Hospital discharge records from the Nationwide Inpatient Sample (NIS) were used for analysis of malaria-related hospitalizations in the United States during 2000–2014. The NIS is sampled from the State Inpatient Databases, and is part of the Healthcare Cost and Utilization Project (HCUP) sponsored by the Agency for Healthcare Research and Quality. The NIS is the largest publically available all-payer inpatient data source in the United States, and each annual NIS dataset contains about 7–8 million hospital discharge records (about 20%) and over 100 clinical and nonclinical data elements, including sociodemographics, admission characteristics, diagnosis type, length of stay, codiagnoses, procedures performed, institutional characteristics, and total charges.12,13 For 2000–2011 data, the NIS was a stratified, single-stage cluster sampling of U.S. community hospitals, in which all of the discharges from the selected hospitals were included in the NIS dataset.12 For 2012–2014 data, the NIS was a stratified random sample of discharges from all community hospitals participating in HCUP.13 Details on the sampling scheme have been described elsewhere.12,13
Cases of malaria from 2000 to 2014 were identified from discharge records in the NIS by the primary and secondary diagnoses, which used the International Classification of Diseases, 9th revision (ICD-9)14 codes of 084.0–084.9 (084.0: falciparum malaria, malignant tertian; 084.1: vivax malaria, benign tertian; 084.2: malariae, quartan; 084.3: ovale malaria; 084.4: other malaria; 084.5: mixed malaria; 084.6: malaria, unspecified; 084.7: induced malaria; 084.8: Blackwater fever; 084.9: other pernicious complications of malaria) and of 647.4 (malaria complicating pregnancy, childbirth, or puerperium).
In this study, the definition of severe malaria was modified from that used by the CDC.5 Since no specific drugs used or laboratory results are available in the NIS, we were unable to use parasitemia ≥ 5% or treatment of severe malaria (i.e., artesunate or quinidine) as specified in the CDC definition.5 Malaria complications were identified using ICD-9 codes and HCUP Clinical Classification Software (CCS)13 categories, which are clinically meaningful categories of ICD-9 codes. Discharge records listing a malaria diagnosis along with one or more of the following criteria were considered as severe malaria cases: 1) neurologic symptoms (cerebral malaria)—CCS codes 82, 83, 85, or 95 (paralysis, epilepsy, convulsions, alteration of consciousness, coma, stupor, brain damage, other nervous system disorders); 2) renal failure—CCS code 157; 3) severe anemia—CCS code 59 (deficiency anemia) with procedural CCS code 222 (blood transfusion); 4) ARDS—CCS code 131; 5) jaundice—ICD-9 code 782.4; or 6) exchange transfusion—ICD-9 code 99.01. Malaria-related hospitalizations with an in-hospital death were also considered as severe malaria cases.
Data analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) with survey procedures that accounted for the complex sampling design. Variables of interest included demographics, infecting species, clinical, financial, institutional, geographic, and seasonal characteristics. National estimates of the frequency of malaria-related hospitalizations were produced using NIS discharge-level sample weights, which are provided by HCUP. Rates per U.S. population16 were calculated using bridged-race U.S. census population estimates. Trends over the period in the counts of malaria cases by different patient characteristics were assessed using negative binomial regression offset by the population.
RESULTS
From 2000 to 2014, there were an estimated 22,029 (95% confidence interval [CI] = 21,001–23,057) malaria-related hospitalizations in the United States, which far outnumbered that of other travel-associated diseases, including filariasis, dengue, schistosomiasis, trypanosomiasis, and leishmaniasis (Table 1).
Table 1.
Number of hospitalizations for selected travel-associated infectious diseases in the United States, NIS 2000–2014
| Disease | Malaria | Strongyloidiasis | Filariasis | Dengue | Schistosomiasis | Trypanosomiasis | Leishmaniasis |
|---|---|---|---|---|---|---|---|
| ICD-9-CM | 084,647.4 | 127.2 | 125 | 061 | 120 | 086 | 085 |
| 2000 | 1,757 | 262 | 535 | 72 | 127 | 114 | 61 |
| 2001 | 1,822 | 325 | 285 | 119 | 157 | 107 | 103 |
| 2002 | 1,278 | 320 | 211 | 134 | 149 | 64 | 70 |
| 2003 | 1,226 | 342 | 304 | 101 | 77 | 158 | 78 |
| 2004 | 1,206 | 309 | 226 | 106 | 187 | 105 | 90 |
| 2005 | 1,488 | 329 | 270 | 236 | 177 | 122 | 76 |
| 2006 | 1,420 | 344 | 252 | 141 | 166 | 135 | 86 |
| 2007 | 1,347 | 404 | 291 | 294 | 222 | 192 | 151 |
| 2008 | 1,093 | 412 | 325 | 344 | 164 | 278 | 81 |
| 2009 | 1,464 | 408 | 321 | 193 | 134 | 241 | 107 |
| 2010 | 2,070 | 481 | 282 | 565 | 236 | 156 | 182 |
| 2011 | 1,626 | 442 | 337 | 242 | 257 | 172 | 108 |
| 2012 | 1,395 | 530 | 390 | 430 | 240 | 185 | 75 |
| 2013 | 1,300 | 490 | 405 | 530 | 165 | 170 | 110 |
| 2014 | 1,540 | 555 | 440 | 380 | 195 | 220 | 95 |
| Annual Average | 1,469 | 397 | 324 | 259 | 175 | 159 | 98 |
ICD-9-CM = International Classification of Diseases, 9th revision, Clinical Modification; NIS = Nationwide Inpatient Sample. All numbers are national estimates based on weighted frequencies.
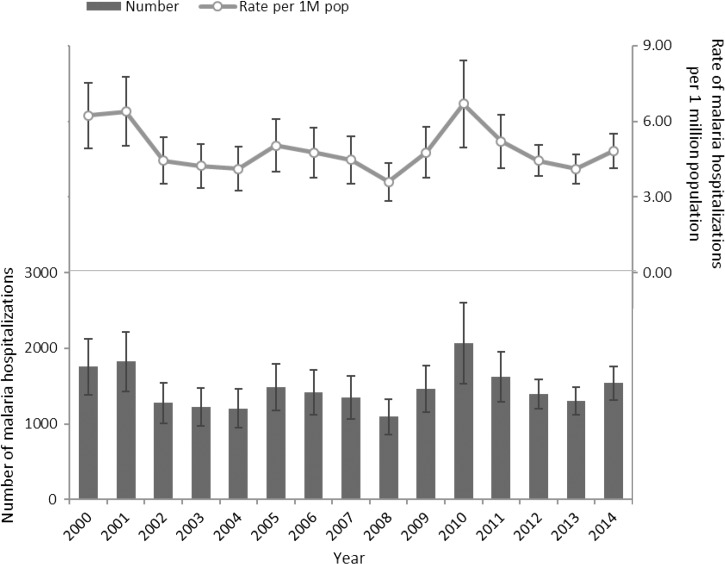
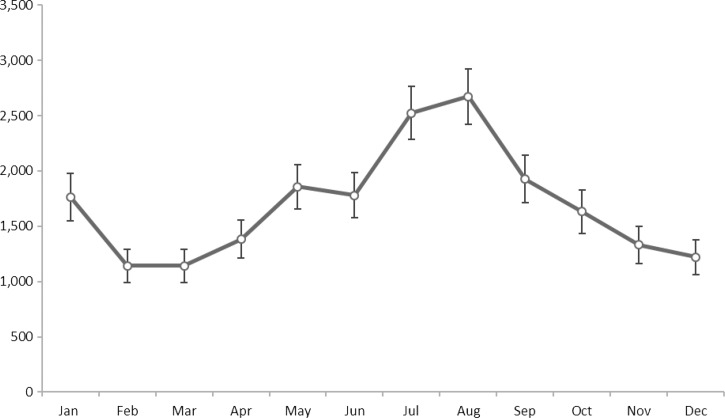
An average of 1,469 malaria-related hospitalizations occurred each year at a rate of 4.88 (95% CI = 4.50–5.26) per 1 million population. The rate of malaria-related hospitalizations did not change significantly over the study period. Malaria-related hospitalizations peaked in 2010, with 2,070 (95% CI = 1,533–2,607) cases and a rate of 6.69 (95% CI = 4.96–8.43) per 1 million population (Figure 1). The largest number of malaria-related hospitalizations occurred in August, followed by a small peak in January (Figure 2).
Figure 1.
Number and rate of malaria hospitalizations in the United States, Nationwide Inpatient Sample, 2000–2014. All numbers are national estimates based on weighted frequencies.
Figure 2.
Number of malaria-related hospitalizations in the United States, by month, Nationwide Inpatient Sample, 2000–2014. All numbers are national estimates based on weighted frequencies and nonmissing data.
Demographics.
The proportion and rate of malaria-related hospitalizations was higher for males (60.1%; 5.97 per 1 million population [95% CI = 5.65–6.28]) than females (39.4%; 3.78 per 1 million population [95% CI = 3.55–4.02]). Pregnant women accounted for 5.5% of the overall, and 14.0% of the female malaria-related hospitalizations. The most common race/ethnic group was black, accounting for over half (52.5%) of all malaria-related hospitalizations with known race information, followed by white (24.0%), Hispanic (6.3%), Asian/Pacific Islander (5.9%), and Native American (0.9%). Blacks (16.98 per 1 million population [95% CI = 15.68–18.28]) also had the highest rates of malaria-related hospitalizations compared with all of the other race/ethnic groups, whereas whites (1.49 per 1 million population [95% CI = 1.38–1.60]) had the lowest rates. Rates for Hispanics decreased over the period (P < 0.01). The mean age of patients with a malaria diagnosis was 37.0 (95% CI = 36.4–37.6). The number and rate of malaria-related hospitalizations increased with age, peaking at 25–44 years, then decreased (Table 2).
Table 2.
Number and rate of malaria-related hospitalizations in the United States, by demographic, NIS, 2000–2014
| Unweighted frequency | Percent of malaria-related hospitalizations | Weighted frequency | Lower 95% CL weight frequency | Upper 95% CL weight frequency | Rate per 1 million population | Lower 95% CL rate | Upper 95% CL rate | |
|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||
| Male | 2,748 | 60.1 | 13,244 | 12,546 | 13,942 | 5.97 | 5.65 | 6.28 |
| Female | 1,803 | 39.4 | 8,684 | 8,148 | 9,220 | 3.78 | 3.55 | 4.02 |
| Pregnant | 252 | 5.5 | 1,220 | 1,007 | 1,432 | 0.53 | 0.44 | 0.62 |
| Missing | 20 | 0.4 | 96 | – | – | – | – | – |
| Race | ||||||||
| White | 925 | 20.2 | 4,453 | 4,125 | 4,781 | 1.49 | 1.38 | 1.60 |
| Black | 2,011 | 44.0 | 9,735 | 8,991 | 10,478 | 16.98 | 15.68 | 18.28 |
| Hispanic | 246 | 5.4 | 1,169 | 986 | 1,351 | 1.70 | 1.43 | 1.96 |
| Asian/Pacific Islander | 231 | 5.1 | 1,103 | 947 | 1,259 | 4.94 | 4.42 | 6.05 |
| Native American | 35 | 0.8 | 174 | 115 | 234 | 4.64 | 3.06 | 6.24 |
| Other | 399 | 8.7 | 1,913 | 1,696 | 2,129 | – | – | – |
| Missing | 725 | 15.9 | 3,483 | – | – | – | – | – |
| Age (years) | ||||||||
| Under 5 | 179 | 3.9 | 863 | 708 | 1,018 | 2.89 | 2.38 | 3.42 |
| 5–14 | 354 | 7.7 | 1,725 | 1,472 | 1,978 | 2.81 | 2.40 | 3.22 |
| 15–24 | 739 | 16.2 | 3,560 | 3,260 | 3,860 | 5.57 | 5.10 | 6.04 |
| 25–44 | 1,688 | 36.9 | 8,148 | 7,656 | 8,639 | 6.53 | 6.14 | 6.92 |
| 45–64 | 1,211 | 26.5 | 5,819 | 5,400 | 6,238 | 5.14 | 4.77 | 5.51 |
| 65–84 | 358 | 7.8 | 1,708 | 1,520 | 1,896 | 3.37 | 3.00 | 3.74 |
| Over 85 | 35 | 0.8 | 166 | 111 | 222 | 2.19 | 1.45 | 2.91 |
| Missing | 8 | 0.2 | 40 | – | – | – | – | – |
| Mean (range) | 37.0 | (0) | (96) | 36.4 | 37.6 | – | – | – |
| Total | 4,572 | 100.0 | 22,029 | 21,001 | 23,057 | 4.88 | 4.50 | 5.26 |
CL = confidence level; NIS = Nationwide Inpatient Sample. Unweighted frequencies are the raw frequencies in the NIS, and weighted raw frequencies represent national estimates.
Species.
Species information was known for about half (52.9%) of the malaria-related hospitalizations. Plasmodium falciparum (72.9%) accounted for the majority of malaria-related hospitalizations with known species information, followed by P. vivax (22.4%), P. malariae (3.1%), and P. ovale (2.8%). Few specified more than one malaria species (1.2%) or listed mixed malaria (2.0%) as a diagnosis. Unspecified malaria accounted for 34.8% of all malaria-related hospitalizations (Table 3).
Table 3.
Clinical characteristics of malaria-related hospitalizations in the United States, NIS, 2000–2014
| Unweighted frequency | Percent of malaria-related hospitalizations | Weighted frequency | Lower 95% CL weight frequency | Upper 95% CL weight frequency | |
|---|---|---|---|---|---|
| Malaria diagnosis | |||||
| Primary | 3,798 | 83.1 | 18,297 | 17,374 | 19,220 |
| Secondary | 774 | 16.9 | 3,732 | 3,416 | 4,048 |
| Clinical classification | |||||
| Uncomplicated malaria | 3,570 | 78.0 | 17,206 | 16,336 | 18,076 |
| Severe malaria | 1,002 | 22.0 | 4,823 | 4,485 | 5,161 |
| Cerebral malaria | 197 | 4.3 | 956 | 819 | 1,093 |
| Malaria with anemia | 330 | 7.2 | 1,587 | 1,409 | 1,766 |
| Malaria with renal failure | 437 | 9.6 | 2,113 | 1,903 | 2,324 |
| Malaria with ARDS | 186 | 4.1 | 900 | 766 | 1,034 |
| Malaria with jaundice | 167 | 3.7 | 808 | 679 | 937 |
| Malaria with in-hospital death | 37 | 0.8 | 182 | 123 | 241 |
| Infecting species | |||||
| Species identified | 2,418 | 52.9 | 11,651 | 10,980 | 12,321 |
| Falciparum | 1,761 | 38.6 | 8,495 | 7,930 | 9,060 |
| Vivax | 544 | 11.9 | 2,612 | 2,362 | 2,861 |
| Malariae | 76 | 1.6 | 358 | 275 | 441 |
| Ovale | 67 | 1.5 | 331 | 248 | 414 |
| Species not identified | 2,154 | 47.1 | 10,379 | 9,795 | 10,962 |
| Mixed malaria | 90 | 2.0 | 430 | 327 | 533 |
| Other malaria | 314 | 6.9 | 1,520 | 1,333 | 1,708 |
| Unspecified malaria | 1,590 | 34.8 | 7,659 | 7,189 | 8,128 |
| Blackwater fever | 17 | 0.4 | 84 | 44 | 124 |
| Complicated malaria | 125 | 2.7 | 599 | 490 | 708 |
| Number of diagnoses | |||||
| 1 | 468 | 10.3 | 2,265 | 2,017 | 2,514 |
| 2–3 | 1,233 | 27.0 | 5,944 | 5,509 | 6,380 |
| 4–6 | 1,443 | 31.5 | 6,935 | 6,502 | 7,368 |
| 7–10 | 967 | 21.1 | 4,653 | 4,302 | 5,003 |
| 11 or more | 461 | 10.1 | 2,232 | 2,010 | 2,454 |
| Missing | 0 | 0.0 | 0 | – | – |
| Number of chronic conditions* | |||||
| 0 | 430 | 20.1 | 2,108 | 1,859 | 2,356 |
| 1–3 | 1,302 | 60.6 | 6,356 | 5,866 | 6,846 |
| 4 or more | 414 | 19.3 | 2,024 | 1,809 | 2,238 |
| Missing | 0 | 0.0 | 0 | – | – |
| Number of procedures performed | |||||
| 0 | 3,065 | 66.9 | 14,746 | 13,992 | 15,501 |
| 1 | 793 | 17.4 | 3,834 | 3,499 | 4,170 |
| 2 or more | 714 | 15.7 | 3,448 | 3,132 | 3,765 |
| Missing | 0 | 0.0 | 0 | – | – |
| Admission source* | |||||
| Admitted from ER | 1,893 | 50.8 | 9,036 | 8,379 | 9,694 |
| Admitted from another hospital | 107 | 2.9 | 512 | 404 | 621 |
| Admitted from another facility | 20 | 0.5 | 97 | 54 | 140 |
| Admitted from routine/birth/other | 700 | 18.8 | 3,304 | 2,927 | 3,680 |
| Missing | 1,005 | 27.0 | 4,845 | – | – |
| Length of stay (days) | |||||
| 0–1 | 683 | 15.0 | 3,300 | 3,009 | 3,591 |
| 2–3 | 1,947 | 42.6 | 9,385 | 8,822 | 9,947 |
| 4–6 | 1,342 | 29.3 | 6,464 | 6,053 | 6,876 |
| 7+ | 600 | 13.1 | 2,880 | 2,631 | 3,129 |
| Missing | 0 | 0 | 0 | – | – |
| Mean (range) | 4.36 | 0 | 135 | 4.21 | 4.50 |
| Total | 4,572 | 100.0 | 22,029 | 21,001 | 23,057 |
ARDS = acute respiratory distress syndrome; CL = confidence level; ER = emergency room; NIS = Nationwide Inpatient Sample. Unweighted frequencies are the raw frequencies in the NIS, and weighted raw frequencies represent national estimates.
Admission source: 2000–2011 data only. Number of chronic conditions 2008–2014 data only.
Clinical characteristics.
The majority (70.0%) of malaria patients with known source of admission were admitted from the emergency room. There were an estimated 4,823 (95% CI = 4,485–5,161) hospitalizations that were classified as having severe malaria, accounting for 22.0% of all malaria-related hospitalizations. Some (21.7%) patients with severe malaria developed multiple complications. Malaria with renal failure (9.6%; 2,113 [95% CI = 1,903–2,324]) was the most common complication, followed by malaria with severe anemia (7.2%; 1,587 [95% CI = 1,409–1,766]), cerebral malaria (4.3%; 956 [95% CI = 819–1,093]), malaria with ARDS (4.1%; 900 [95% CI = 766–1,034]), malaria with jaundice (3.7%; 808 [95% CI = 679–937]), and malaria with an in-hospital death (0.8%; 182 [95% CI = 123–241]) (Table 3). The proportion and rate of malaria-related hospitalizations classified as severe increased over the period (P < 0.01).
Discharge records with a malaria-related diagnosis listed a mean of 5.5 (95% CI = 5.4–5.7) diagnoses, with a mean of 2.1 (95% CI = 2.1–2.2) chronic conditions.15 Most (83.1%) malaria-related discharge records listed malaria as the primary diagnosis. Among discharge records with malaria listed as the primary diagnosis, the most common codiagnoses were thrombocytopenia (31.5%), anemia (17.8%), hypokalemia (12.6%), and hypertension (12.5%). Among discharge records with malaria listed as a secondary diagnosis, the most common primary diagnoses were unspecified septicemia (13.9%), fever (4.6%), and other specified septicemia (3.9%). The most common procedures performed for malaria patients were blood transfusion (10.0%), other therapeutic procedures (6.9%), diagnostic spinal tap (5.2%), nonheart vascular catheterization (5.1%), and respiratory intubation and mechanical ventilation (3.3%). Malaria patients stayed an average of 4.36 (95% CI = 4.21–4.50) days.
Financial characteristics.
The mean hospital charge for malaria-related hospitalizations was $25,789 (95% CI = 24,478–27,101). Over half of patients resided in a zip code with median household incomes above the national median. Private insurance (45.1%) was the largest primary payer for malaria-related hospitalizations, followed by out-of-pocket payers (21.6%), Medicaid (19.3%), and Medicare (6.9%) (Table 4).
Table 4.
Financial characteristics of malaria-related hospitalizations in the United States, NIS, 2000–2014
| Unweighted frequency | Percent of malaria-related hospitalizations | Weighted frequency | Lower 95% CL weight frequency | Upper 95% CL weight frequency | |
|---|---|---|---|---|---|
| Hospital charges* | |||||
| Mean (range) | 25,789 | (194) | (1,021,738) | 24,478 | 27,101 |
| Missing | 104 | 0.0 | 492 | – | – |
| Income* | |||||
| High | 2,492 | 54.3 | 11,955 | 11,274 | 12,636 |
| Low | 1,806 | 39.7 | 8,742 | 8,108 | 9,375 |
| Missing | 274 | 6.1 | 1,333 | – | – |
| Primary payer | |||||
| Medicare | 317 | 6.9 | 1,516 | 1,338 | 1,694 |
| Medicaid | 871 | 19.3 | 4,260 | 3,828 | 4,692 |
| Private insurance | 2,072 | 45.1 | 9,936 | 9,392 | 10,481 |
| Self-pay | 989 | 21.6 | 4,762 | 4,320 | 5,203 |
| No charge | 93 | 2.0 | 451 | 320 | 581 |
| Other | 217 | 4.7 | 1,043 | 884 | 1,202 |
| Missing | 13 | 0.3 | 62 | – | – |
| Total | 4,572 | 100.0 | 22,029 | 21,001 | 23,057 |
CL = confidence level; NIS = Nationwide Inpatient Sample. Unweighted frequencies are the raw frequencies in the NIS, and weighted raw frequencies represent national estimates.
Adjusted for inflation to 2015 US dollars. Income category is based on median household income by national quartiles for patient zip code.
Institutional characteristics.
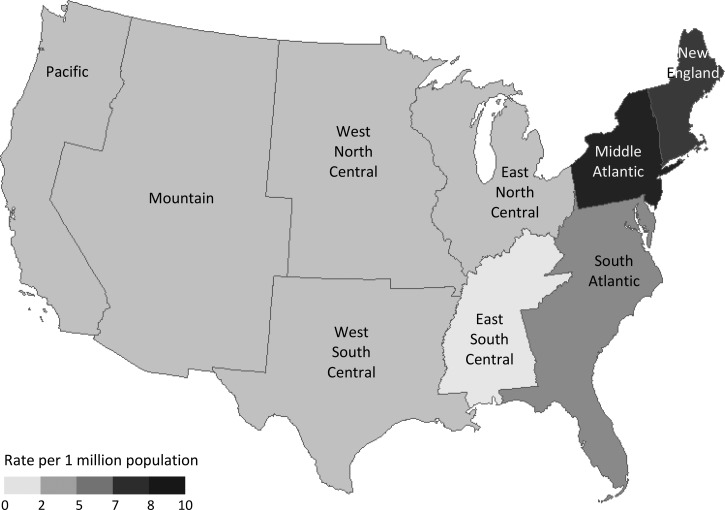
From 2000 to 2014, the southern (37.1%) and northeastern (34.2%) regions of the United States had the highest numbers of malaria-related hospitalizations, followed by the western (14.6%) and midwestern (14.2%) regions. The Middle Atlantic (27.2%; 9.82 per 1 million population [95% CI = 8.76–10.87]) and South Atlantic (24.9%; 6.33 per 1 million population [95% CI = 5.65–7.00]) census divisions accounted for over half of all malaria-related hospitalizations over the study period. The East South Central census division had the lowest rate of malaria-related hospitalizations (2.1%; 1.70 per 1 million population [95% CI = 1.24–2.16]) (Figure 3).
Figure 3.
Rate of malaria-related hospitalizations in the United States, by division, Nationwide Inpatient Sample, 2000–2014. All numbers are national estimates based on weighted frequencies.
Patients with a malaria diagnosis were more often hospitalized at urban teaching hospitals (67.7%), than urban nonteaching (28.7%) or rural (3.7%) hospitals; at private, not-for-profit hospitals (73.1%), than government, nonfederal (18.8%) and private, investor-owned (8.2%) hospitals; and at hospitals with large bed size (60.9%) than medium (28.4%) or small (10.7%) bed size.
DISCUSSION
Malaria hospitalizations routinely occur in the United States, and the associated burden from morbidity and mortality is substantial. From 2000 to 2014, hospitalizations for malaria far outnumbered that of other travel-associated diseases. Although we are unable to ascertain travel status, it is likely that most malaria hospitalizations represent travel-related cases, which are largely preventable.
This study indicates possible underreporting and underascertainment of malaria cases and deaths, which are expected in passive surveillance systems. In some years (2000, 2001, and 2010) during the study period, the number of malaria-related hospitalizations exceeded the total number of malaria cases reported by the malaria surveillance system,5 which includes both inpatient and outpatient cases. If it is assumed that hospital cases represented about 69% of all malaria cases, as was consistently reported in recent malaria surveillance reports,5,17,18 and the actual number of hospitalizations are as reported in this study, then the total number of malaria cases occurring in the United States may be closer to about 2,128 each year on average. From 2000 to 2013 (most recent surveillance data available), the total number of malaria-related in-hospital deaths in the NIS was slightly higher than the total number of malaria-related deaths in the national Multiple Cause of Death data from U.S. death certificates (N = 168),19 which was nearly double the number reported in the national malaria surveillance reports (N = 86).5,17,18,20–30 Further, only about 64% of death certificates listing malaria as a cause of death reported an inpatient medical facility as the place of death on the death certificate.19
A strong overall increasing trend in the number of reported malaria cases in the United States5 and other countries6 that eliminated malaria has been reported since the early 1970s, which is likely due to the increase in international travel to malaria-endemic regions.31 Although we did not find a statistically significant trend in malaria-related hospitalizations from 2000 to 2014, the overall numbers generally reflected the patterns reported in surveillance reports.11
The overrepresentation of malaria among men has been well documented. Compared with women, men may be less likely to seek pretravel advice, less likely to adhere to appropriate personal vector avoidance and chemoprophylaxis, suffer more mosquito bites, and exhibit other high-risk behaviors.32,33 The relatively large proportion of pregnant women among female malaria-related hospitalizations is concerning as malaria during pregnancy can be dangerous for the mother and fetus.3 The age distribution of malaria patients, which may reflect the age distribution of international travelers, is also consistent with previous reports.5 The race/ethnicity distribution of malaria in the United States has been infrequently reported. The overrepresentation of malaria among blacks may reflect the distribution of international travel among visitors, immigrants, or expatriates from endemic areas, including countries in the Caribbean and Africa.5 Some immigrants may be more likely to return to their countries of origin with endemic malaria to visit friends or relatives, and tend to have longer stays or stay at familial communities with higher risk lodging that increases their exposure to vectors.6,34 Importantly, these individuals may perceive themselves to be immune or at low risk, and may forego malaria prevention measures.34
The predominant infecting species for malaria-related hospitalizations with known species information was P. falciparum, consistent with its well-documented relative virulence and global distribution.5 Although all malaria species can cause severe malaria, infection by P. falciparum is more likely to result in severe disease because it lacks preference for particular red blood cell age classes,35 and is associated with sequestration and cytoadherence.36 Although P. falciparum is recognized for its ability to cause serious morbidity and mortality and is confirmed as the primary threat in this study, the disease burden caused by P. vivax is sizeable and should not be discounted.37,38 The reasons for not ascertaining the infecting species for nearly half of malaria-related hospitalizations are not known, as species information is important in determining the appropriate treatment regimen.39 Improved reporting or additional laboratory training on malaria microscopy may be needed to improve the rates or capacity of species identification in the United States.
The proportion of hospitalizations classified as having severe malaria was slightly higher than that reported from the surveillance system in recent years,11 which is expected since hospitalized cases are selected for severity and exclude milder outpatient cases. The relative proportions of specific complications that defined severe malaria observed were consistent with surveillance data. The number of in-hospital deaths observed underscores the importance of prevention and early diagnosis. Available treatment regimens for malaria are highly effective when properly and promptly administered, and symptoms can resolve within days.39
Large geographical disparities consistent with surveillance data were found along the east coast, which may be due to the popularity of the northeastern region states as destinations for immigrants from malaria-endemic countries, and thus an increased popularity of malaria-endemic countries as travel destinations for those visiting friends and relatives among residents of the northeastern region.40,41 Additional provider training for malaria awareness, diagnosis, and management may be warranted in these areas. Improved public health messages and adequate access to quality pretravel clinical care are needed to increase the use of pretravel consultations, chemoprophylaxis, and vector avoidance,32,42–44 particularly among those returning to countries of origin to visit friends or relatives.6,36 Improvements in secondary prevention by recognition, diagnosis, and treatment in primary care are also needed to avert the development of more severe conditions requiring hospitalization. Prompt diagnosis and treatment is crucial for good prognosis. Febrile illness with a history of international travel must always include malaria as a diagnostic consideration. Physician index of suspicion should be especially heightened during the summer and winter holiday seasons, which are popular travel times of the year to Africa.45
Some limitations of this study should be considered. The national estimates from the NIS are subject to coding and sampling errors, and the actual number of malaria-related hospitalizations may be greater or less than that reported here. Error in the estimation of the malaria-related hospitalization frequency and rate is possible due to misdiagnoses as malaria is a rare disease in the United States often presenting with nonspecific clinical manifestations. Underestimates are possible since malaria can be difficult to diagnose, often requiring a skilled microscopist.46 Overestimates may result from incorrect diagnoses based on clinical suspicion alone without laboratory confirmation or misinterpretation of inappropriate diagnostic tests (e.g., enzyme-linked immunosorbent assay antibody test). Underreporting of potentially relevant diagnoses and the high proportion of clinically imprecise ICD diagnosis codes, such as those corresponding to “general,” “other,” “unspecified,” or “mixed” conditions, or those corresponding to conditions that can have a wide clinical spectrum (e.g., renal failure), precludes accurate identification of all malaria-related hospitalizations, of relevant clinical manifestations for classifying severe malaria cases, and of the infecting species. Since this administrative data are discharge record based and not patient based, hospital transfers and recrudescent malaria cases that result in rehospitalizations may lead to multiple counting. Although the random and intentional (state-level suppression) missing data on race may not affect relative proportions, race-specific frequency and rate estimates are underestimated since only cases with known race were included in the calculations. Factors of interest not available in the NIS included laboratory results, specific diagnostic and treatment method, and other items on medical charts that could indicate possible coding errors. Travel history, place of birth and immigration status, destination and purpose of international travel, and chemoprophylaxis use were also not available in NIS data to assess as possible risk factors.
In conclusion, this study brings to light the sizeable burden of malaria hospitalizations in the United States. Despite the reduction of malaria incidence in developing countries, malaria continues to be an important public health problem in the United States despite its elimination in the early 1950s, and the disease burden remains substantial. Malaria hospitalizations and deaths are largely preventable through use of personal protective measures, adherence to correct chemoprophylactic regimens, and ensuring rapid and correct diagnosis and treatment. CDC provides free guidance on malaria risk and prophylaxis by region, and staff is available for guidance on therapy.
Acknowledgment:
We would like to thank Paul Arguin for reviewing this manuscript.
REFERENCES
- 1.World Health Organization , 2016. World Malaria Report 2016. Geneva, Switzerland: World Health Organization; Available at: http://apps.who.int/iris/bitstream/10665/252038/1/9789241511711-eng.pdf?ua=1. [Google Scholar]
- 2.Centers for Disease Control and Prevention , 2016. Malaria. Available at: http://www.cdc.gov/malaria/. Accessed April 15, 2015.
- 3.Guyatt HL, Snow RW, 2004. Impact of malaria during pregnancy on low birth weight in sub-Saharan Africa. Clin Microbiol Rev 17: 760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steketee RW, Campbell CC, 2010. Impact of national malaria control scale-up programmes in Africa: magnitude and attribution of effects. Malar J 9: 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cullen KA, Mace KE, Arguin PM, 2016. Malaria surveillance: United States, 2013. MMWR Surveill Summ 65: 1–22. [DOI] [PubMed] [Google Scholar]
- 6.Checkley AM, Smith A, Smith V, Blaze M, Bradley D, Chiodini PL, Whitty CJM, 2012. Risk factors for mortality from imported falciparum malaria in the United Kingdom over 20 years: an observational study. BMJ 344: e2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagmann SHF, Van PV, Stauffer WM, Miller AO, Connor BA, Hale DC, Coyle CM, Cahill JD, Marano C, Esposito DH, Kozarsky PE, 2014. Travel-associated disease among US residents visiting US GeoSentinel clinics after return from international travel. Fam Pract 31: 678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinka ME, Bangs MJ, Manguin S, Rubio-Palis Y, Chareonviriyaphap T, Coetzee M, Mbogo CM, Hemingway J, Patil AP, Temperley WH, Gething PW, Kabaria CW, Burkot TR, Harbach RE, Hay SI, 2012. A global map of dominant malaria vectors. Parasit Vectors 5: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zucker JR, 1996. Changing patterns of autochthonous malaria transmission in the United States: a review of recent outbreaks. Emerg Infect Dis 2: 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abanyie FA, Arguin PM, Gutman J, 2011. State of malaria diagnostic testing at clinical laboratories in the United States, 2010: a nationwide survey. Malar J 10: 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doyle TJ, Glynn MK, Groseclose SL, 2002. Completeness of notifiable infectious disease reporting in the United States: an analytical literature review. Am J Epidemiol 155: 866–874. [DOI] [PubMed] [Google Scholar]
- 12.Healthcare Cost and Utilization Project (HCUP) , 2013. Introduction to the HCUP Nationwide Inpatient Sample (NIS) 2011. Rockville, MD: Agency for Healthcare Research and Quality; Available at: http://www.hcup-us.ahrq.gov/db/nation/nis/NIS_Introduction_2011.jsp. Accessed March 26, 2016. [Google Scholar]
- 13.Healthcare Cost and Utilization Project (HCUP) , 2014. Introduction to the HCUP Nationwide Inpatient Sample (NIS) 2012. Rockville, MD: Agency for Healthcare Research and Quality; Available at: http://www.hcup-us.ahrq.gov/db/nation/nis/NIS_Introduction_2012.jsp. Accessed March 26, 2016. [Google Scholar]
- 14.Centers for Disease Control and Prevention , 2015. Classification of Diseases, Functioning, and Disability. Conversion Table. Available at: http://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed September 12, 2014.
- 15.Healthcare Cost and Utilization Project (HCUP) , 2015. Clinical Classifications Software (CCS) for ICD-9-CM and Chronic Condition Indicator. HCUP Tools and Software. Rockville, MD: Agency for Healthcare Research and Quality; Available at: https://www.hcup-us.ahrq.gov/tools_software.jsp. Accessed June 26, 2016. [PubMed] [Google Scholar]
- 16.U.S. Department of Health and Human Services (US DHHS), Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS) , 2016. Bridged-Race Population Estimates, United States July 1st Resident Population by State, County, Age, Sex, Bridged-Race, and Hispanic Origin. Available at: http://wonder.cdc.gov/bridged-race-v2014.html. Accessed April 15, 2016.
- 17.Cullen KA, Arguin PM, 2013. Malaria surveillance: United States, 2011. MMWR Surveill Summ 62: 1–17. [PubMed] [Google Scholar]
- 18.Cullen KA, Arguin PM, Centers for Disease Control an Prevention (CDC) , 2014. Malaria surveillance: United States, 2012. MMWR Surveill Summ 63: 1–22. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention, National Center for Health Statistics , 2016. Multiple Cause of Death 1999–2014. Available at: http://wonder.cdc.gov/mcd-icd10.html. Accessed on June 11, 2016.
- 20.Causer LM, Newman RD, Barber AM, Roberts JM, Stennies G, Bloland PB, Parise ME, Steketee RW, 2002. Malaria surveillance: United States, 2000. MMWR Surveill Summ 51: 9–21. [Google Scholar]
- 21.Filler S, Causer LM, Newman RD, Barber AM, Roberts JM, MacArthur J, Parise ME, Steketee RW, 2003. Malaria surveillance: United States, 2001. MMWR Surveill Summ 52: 1–14. [PubMed] [Google Scholar]
- 22.Shah S, Filler S, Causer LM, Rowe AK, Bloland PB, Barber AM, Roberts JM, Desai MR, Parise ME, Steketee RW, 2004. Malaria surveillance: United States, 2002. MMWR Surveill Summ 53: 21–34. [PubMed] [Google Scholar]
- 23.Eliades MJ, Shah S, Ngyuen-Dinh P, Newman RD, Barber AM, Roberts JM, Mail S, Parise ME, Steketee RW, 2005. Malaria surveillance: United States, 2003. MMWR Surveill Summ 54: 25–39. [PubMed] [Google Scholar]
- 24.Skarbinski J, James EM, Causer LM, Barber AM, Mali S, Nguyen-Dinh P, Roberts JM, Parise ME, Slutsker L, Newman RD, 2006. Malaria surveillance: United States, 2004. MMWR Surveill Summ 55: 23–37. [PubMed] [Google Scholar]
- 25.Thwing J, Skarbinski J, Newman RD, Barber AM, Mali S, Roberts JM, Slutsker L, Arguin PM, 2007. Malaria surveillance: United States, 2005. MMWR Surveill Summ 56: 23–38. [PubMed] [Google Scholar]
- 26.Mali S, Steele S, Slutsker L, Arguin PM, 2008. Malaria surveillance: United States, 2006. MMWR Surveill Summ 57: 24–39. [PubMed] [Google Scholar]
- 27.Mali S, Steele S, Slutsker L, Arguin PM, 2009. Malaria surveillance: United States, 2007. MMWR Surveill Summ 58: 1–16. [PubMed] [Google Scholar]
- 28.Mali S, Steele S, Slutsker L, Arguin PM, 2010. Malaria surveillance: United States, 2008. MMWR Surveill Summ 59: 1–15. [PubMed] [Google Scholar]
- 29.Mail S, Tan KR, Arguin PM, 2011. Malaria surveillance: United States, 2009. MMWR Surveill Summ 60: 1–15. [PubMed] [Google Scholar]
- 30.Mali S, Kachur SP, Arguin PM, 2012. Malaria surveillance: United States, 2009. MMWR Surveill Summ 61: 1–17. [PubMed] [Google Scholar]
- 31.World Tourism Organization , 2014. UNWTO Tourism Highlights, 2014 edition Madrid, Spain: World Tourism Organization; Available at: http://mkt.unwto.org/publication/unwto-tourism-highlights-2014-edition. Accessed March 25, 2015. [Google Scholar]
- 32.Schlagenhauf P, Weld L, Goorhuis A, Gautret P, Weber R, von Sonnenburg F, Lopez-Velez R, Jensenius M, Cramer JP, Field VK, Odolini S, Gkrania-Klotsas E, Chappuis F, Malvy D, van Genderen PJJ, Mockenhaupt F, Jaureguiberry S, Smith C, Beeching NJ, Ursing J, Rapp C, Parola P, Grobusch MP, 2015. Travel-associated infection presenting in Europe (2008–2012): an analysis of EuroTravNet longitudinal, surveillance data, and evaluation of the effect of the pre-travel consultation. Lancet 15: 55–64. [DOI] [PubMed] [Google Scholar]
- 33.Schlagenhauf P, Chen LH, Wilson ME, Freedman DO, Tcheng D, Schwartz E, Pandey P, Weber R, Nadal D, Berger C, von Sonnenburg F, Keystone J, Leder K, 2010. Sex and gender differences in travel-associated disease. Clin Infect Dis 50: 826–832. [DOI] [PubMed] [Google Scholar]
- 34.Leder K, Torresi J, Libman MD, Cramer JP, Castelli F, Schlagenhauf P, Wilder-Smith A, Wilson ME, Keystone JS, Schwartz E, Barnett ED, von Sonnenburg F, Brownstein JS, Cheng AC, Sotir MJ, Esposito DH, Freedman DO, 2013. GeoSentinel surveillance of illness in returned travelers, 2007–2011. Ann Intern Med 158: 456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerlin DH, Gatton ML, 2013. Preferential invasion by Plasmodium merozoites and the self-regulation of parasite burden. PLoS One 8: e57434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM, 2014. Malaria. Lancet 383: 723–735. [DOI] [PubMed] [Google Scholar]
- 37.Hwang J, Cullen KA, Kachur SP, Arguin PM, Baird JK, 2014. Severe morbidity and mortality risk from malaria in the United States, 1985–2011. Open Forum Infect Dis 1: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendis K, Sina BJ, Marchesini P, Carter R, 2001. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg 64 (Suppl): 97–106. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention , 2013. Treatment of Malaria. Available at: http://www.cdc.gov/malaria/resources/pdf/clinicalguidance.pdf. Accessed March 26, 2016.
- 40.International Trade Administration , 2013. National Travel and Tourism Office. US Department of Commerce. US Travel and Tourism Statistics (Inbound). Region of origin profiles, p. 6. Africa (Lond) 6 Available at: http://tinet.ita.doc.gov/outreachpages/download_data_table/2012_Africa_Market_Profile.pdf. Accessed June 28, 2016. [Google Scholar]
- 41.International Trade Administration , 2013. National Travel and Tourism Office. US Department of Commerce. US Travel and Tourism Statistics (US Resident Outbound). US to overseas for leisure/visit friends and relatives (VFR). Page 7 Available at: http://tinet.ita.doc.gov/outreachpages/download_data_table/2012-US-Leisure-VFR.pdf. Accessed June 28, 2016.
- 42.Hamer DH, Connor BA, 2004. Travel health knowledge, attitudes and practices among United States travelers. J Travel Med 11: 23–26. [DOI] [PubMed] [Google Scholar]
- 43.LaRocque RC, Rao SR, Tsibris A, Lawton T, Barry MA, Marano N, Brunette G, Yanni E, Ryan ET, 2010. Pre-travel health advice-seeking behavior among US international travelers departing from Boston Logan International Airport. J Travel Med 17: 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention , 2015. Malaria. Yellow Book 2015. Available at: http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/malaria. Accessed April 15, 2016.
- 45.International Trade Administration , 2016. National Travel and Tourism Office. US Department of Commerce. Monthly arrivals to the United States, 1996–2015. Available at: http://travel.trade.gov/research/monthly/arrivals/. Accessed June 28, 2016.
- 46.Ash LR, Orihel TC, 2007. Atlas of Human Parasitology, 5th edition Chicago, IL: ASCP Press. [Google Scholar]