Abstract.
Rift Valley fever virus (RVFV) causes severe disease in both animals and humans, resulting in significant economic and public health damages. The objective of this study was to measure RVFV seroprevalence in six coastal Kenyan villages between 2009 and 2011, and characterize individual-, household-, and community-level risk factors for prior RVFV exposure. Sera were tested for anti-RVFV IgG via enzyme-linked immunosorbent assay. Overall, 51 (1.8%; confidence interval [CI95] 1.3–2.3) of 2,871 samples were seropositive for RVFV. Seroprevalence differed significantly among villages, and was highest in Jego Village (18/300; 6.0%; CI95 3.6–9.3) and lowest in Magodzoni (0/248). Adults were more likely to be seropositive than children (P < 0.001). Seropositive subjects were less likely to own land or a motor vehicle (P < 0.01), suggesting exposure is associated with lower socioeconomic standing (P = 0.03). RVFV exposure appears to be low in coastal Kenya, although with some variability among villages.
BACKGROUND
Rift Valley fever virus (RVFV) is a zoonotic phlebovirus that can be transmitted to livestock and humans by a number of mosquito species, including Aedes ochraceus, Aedes mcintoshi, Culex tritaeniorhynchus, and Aedes vexans, or by direct contact with, or aerosols from, contaminated fluids and tissues.1–7 A majority of infected humans experience mild disease, with roughly 1% of cases suffering severe symptoms such as hemorrhagic fever and encephalitis.2,4,5,8 RVFV is highly pathogenic in domestic livestock, specifically goats, sheep, and cattle.6,9 Outbreaks have had detrimental impacts on livestock trade, and meat and dairy industries, as infection can cause a catastrophic decline in animal breeding and productivity.6
It is difficult to determine the true public health impact of RVFV and principle risk factors associated with exposure and disease, as human cases are not reliably reported. The weight of many factors, specifically as biological sex and gender dynamics, differ by study scope and regional focus.10 Similarly, community knowledge and perception of described risks, methods of transmission, and symptoms and severity of disease vary by study region, populations surveyed, and access to health interventions and public health efforts to minimize disease.11–13 The objective of this study was to measure RVFV prevalence in six coastal villages in Kenya. Variability in prevalence between these villages was used to identify risk factors associated with RVFV exposure by linking seropositivity to demographic data such as socioeconomic standing (SES), occupation, and clinical history. Household mosquito abundance was also measured to test for correlation between mosquito exposure, in and near the home, and seropositivity for RVFV.
MATERIALS AND METHODS
This study was part of a larger project on polyparasitism in communities on the southern coast of Kenya.14,15 Study participants were recruited in 2009–2011 from the rural villages of Jego, Kinango, Magodzoni, Milalani, Nganja, and Vuga located in the southeastern corner of Kenya, in Kwale County (Figure 1).14 Jego, the southern-most village, is located on the border with Tanzania. Milalani, Nganja, Magadzoni, Vuga, and Jego are very close to the Indian Ocean, whereas Kinango is situated inland by 50 km. Kinango, located in a semiarid inland area, has a much drier climate compared with villages such as Milalani, Nganja, and Magadzoni located in the coastal plains region.15,16
Figure 1.
Map of study area; inset: map of Kenya showing study sites within Kwale County.
Biobanked sera collected from 2,871 study participants during the 2009–2011 recruitment phase were tested for anti-RVFV IgG by standardized indirect enzyme-linked immunosorbent assay (ELISA) protocols as previously described.2–5 Institutional Review Board (IRB) approval was obtained for this project from Children’s Hospital of Oakland Research Institute (IRB No. 2013-023), University Hospitals Case Medical Center of Cleveland (IRB No. 11-07-42), and Kenya Medical Research Institute (IRB SSC No. 087) before testing biobanked samples. All consenting long-term residents of designated study villages in Kwale District, Coast Province, Kenya, ≥ 5 years of age, were selected for inclusion. Those who refused consent or assent, and/or were not a long-term resident of the study area were excluded from participation.
Demographic, household inventory, and environmental exposure questionnaires were administered to all participants at the time of enrollment. Questions referred to SES, occupation, basic clinical history, livestock exposures, and exposure to mosquitoes. An SES index was established with the use of Principal Component Analysis of demographic and household data relating to land ownership, homestead construction (i.e., materials used for roof and floor), light sources available, mobility (i.e., ownership of a bicycle or motor vehicle), drinking water sources, and the type of primary latrine and its proximity to their homestead (Table 1).17
Table 1.
Risk factor analysis of demographic and lifestyle factors
| Factor | Level | Nganja |
Milalani |
Vuga |
Jego | Kinango | Magodzoni |
Total |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | P value | Negative | Positive | P value | Negative | Positive | P value | Negative | Positive | P value | Negative | Positive | P value | Negative | Positive | Negative | Positive | P value | Total | Test | ||
| N | 397 (13.8%) | 8 (0.3%) | 541 (18.8%) | 12 (0.4%) | 842 (29.3%) | 8 (0.3%) | 282 (9.8%) | 16 (0.6%) | 516 (18%) | 5 (0.2%) | 247 (8.6%) | 2,825 (98.3%) | 49 (1.7%) | 2,874 (100%) | |||||||||
| Age, median (IQR) | 20 (11, 36) | 49.5 (37.5, 53) | 0.003* | 17 (11, 35) | 44.5 (31.5, 75) | < 0.001* | 17 (12, 36) | 37.5 (21.5, 51) | 0.021 | 20 (14, 34) | 44 (29.5, 61.5) | < 0.001* | 14 (10, 28) | 63 (53, 66) | 0.002* | 14 (9, 30) | 17 (11, 34) | 48 (30, 61) | < 0.001* | 16 (8, 33) | Wilcoxon rank-sum | ||
| Sex | Female | 213 (12.9%) | 5 (0.3%) | 0.73 | 317 (19.2%) | 7 (0.4%) | 1.00 | 473 (28.6%) | 7 (0.4%) | 0.15 | 149 (9%) | 8 (05%) | 1.00 | 330 (19.9%) | 3 (0.2%) | 1.00 | 143 (8.6%) | 1,625 (98.2%) | 30 (1.8%) | 0.77 | 1,655 (100%) | Fisher’s exact | |
| Male | 183 (15.3%) | 3 (0.3%) | 224 (18.8%) | 5 (0.4%) | 356 (29.8%) | 1 (0.1%) | 133 (11.1%) | 8 (0.7%) | 182 (15.3%) | 2 (0.2%) | 96 (8%) | 1,174 (98.4%) | 19 (1.6%) | 1,193 (100%) | |||||||||
| Tribe | Digo | 354 (17.2%) | 8 (0.4%) | 1.00 | 513 (25%) | 12 (0.6%) | 1.00 | 738 (35.9%) | 6 (0.3%) | 0.34 | 149 (7.3%) | 4 (0.2%) | 0.063 | 51 (2.5%) | 0 (0.0%) | 1.00 | 219 (10.7%) | 2,024 (98.5%) | 30 (1.5%) | 0.28 | 2,054 (100%) | Fisher’s exact | |
| Diriama | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (100%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (100%) | 0 (0.0%) | 1 (100%) | |||||||||
| Duruma | 20 (3.3%) | 0 (0.0%) | 14 (2.3%) | 0 (0.0%) | 66 (10.9%) | 2 (0.3%) | 96 (15.9%) | 8 (1.3%) | 380 (62.8%) | 5 (0.8%) | 14 (2.3%) | 590 (97.5%) | 15 (2.5%) | 605 (100%) | |||||||||
| Kamba | 7 (12.1%) | 0 (0.0%) | 3 (5.2%) | 0 (0.0%) | 7 (12.1%) | 0 (0.0%) | 12 (20.7%) | 2 (3.4%) | 25 (43.1%) | 0 (0.0%) | 2 (3.4%) | 56 (96.5%) | 2 (3.5%) | 58 (100%) | |||||||||
| Swahili | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 7 (58.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 5 (41.7%) | 0 (0.0%) | 0 (0.0%) | 12 (100%) | 0 (0.0%) | 12 (100%) | |||||||||
| Other | 15 (12.6%) | 0 (0.0%) | 11 (9.2%) | 0 (0.0%) | 13 (10.9%) | 0 (0.0%) | 25 (21%) | 2 (1.7%) | 51 (42.9%) | 0 (0.0%) | 2 (1.7%) | 117 (98.3%) | 2 (1.7%) | 119 (100%) | |||||||||
| Socioeconomic status level | 0–25% | 81 (11.6%) | 3 (0.4%) | 0.64 | 235 (33.7%) | 8 (1.1%) | 0.58 | 138 (19.8%) | 1 (0.1%) | 1.00 | 94 (13.5%) | 6 (0.9%) | 0.62 | 59 (8.5%) | 2 (0.3%) | 0.15 | 71 (10.2%) | 678 (97.1%) | 20 (2.9%) | 0.030 | 698 (100%) | Fisher’s exact | |
| 25–50% | 109 (13.4%) | 2 (0.2%) | 143 (17.6%) | 2 (0.2%) | 234 (28.7%) | 2 (0.2%) | 101 (12.4%) | 7 (0.9%) | 142 (17.4%) | 2 (0.2%) | 70 (8.6%) | 799 (98.2%) | 15 (1.8%) | 814 (100%) | |||||||||
| 50–75% | 53 (9.7%) | 0 (0.0%) | 63 (11.5%) | 1 (0.2%) | 218 (39.8%) | 2 (0.4%) | 54 (9.9%) | 3 (0.5%) | 97 (17.7%) | 0 (0.0%) | 57 (10.4%) | 542 (98.9%) | 6 (1.1%) | 548 (100%) | |||||||||
| 75–100% | 154 (18.9%) | 3 (0.4%) | 100 (12.3%) | 1 (0.1%) | 252 (31%) | 3 (0.4%) | 33 (4.1%) | 0 (0.0%) | 218 (26.8%) | 1 (0.1%) | 49 (6%) | 806 (99.0%) | 8 (0.98%) | 814 (100%) | |||||||||
| Highest level of education completed | None | 34 (16.1%) | 2 (0.9%) | 1.00 | 37 (17.5%) | 4 (1.9%) | 0.31 | 54 (25.6%) | 2 (0.9%) | 0.86 | 24 (11.4%) | 4 (1.9%) | 0.55 | 39 (18.5%) | 1 (0.5%) | 1.00 | 10 (4.7%) | 198 (93.8%) | 13 (6.2%) | 0.28 | 211 (100%) | Fisher’s exact | |
| Primary | 51 (16.5%) | 3 (1%) | 64 (20.6%) | 2 (0.6%) | 95 (30.6%) | 2 (0.6%) | 28 (9%) | 2 (0.6%) | 42 (13.5%) | 2 (0.6%) | 19 (6.1%) | 299 (96.4%) | 11 (3.5%) | 310 (100%) | |||||||||
| Secondary | 12 (15.4%) | 1 (1.3%) | 18 (23.1%) | 1 (1.3%) | 20 (25.6%) | 0 (0.0%) | 4 (5.1%) | 1 (1.3%) | 19 (24.4%) | 0 (0.0%) | 2 (2.6%) | 75 (96.1%) | 3 (3.8%) | 78 (100%) | |||||||||
| University/adult education | 9 (11.3%) | 0 (0.0%) | 24 (30%) | 0 (0.0%) | 24 (30%) | 0 (0.0%) | 9 (11.3%) | 1 (1.3%) | 7 (8.8%) | 0 (0.0%) | 6 (7.5%) | 79 (98.8%) | 1 (1.3%) | 80 (100%) | |||||||||
| Mosquito avoidance behavior | None | 27 (9.7%) | 1 (0.4%) | 0.71 | 48 (17.3%) | 2 (0.7%) | 0.40 | 107 (38.6%) | 0 (0.0%) | 0.91 | 28 (10.1%) | 1 (0.4%) | 0.58 | 43 (15.5%) | 1 (0.4%) | 0.35 | 19 (6.9%) | 272 (98.2%) | 5 (1.8%) | 0.38 | 277 (100%) | Fisher’s exact | |
| 0–33% | 202 (14%) | 4 (0.3%) | 328 (22.7%) | 9 (0.6%) | 383 (26.5%) | 4 (0.3%) | 151 (10.4%) | 11 (0.8%) | 220 (15.2%) | 1 (0.1%) | 133 (9.2%) | 1,417 (97.9%) | 29 (2.0%) | 1,446 (100%) | |||||||||
| 33–66% | 128 (15.3%) | 3 (0.4%) | 117 (14%) | 1 (0.1%) | 242 (29%) | 3 (0.4%) | 72 (8.6%) | 4 (0.5%) | 188 (22.5%) | 2 (0.2%) | 74 (8.9%) | 821 (98.4%) | 13 (1.6%) | 834 (100%) | |||||||||
| 66–100% | 40 (12.6%) | 0 (0.0%) | 48 (15.1%) | 0 (0.0%) | 110 (34.7%) | 1 (0.3%) | 31 (9.8%) | 0 (0.0%) | 65 (20.5%) | 1 (0.3%) | 21 (6.6%) | 315 (99.4%) | 2 (0.63) | 317 (100%) | |||||||||
| Yellow fever vaccine | No | 90 (16.5%) | 1 (0.2%) | 1.00 | 51 (9.3%) | 0 (0.0%) | 1.00 | 121 (22.2%) | 0 (0.0%) | 1.00 | 42 (7.7%) | 0 (0.0%) | 1.00 | 136 (24.9%) | 1 (0.2%) | 1.00 | 104 (19%) | 544 (99.6%) | 2 (0.4%) | 1.00 | 546 (100%) | Fisher’s exact | |
| Yes | 37 (39.4%) | 0 (0.0%) | 8 (8.5%) | 0 (0.0%) | 5 (5.3%) | 0 (0.0%) | 7 (7.4%) | 0 (0.0%) | 35 (37.2%) | 0 (0.0%) | 2 (2.1%) | 94 (100%) | 0 (0.0%) | 94 (100%) | |||||||||
| No data | 270 (9.4%) | 7 (0.25%) | 482 (16.8%) | 12 (0.42%) | 716 (24.9%) | 8 (0.28%) | 233 (8.1%) | 16 (0.56%) | 345 (12%) | 4 (0.14%) | 247 (8.74%) | 2,825 (98.3%) | 47 (1.7%) | 2,874 (100%) | |||||||||
Indicates a statistically significant finding, based on the P-value (reported).
Resting mosquitoes were captured inside study households in the early morning using the Pyrethrum Spray Catch (PSC) method, and outside using clay pot traps and Prokopack aspirators.10 PSC collections were performed monthly in 10 randomly selected households from April 2009 to 2013.14,16 Approximately 95% of the collected mosquitoes were culicine (Culex spp.).14 Culicine density per household was tested for association with household RVFV seroprevalence.
The relationship of each potential predictor with odds of RVFV seropositivity was assessed in bivariate analysis with the use of χ2 test.
RESULTS
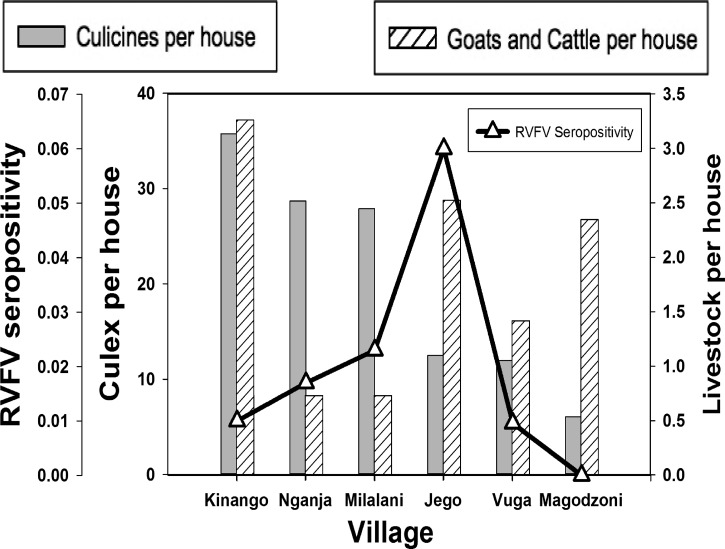
Of the 2,871 serum samples tested, 51 (1.8%; confidence interval [CI95] 1.3–2.3) were RVFV seropositive (Table 1). Distribution of seropositives was significantly different among the six villages (P < 0.001). Jego had the highest seroprevalence (18/300; 6.0%; CI95 3.6–9.3), whereas Magodzoni had the lowest (0/248). The four other villages also had low seroprevalence, with only 1.0% of residents in Vuga (8/835; CI95 0.4–1.9), 1.0% in Kinango (5/524; CI95 0.3–2.2), 1.7% in Nganja (7/404; CI95 0.1–3.5), and 2.3% in Milalani (13/560; CI95 1.2–3.9) having positive tests (Table 1 and Figure 2). Adults were more likely to be seropositive than children (1.7% and 0.1%, respectively; P < 0.001) (Table 1). Participants who tested seropositive for anti-RVFV antibodies ranged from 13 to > 90 old. No statistically significant difference in seropositivity was noted between genders.
Figure 2.
Culicine and livestock densities per house and Rift Valley fever virus (RVFV) seropositivity per village.
Questionnaire data identified few statistically significant correlations between subject lifestyle, behaviors, and health history, and RVFV exposure. Seropositive subjects were less likely to own land or a motor vehicle (P < 0.01). Seropositivity was not associated with documented livestock exposure at the household (Figure 2).
DISCUSSION
Our data suggest that RVFV exposure is not common in the extreme southern part of rural coastal Kenya. Studies conducted in rural northeastern Kenya in 2006 reported seroprevalence rates of > 13% in two areas of the semi-arid Ijara District,2 located north of our present study region (coastal Kwale County).2–4,18 A study by Mohamed and others described a 2007 outbreak of RVFV in Tanzania, located south of our study region, with 511 suspected cases, 36.4% of which were laboratory confirmed.19 Previous reports from Kwale County indicate that there have been at least 21 RVFV outbreaks in that location since the first recorded case in 1961.20
Historical outbreak data compared with the average age of seropositive subjects may elucidate the frequency of outbreaks in Kwale County villages. Median age of seropositive subjects was significantly higher than those who tested seronegative in Nganja (P = 0.003), Milalani (P < 0.001), Jego (P < 0.001), and Kinango (P = 0.002), and in the total study cohort overall (P < 0.001). Across the villages surveyed in our study, adults were 1.7 times more likely to be seropositive than children. The higher likelihood for seropositivity in adults may due to exposure earlier in their lives, such as through an outbreak that occurred before many of the children of our study population were born, or, alternatively, through age-specific occupational exposures.8,13 Our data suggest that cumulative exposure has been relatively low, despite the number of previous outbreaks in Kwale County, and nationally over time.
Low rates of RVFV exposure on the coast may be attributed to diversity in the types of jobs available in those regions. Human seroprevalence was not significantly correlated with household livestock in our study. In contrast, a study in the northeastern province by Munyua and others indicates that livestock infections typically multiply before human exposure.9 Most studies analyzing the zoonotic nature of RVFV have shown a significant link between exposure to animals and seropositivity for RVFV.8 Although number of animals kept was not significantly associated with RVFV exposure, occupational exposures that may contribute more significantly to RVFV exposure (e.g., herding, butchering) may be more influential than keeping animals at home. A 2010 study of a population of nomadic herders by Aagaard-Hansen and others confirms a higher risk of disease exposure in individuals with occupational handling of animals.21 Additionally, studies by Ng’ang’a and others (2016) and Owange and others (2014) suggest that these risks may also be due to specific perceptions of risk and overall disease impact in these populations.12,13
Gender was not a significant factor for exposure in our study, which may be a limitation of our sample size. Many studies that indicate gender as a risk factor for RVFV exposure have shown that males are more likely to be seropositive for RVFV than females, with differences in prevalence ranging between 5% and 14% between genders.5,18 This may suggest differential exposure to RVFV through labor distribution and gender roles, with the expectation of significant regional and cultural variation. In contrast, other studies do not report gender as a risk factor for RVFV exposure.5,10,22
The World Health Organization (WHO) has declared that RVFV is a significant emerging infectious disease that impacts primarily poor and marginalized populations.23 Although there are many individual- and household-level factors that are associated with lower SES and poverty that may create a higher likelihood of exposure to mosquito-borne diseases, many of the traditional risk factors identified in previous studies of RVFV exposure were not significant risk factors for our populations. SES data in this study indicated that subjects of lower standing were more likely to be seropositive, yet occupation could not be correlated with seropositivity.
Human seroprevalence was not significantly correlated to household Culex density in our study. Culex density near the homestead may not be representative of overall mosquito exposure during daytime. Overall, households in Kinango, the most inland village surveyed, had the highest density of Culex in the homestead, but some of the lowest rates of seropositivity. Further research is required to determine whether this may suggest exposure to RVFV can be attributed to other modes of transmission or by other mosquito species.
Our data indicate a significant variation in exposure at the village level, with the highest seroprevalence found in Jego. The higher seroprevalence in Jego may be related to periodic flooding of the area, which is predominantly low-lying estuary. Jego is also adjacent to a herding community that had exceptionally high livestock and cattle numbers. Historically, RVFV outbreaks have occurred during years with significant and extensive rainfall, which creates new mosquito habitats through flooding, thus increasing mosquito populations.6,24 The importance of heavy rainfall and flooding for mosquito-borne diseases has also been identified at the community level. Owange and others report that high rainfall and the creation of dambos from flooding are perceived by community members as one of the most important risk factors of the RVFV disease pathway.13
There were a number of limitations to our study. This cross-sectional study of selected villages does not represent extensive variations in climate other than rainfall. Data collected using questionnaires, addressing health history, environment, and lifestyle factors, were self-reported and subject to reporting bias. Mosquito analysis only included the most abundant Culex spp., and did not include testing to detect RVFV in these vectors. Analysis of livestock included only goats and cattle, as other species were too rare for meaningful analysis.
In conclusion, the results presented suggest that RVFV is transmitting at low levels on the coast of Kenya, with exposure varying by village. Despite the lack of a significant correlation between culicine density, household livestock, and RVFV prevalence, our data did illustrate the impacts of age and SES on exposure to RVFV. Additionally, there are public health implications highlighted by our findings in this region, specifically environmental and occupational risks that may be higher for adults or those of lower SES. These findings point to the need for more extensive local and regional studies to further elucidate the influence of mosquito exposure, occupational exposure, and livestock trade on RVFV transmission.
Acknowledgments:
We sincerely thank the study participants and the Kenyan field team. We also thank the Pacific North West Regional Center of Excellence for funding this project.
REFERENCES
- 1.Arum SO, Weldon CW, Orindi B, Landmann T, Tchouassi DP, Affognon HD, Sang R, 2015. Distribution and diversity of the vectors of Rift Valley fever along the livestock movement routes in the northeastern and coastal regions of Kenya. Parasit Vectors 8: 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LaBeaud AD, Muchiri EM, Ndzovu M, Mwanje MT, Muiruri S, Peters CJ, King CH, 2008. Interepidemic Rift Valley fever virus seropositivity, northeastern Kenya. Emerg Infect Dis 14: 1240–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LaBeaud AD, Muiruri S, Sutherland LJ, Dahir S, Gildengorin G, Morrill J, Muchiri EM, Peters CJ, King CH, 2011. Postepidemic analysis of Rift Valley fever virus transmission in northeastern Kenya: a village cohort study. PLoS Negl Trop Dis 5: e1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LaBeaud AD, Ochiai Y, Peters CJ, Muchiri EM, King CH, 2007. Spectrum of Rift Valley fever virus transmission in Kenya: insights from three distinct regions. Am J Trop Med Hyg 76: 795–800. [PMC free article] [PubMed] [Google Scholar]
- 5.LaBeaud AD, Pfeil S, Muiruri S, Dahir S, Sutherland LJ, Traylor Z, Gildengorin G, Muchiri EM, Morrill J, Peters CJ, Hise AG, Kazura JW, King CH, 2015. Factors associated with severe human Rift Valley fever in Sangailu, Garissa County, Kenya. PLoS Negl Trop Dis 9: e0003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linthicum KJ, Britch SC, Anyamba A, 2016. Rift Valley fever: an emerging mosquito-borne disease. Annu Rev Entomol 61: 395–415. [DOI] [PubMed] [Google Scholar]
- 7.Tchouassi DP, Bastos AD, Sole CL, Diallo M, Lutomiah J, Mutisya J, Mulwa F, Borgemeister C, Sang R, Torto B, 2014. Population genetics of two key mosquito vectors of Rift Valley fever virus reveals new insights into the changing disease outbreak patterns in Kenya. PLoS Negl Trop Dis 8: e3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahlon SS, Peters CJ, Leduc J, Muchiri EM, Muiruri S, Njenga MK, Breiman RF, White AC, Jr, King CH, 2010. Severe Rift Valley fever may present with a characteristic clinical syndrome. Am J Trop Med Hyg 82: 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munyua P, Murithi RM, Wainwright S, Githinji J, Hightower A, Mutonga D, Macharia J, Ithondeka PM, Musaa J, Breiman RF, Bloland P, Njenga MK, 2010. Rift Valley fever outbreak in livestock in Kenya, 2006–2007. Am J Trop Med Hyg 83: 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muga GO, Onyango-Ouma W, Sang R, Affognon H, 2015. Sociocultural and economic dimensions of Rift Valley fever. Am J Trop Med Hyg 92: 730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jost CCNS, Kihu S, Bett B, Njogu G, Swai ES, Mariner JC, 2010. Epidemiological assessment of the Rift Valley fever outbreak in Kenya and Tanzania in 2006 and 2007. Am J Trop Med Hyg 83: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng’ang’a CMBS, Bett BK, 2016. Lay perceptions of risk factors for Rift Valley fever in a pastoral community in northeastern Kenya. BMC Public Health 16: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owange NOOW, Kasiiti J, Gathura PB, Okuthe S, Sang R, Affognon H, Onyango-Ouma W, Landmann TT, Mbabu M, 2014. Perceived risk factors and risk pathways of Rift Valley fever in cattle in Ijaea district, Kenya. Onderstepoort J Vet Res 81. [DOI] [PubMed] [Google Scholar]
- 14.Bisanzio D, Mutuku F, Bustinduy AL, Mungai PL, Muchiri EM, King CH, Kitron U, 2014. Cross-sectional study of the burden of vector-borne and soil-transmitted polyparasitism in rural communities of Coast Province, Kenya. PLoS Negl Trop Dis 8: e2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mbogo CM, Mwangangi JM, Nzovu J, Gu W, Yan G, Gunter JT, Swalm C, Keating J, Regens JL, Shililu JI, Githure JI, Beier JC, 2003. Spatial and temporal heterogeneity of Anopheles mosquitoes and Plasmodium falciparum transmission along the Kenyan coast. Am J Trop Med Hyg 68: 734–742. [PubMed] [Google Scholar]
- 16.Mutuku FM, King CH, Mungai P, Mbogo C, Mwangangi J, Muchiri EM, Walker ED, Kitron U, 2011. Impact of insecticide-treated bed nets on malaria transmission indices on the south coast of Kenya. Malar J 10: 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gwatkin DRRS, Johnson K, Suliman E, Wagstaff A, Amouzou A, 2007. Socio-economic differences in health, nutrition, and population: Kenya 1993, 1998, 2003. Country reports on HNP and poverty. Washington, DC. World Bank Group 1: 1–150. [Google Scholar]
- 18.Muiruri S, Kabiru EW, Muchiri EM, Hussein H, Kagondu F, LaBeaud AD, King CH, 2015. Cross-sectional survey of Rift Valley fever virus exposure in Bodhei village located in a transitional coastal forest habitat in Lamu county, Kenya. Am J Trop Med Hyg 92: 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohamed M, Mosha F, Mghamba J, Zaki SR, Shieh WJ, Paweska J, Omulo S, Gikundi S, Mmbuji P, Bloland P, Zeidner N, Kalinga R, Breiman RF, Njenga MK, 2010. Epidemiologic and clinical aspects of a Rift Valley fever outbreak in humans in Tanzania, 2007. Am J Trop Med Hyg 83: 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murithi RM, Munyua P, Ithondeka PM, Macharia JM, Hightower A, Luman ET, Breiman RF, Njenga MK, 2011. Rift Valley fever in Kenya: history of epizootics and identification of vulnerable districts. Epidemiol Infect 139: 372–380. [DOI] [PubMed] [Google Scholar]
- 21.Aagaard-Hansen JNN, Alvar J, 2010. Population movement: a key factor in the epidemiology of neglected tropical diseases. Trop Med Int Health 15: 1281–1288. [DOI] [PubMed] [Google Scholar]
- 22.Gray GC, Anderson BD, LaBeaud AD, Heraud JM, Fevre EM, Andriamandimby SF, Cook EA, Dahir S, de Glanville WA, Heil GL, Khan SU, Muiruri S, Olive MM, Thomas LF, Merrill HR, Merrill ML, Richt JA, 2015. Seroepidemiological study of interepidemic Rift Valley fever virus Infection among persons with intense ruminant exposure in Madagascar and Kenya. Am J Trop Med Hyg 93: 1364–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO, 1998. Proceedings of the International Conference on Emerging Infectious Diseases. Atlanta, Georgia, USA March 8–11, 1998. Emerg Infect Dis 4: 353–505. [PMC free article] [PubMed] [Google Scholar]
- 24.Anyamba A, Chretien JP, Small J, Tucker CJ, Formenty PB, Richardson JH, Britch SC, Schnabel DC, Erickson RL, Linthicum KJ, 2009. Prediction of a Rift Valley fever outbreak. Proc Natl Acad Sci USA 106: 955–959. [DOI] [PMC free article] [PubMed] [Google Scholar]