Abstract
Avian reovirus (ARV) is an economically significant pathogen of broiler chickens. Our objective was to determine the prevalence, geographical distribution, and seasonal variation of ARV infection among commercial broiler flocks in Ontario, Canada during grow-out. A cross-sectional study of 231 randomly selected flocks was conducted from July 2010 to January 2012. Fifteen blood samples, 15 whole intestines, and 15 cloacal swabs per flock were collected at slaughter; ELISA and PCR were used to determine a flock’s ARV exposure status. Avian reovirus prevalence was 91% (95% CI: 87 to 94). District alone did not significantly explain the overall variation in the prevalence of ARV (univariable logistic regression; P = 0.073), although geographical differences were identified. The odds of ARV presence were significantly lower in the summer/autumn compared to the winter/spring (univariable exact logistic regression; P < 0.001). There was no association between flock mortality and flock ELISA mean titer or PCR status.
Résumé
Prévalence au niveau du troupeau, distribution géographique et variations saisonnières du réovirus aviaire parmi les troupeaux de poulets à griller en Ontario. Le réovirus aviaire (RVA) est un agent pathogène important sur le plan économique pour les poulets à griller. Notre objectif consistait à déterminer la prévalence, la distribution géographique et les variations saisonnières de l’infection par le RVA parmi les troupeaux de poulets à griller commerciaux en Ontario, au Canada, durant la phase d’engraissement. Une étude transversale de 231 troupeaux choisis au hasard a été réalisée de juillet 2010 à janvier 2012. Quinze échantillons sanguins, 15 intestins complets et 15 écouvillons cloacaux ont été prélevés à l’abattage; des tests ELISA et ACP ont été utilisés pour déterminer l’état d’exposition du troupeau au RVA. La prévalence du réovirus aviaire était de 91 % (IC de 95 % : de 87 à 94). Le district à lui seul ne peut pas expliquer significativement la variation générale dans la prévalence du RVA (régression logistique univariable; P = 0,073), quoique des différences géographiques ont été identifiées. Les risques de la présence du RVA étaient significativement inférieurs à l’été/automne comparativement à l’hiver/printemps (régression logistique exacte univariable; P < 0,001). Il n’y avait aucune association entre la mortalité du troupeau et le titre moyen ELISA du troupeau ou de son état d’ACP.
(Traduit par Isabelle Vallières)
Introduction
Avian reoviruses (ARVs) are members of the Orthoreovirus genus in the family Reoviridae. Avian reoviruses are associated with various diseases in chickens, such as respiratory and enteric disease, hydropericardium, pericarditis, myocarditis, and hepatitis (1). However, the most recognized form of ARV-associated diseases, and also a significant cause of lameness, is tenosynovitis (1).
Tenosynovitis, also known as viral arthritis, is predominantly a problem in broiler chickens, and results in swelling of the hock joint(s). Depending on the severity of the inflammation, affected birds are unable to move toward feed and water resulting in poor growth, culling, or death. Birds that survive to slaughter may be downgraded because of inflamed hock joints. This is a serious economic concern for broiler producers due to the costs associated with feed, labor, and lost production.
Avian reovirus infection in broiler chicken flocks appears to be high. Owoade et al (2) reported a seroprevalence of 41.0% among Nigerian broiler flocks, whereas studies conducted in Iran (3), Turkey (4), and Romania (5) reported higher seroprevalences (70.6% to 98.3%). At present, there is insufficient information on the prevalence or distribution of ARV in the Ontario broiler industry. An understanding of the baseline prevalence is the first step toward developing disease control strategies and determining the effectiveness of current biosecu-rity and management practices on the risk of ARV infection. Thus, the objectives of this study were to establish the baseline flock-level prevalence, geographical distribution, and seasonal variation of ARV among commercial broiler chicken flocks in Ontario during the grow-out period.
Materials and methods
Sampling
This study was part of a larger project designed to estimate the flock-level prevalence and risk factors for 13 pathogens of poultry health significance in the Ontario broiler industry. The sampling frame was all broiler producers in Ontario who were contracted with 6 major processing plants (5 federal and 1 provincial). These plants represent approximately 70% of broiler chickens processed in the province. Sample size estimates were based on identifying risk factors. Thus, using 95% confidence, 80% power, and a difference of 20% between the proportions of exposed and unexposed flocks with an estimated baseline flock-level (i.e., between-flock) prevalence of 20%, the target sample size to simultaneously evaluate multiple risk factors was 240 flocks. Our estimated baseline prevalence is lower than that of ARV studies conducted elsewhere (41% to 98%) (2–5); however, because we were estimating the flock-level prevalence of 13 pathogens, we chose a single, relatively conservative value of 20% for all pathogens. Approximately equal numbers of randomly selected flocks were enrolled every 4 wk during the sampling period (July 2010 to January 2012) to account for potential seasonal variation in pathogen prevalence. The number of flocks sampled from each processing plant was proportional to the plant’s market share of Ontario’s broiler processing. The days on which each plant was visited during each 4-week period were randomized using MiniTab 14 statistical software (Minitab, State College, Pennsylvania, USA). For each sampling day, 1 flock was randomly selected using numbered coins from the list of flocks scheduled to be slaughtered that day, and the corresponding producer was phoned and invited to participate in the study. The number of flocks sampled per farm was limited to 1; if > 1 flock was selected from the same farm, only the first flock was considered for inclusion in the study. Flocks originating from Quebec were excluded from the study.
The number of samples required per flock to detect ARV within the flock was determined using the formula n = lnα/lnq (6), with an a priori within-flock prevalence estimate of 15% and a confidence level of 90%. This a priori estimate was deemed sufficient to detect all pathogens of interest in the context of the larger project. At the processing plants, 15 blood samples, 15 cloacal swabs pooled into 3 samples (5 swabs per sample), and 15 whole intestines (duodenum to cloaca) were collected conveniently from each flock. Each blood sample contained blood from 1 or more birds depending on the line-speed and set-up of the plant. If a flock was shipped by more than 1 truck, the number of samples collected from each truck-load of birds was approximately equal. For example, if 5 trucks were used to ship a flock, 3 samples were collected from each truckload of birds to ensure representative sampling of the flock.
Sample processing
Samples were transported in coolers with ice packs to the Animal Health Laboratory, Guelph, Ontario. The samples were then further processed before submission for laboratory testing; for cecal tonsil tissue samples, 3 pools of 5 cecal tonsil tissues were collected from whole intestines. Instruments were autoclaved between flocks.
Enzyme-linked immunosorbent assay (ELISA)
A commercial ELISA kit (IDEXX Laboratories, Westbrook, Maine, USA) was used to detect ARV antibodies in 15 serum samples. Samples were diluted 1 in 500 in the sample diluent buffer provided by the manufacturer. A 100-μL volume of diluted sample was added to the wells of the ELISA microplate and incubated for 30 min at room temperature. The liquid content of the wells was then removed and each well was washed 5 times with 300 μL of distilled water. A 100-μL volume of (goat) anti-chicken horseradish peroxidase conjugate was then dispensed into each well and incubated for 30 min at room temperature. The liquid content of the wells was then removed and each well was washed 5 times with 300 μL of distilled water. A 100-μL volume of 3,3′,5,5′-tetramethylbenzidine substrate solution was added and incubated for 15 min at room temperature. After incubation, 100 μL of “Stop Solution” was dispensed to stop the reaction and absorbance values were then measured at 650 nm.
Virus isolation
Three pools of cloacal swabs and 3 pools of cecal tonsils were used for virus isolation in cell culture to detect viral shedding and persistence, respectively. Virus isolation was conducted in Leghorn male hepatoma cells obtained from American Type Culture Collection (ATCC#CRL-2117). The cells were propagated in Dulbecco’s Modified Eagle’s Medium -F12 supplemented with 5% fetal bovine serum, 2 mM L-glutamine, 100 units/mL penicillin, and 100 mg/mL streptomycin (Gibco, Carlsbad, California, USA). Cells were monitored daily for the appearance of syncytia suggestive of reovirus replication and 1 additional blind passage was carried out.
Polymerase chain reaction (PCR)
Polymerase chain reaction was carried out on cell culture fluids after the second passage. Total nucleic acids for ARV-specific real-time PCR were extracted using MagMAX-96 Viral RNA Isolation Kit in a MagMAX Express-96 Magnetic Particle Processor (Applied Biosystems, Foster City, California, USA) according to the manufacturer’s instructions. Viral RNA was detected using a real-time PCR assay with primers and a probe designed to amplify a conserved 63 bp fragment of reovirus S3 segment (Table 1a); the test does not differentiate between “pathogenic” and “non-pathogenic” strains. The PCR amplification was carried out in 25 μL reactions in a LightCycler 480 Real-Time PCR System (Roche, Laval, Quebec) using AgPath-ID One-Step RT-PCR Kit (Applied Biosystems) under standard conditions (Table 1b).
Table 1.
| a. Primer and probe sequences | ||||
|---|---|---|---|---|
|
| ||||
| Oligo name | Sequence 5′-3′ | Final concentration | ||
| AReoV_For_111027 | AGCGTGCAAGCCGCAAT | 0.25 μM | ||
| AReoV_Pr_111027 | GGAGGTACGTGTGCCAAACTTT | 0.50 μM | ||
| AReoV_Rev_111027 | TGTWATDCCTTCAACRAAMGAGTG | 0.25 μM | ||
|
| ||||
| b. Real-time PCR cycling parameters | ||||
|
| ||||
| Step | Temperature (°C) |
Hold (min:s) |
Ramp (°C/s) |
Cycles (X) |
|
| ||||
| Reverse transcription | 45 | 10:00 | 4.4 | 1 |
| Activation | 95 | 10:00 | 4.4 | 1 |
| Denaturation | 94 | 00:05 | 2.2 | 45 |
| Annealing/extension | 60 | 01:00 | 2.2 | |
| Cooling | 40 | 10:00 | 2.2 | 1 |
Data analysis
The total number of samples tested per flock using PCR was 6 (3 pooled samples of cecal tonsil tissues and 3 pooled samples of cloacal swabs); if ≥ 1 of 6 samples tested positive, the flock was considered to be PCR-positive. Criteria used to classify flocks as positive or negative for ARV based on ELISA and PCR results are shown in Table 2. For ELISA mean titers, the cut-point used (396) was that specified in the IDEXX reference guide. Titer groups provided by IDEXX are shown in Appendix 1. Flocks were further stratified by ELISA and PCR test results and the average flock mortality per stratum was estimated (Table 3). For each flock, mortality was calculated as the number of chicks placed minus the number of birds shipped, divided by the number of chicks placed, and multiplied by 100. For each stratum, average mortality was calculated as the sum of flock mortality in the stratum divided by the number of flocks in the stratum. A bi-variable linear regression model was used to determine if there was an association between flock mortality (dependent variable) and flock ELISA mean titer (categorical) and flock PCR status (dichotomous) (Table 3); model assumptions of normality and homoscedasticity were evaluated visually using a normal quantile plot and a plot of the standardized residuals against the predicted outcome, respectively.
Table 2.
Criteria used to classify commercial broiler chicken flocks sampled at processing between July 2010 and January 2012 in Ontario, Canada, as positive or negative for avian reovirus (ARV) based on enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR) results, and the number and percentage of flocks per criterion (n = 231 flocks)
| ELISAa | PCR positiveb | PCR negative |
|---|---|---|
| Mean titer > 396 | Flock ARV positive | Flock ARV positive |
| 76 (32.9%) | 70 (30.3%) | |
| Mean titer ≤ 396 and % samples ≥ 10%c | Flock ARV positive | Flock ARV positive |
| 31 (13.4%) | 19 (8.2%) | |
| Mean titer ≤ 396 and % samples < 10%d | Flock ARV positive | Flock ARV negative |
| 13 (5.6%) | 22 (9.5%) |
The cut-point used for the ELISA mean titer was that specified in the IDEXX reference guide (IDEXX Laboratories, Westbrook, Maine, USA).
The total number of samples tested per flock using PCR was 6 (3 pooled samples of cecal tonsil tissues and 3 pooled samples of cloacal swabs); if ≥ 1 of 6 samples tested positive, the flock was considered to be PCR-positive.
At least 10% of the blood samples were in profile group 1 (titers of 397 to 999) or higher. Profile groups are based on titer levels; as the level of antibody titer in a sample increases, the group number increases. The profile groups for this test range from 0 to 18 (Appendix 1).
The percentage of blood samples in profile group 1 or higher was less than 10%.
Appendix 1.
Titer groups provided by IDEXX (IDEXX Laboratories, Westbrook, Maine, USA) for their enzyme-linked immunosorbent assay (ELISA)
| Avian reovirus | |
|---|---|
| Titer 1: 397 | Titer 10: 12 000 |
| Titer 2: 1000 | Titer 11: 14 000 |
| Titer 3: 2000 | Titer 12: 16 000 |
| Titer 4: 3000 | Titer 13: 18 000 |
| Titer 5: 4000 | Titer 14: 20 000 |
| Titer 6: 5000 | Titer 15: 22 000 |
| Titer 7: 6000 | Titer 16: 24 000 |
| Titer 8: 8000 | Titer 17: 28 000 |
| Titer 9: 10 000 | Titer 18: 32 000 |
Table 3.
Number of commercial broiler chicken flocks sampled at processing between July 2010 and January 2012 in Ontario, Canada stratified by enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR) test results for avian reovirus (n = 231 flocks); the average flock mortalitya per stratum is provided
| ELISA mean titer | PCR positiveb | Average (range) flock mortality (%) | PCR negative | Average (range) flock mortality (%) |
|---|---|---|---|---|
| 5000 to 5999 | 1 | 3.1 | 0 | — |
| 4000 to 4999 | 2 | 3.2 (0.8 to 5.7) | 0 | — |
| 3000 to 3999 | 2 | 3.0 (2.4 to 3.5) | 2 | 3.0 (2.9 to 3.2) |
| 2000 to 2999 | 8 | 3.9 (2.3 to 7.6) | 5 | 4.8 (2.2 to 9.4) |
| 1000 to 1999 | 18 | 3.6 (0.3 to 6.3)c | 22 | 3.5 (1.7 to 6.4)g |
| 500 to 999 | 31 | 3.6 (0.6 to 5.8)d | 26 | 3.6 (1.3 to 7.6)h |
| 397 to 499 | 14 | 3.3 (0.8 to 8.9)e | 15 | 4.4 (2.1 to 6.4)i |
| 0 to 396 | 44 | 3.2 (1.9 to 5.5)f | 41 | 3.4 (0.6 to 12.7)j |
For each flock, mortality was estimated as the number of chicks placed minus the number of birds shipped, divided by the number of chicks placed, and multiplied by 100. For each stratum, average mortality was calculated as the sum of flock mortality in the stratum divided by the number of flocks in the stratum.
The total number of samples tested per flock using PCR was 6 (3 pooled samples of caecal tonsil tissues and 3 pooled samples of cloacal swabs); if ≥ 1 of 6 samples tested positive, the flock was considered to be PCR-positive.
Due to missing mortality data the number of flocks used for the calculations were c16 of 18, d23 of 31, e11 of 14, f37 of 44, g18 of 22, h23 of 26, i14 of 15, j38 of 41.
To explore potential seasonal variation in ARV prevalence, each flock was categorized into 1 of 4 seasons (summer = June 21 to September 20; autumn = September 21 to December 20; winter = December 21 to March 20; spring = March 21 to June 20). For example, a flock was considered to have been raised in the summer if the half age of the flock (19 d on average in the study population) was before the first calendar day of autumn. Half age of the flock was chosen because broiler breeders in Ontario are vaccinated against ARV, and maternal immunity is expected to decrease after approximately 14 d of age, leaving the chicks vulnerable to infection if exposed to the virus. Of 231 flocks, 4 were excluded from the seasonal analysis due to incomplete data on age at processing. For each season, prevalence was calculated as the number of flocks that were positive for ARV during the season divided by the number of flocks sampled during the season. Initially, a univariable logistic regression model was used to determine if there were statistically significant differences in prevalence between seasons. However, a model using season as a categorical independent variable could not be built due to perfect prediction (100% prevalence of ARV in the winter and spring). Season was therefore re-categorized into a dichotomous variable; winter and spring were collapsed into 1 category, and summer and autumn, which had similar prevalence, were collapsed into another category (the referent category), and the 2 categories were compared using a univari-able exact logistic regression model.
For each broiler district (administrative area), prevalence was calculated as the number of flocks that were positive for ARV in the district divided by the number of flocks sampled in the district. A choropleth map of the geographical distribution of ARV prevalence in Ontario was created using ArcGIS 10.4 (Esri, Redlands, California, USA). A reference map of Ontario’s 9 broiler districts was obtained from the Chicken Farmers of Ontario (CFO; Ontario’s chicken marketing board) and used to create the prevalence map; a manual classification method was used to create prevalence classes with ranges of equal size in ArcMap (ArcGIS 10.4; Esri, Redlands, California, USA). This method was selected because of the very high prevalence and relatively low variation of ARV across the province coupled with the uneven distribution of flocks per district. A univariable logistic regression model was used to determine if there were statistically significant differences in prevalence between districts. However, a model using district as a categorical independent variable (districts 1 through 9) could not be developed because district 8, which had a prevalence of 100%, dropped out of the model due to perfect prediction. Therefore, districts 8 and 2 (second highest prevalence and adjacent to district 8) were combined and used as the category to which all other districts were compared. A Hosmer-Lemeshow test was used to assess the fit of the model, with P ≤ 0.05 indicating a lack of fit. A Hosmer-Lemeshow test was chosen because the Pearson Chi-squared goodness-of-fit test could not estimate the P-value for the test statistic, most likely due to a low number of data points for some covariate patterns. All statistical analyses (α = 0.05, 2-tailed) were performed using Stata Intercooled version 13 (StataCorp, College Station, Texas, USA).
Results
In total, 231 flocks were sampled between July 2010 and January 2012. One flock had 16 blood samples collected, 2 flocks had 10 blood samples collected, 11 had 14 collected, and the remainder (217, 93.9%) had 15 blood samples collected.
Characteristics of the study population
Four producers who allowed sample collection at the processing plant did not complete an interview. Consequently, farm/flock-level data were available for 227 flocks. None of the study flocks were vaccinated for ARV during the grow-out period. The mean and median weight of birds at processing was 2.2 kg (range: 1.7 to 3.1 kg). The median flock age at shipment was 38 d (range: 31 to 53 d). The mean mortality per flock was 3.5% (range: 0.3% to 12.7%); this included both natural death and culling. The median flock size was 25 092 birds (range: 7242 to 104 040 birds; flock size was available for 200 flocks). The number of flocks raised using an all-in-all-out system of production at the flock level (i.e., all birds within a flock were placed on the same date and also shipped for processing simultaneously) was 223 of 227 flocks (98.2%). The number of barns in which the flock was raised varied: 1 barn (168/227 flocks; 74.0%), 2 barns (46/227 flocks; 20.3%), 3 barns (8/227 flocks; 3.5%), or 4 barns (5/227 flocks; 2.2%).
Avian reovirus prevalence
In total, 117 of 692 cloacal swabs (16.9%) and 169 of 691 cecal tonsils (24.5%) were positive for ARV on real-time PCR, and 1756 of 3445 blood samples (51.0%) were in profile group 1 (titers of 397 to 999) or higher. Overall, 209 of 231 flocks (90.5%, 95% CI: 86.8 to 94.3) were positive for ARV. The number and percentage of flocks per classification criterion are shown in Table 2. The ELISA mean titers ranged from 1 to 5222; 120 flocks (51.9%) tested positive and 111 flocks (48.1%) tested negative on PCR (Table 3). The average flock mortality stratified by ELISA mean titer and PCR status ranged from 3.0% to 4.8% (Table 3); the stratum-specific ranges were variable in both the PCR-positive and -negative groups, with very high flock-level mortality on some farms. There was no association between flock mortality, and flock ELISA and PCR results (Table 4), and the assumptions of linear regression were met.
Table 4.
Final regression models for the associations between flock mortality and flock avian reovirus (ARV) ELISAa and PCRb test results (linear), ARV presence and season of grow-out (exact logistic), and ARV presence and district of grow-out (logistic), in commercial broiler chicken flocks in Ontario
| Linear regression: flock mortality and flock ARV ELISA and PCR test results (n = 198 flocks) | |||||
|---|---|---|---|---|---|
|
| |||||
| Variable | Coefficient | 95% CI of coefficient | P-value (t-test) | P-value (partial F-test) | |
| Flock ELISA mean titer | 0.634 | ||||
| 5000 to 5999 | 2.70 | (−0.60, 6.00) | 0.108 | ||
| 4000 to 4999 | 0.96 | (−1.39, 3.30) | 0.421 | ||
| 3000 to 3999 | 0.34 | (−1.34, 2.02) | 0.687 | ||
| 2000 to 2999 | −0.21 | (−1.23, 0.81) | 0.686 | ||
| 1000 to 1999 | 0.09 | (−0.59, 0.77) | 0.794 | ||
| 500 to 999 | 0.11 | (−0.50, 0.73) | 0.716 | ||
| 397 to 499 | −0.38 | (−1.15, 0.39) | 0.334 | ||
| 0 to 396 | Referent | ||||
| Flock positive to ARV on PCRc | |||||
| Yes | −0.16 | (−0.63, 0.32) | 0.519 | ||
| No | Referent | ||||
| Intercept | 3.62 | (3.15, 4.10) | − 0.001 | ||
| Significance of overall model | 0.716; R2 = 0.028 | ||||
|
| |||||
| Exact logistic regression: ARV presence and season (n = 227 flocks) | |||||
|
| |||||
| Variable | Odds ratiod | 95% CI of OR (exact) | P-value (exact) | ||
|
| |||||
| Season | |||||
| Winter and spring | 17.53 | (2.98, + ∞) | 0.0002 | ||
| Summer and autumn | Referent | ||||
|
| |||||
| Logistic regression: ARV presence and district (n = 231 flocks) | |||||
|
| |||||
| Variable | Odds ratio | Coefficient | 95% CI of coefficient | P-value (Wald’s test) | P-value (Likelihood ratio test) |
|
| |||||
| Districte | 0.073 | ||||
| District 1 | 0.08 | −2.54 | (−4.87, −0.22) | 0.032 | |
| District 3 | 0.09 | −2.43 | (−4.58, −0.28) | 0.027 | |
| District 4 | 0.07 | −2.60 | (−4.93, −0.28) | 0.028 | |
| District 5 | 0.06 | −2.89 | (−5.23, −0.55) | 0.016 | |
| District 6 | 0.11 | −2.20 | (−5.06, 0.70) | 0.133 | |
| District 7 | 0.19 | −1.64 | (−4.08, 0.80) | 0.188 | |
| District 9 | 0.08 | −2.48 | (−4.80, −0.16) | 0.036 | |
| District 2 + 8 | Referent | ||||
| Intercept | 4.28 | (2.30, 6.25) | < 0.001 | ||
ELISA = enzyme-linked immunosorbent assay.
PCR = polymerase chain reaction.
At least 1 of 6 pooled cecal tonsil tissue or pooled cloacal swab samples tested positive to ARV on PCR.
Median unbiased estimate.
The Chicken Farmers of Ontario, a producer-run, non-profit organization representing registered Ontario chicken producers, partitions Ontario into 9 administrative/geographical areas referred to as broiler districts. Districts 2 and 8 were chosen as the referent because the highest prevalence was observed in these districts.
The prevalence of ARV by season was 88.4% in the summer (61/69 flocks), 84.0% in the autumn (68/81), and 100% in the winter (48/48) and the spring (29/29). The results of the exact logistic regression showed that flocks raised in the winter and spring were at higher odds of testing positive for ARV compared with flocks raised in the summer and autumn [OR (median unbiased estimate) = 17.53, exact 95% CI: 2.98 to + ∞, exact P = 0.0002; Table 4].
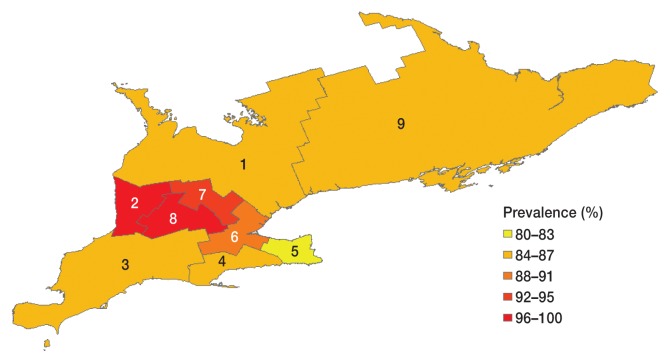
The prevalence of ARV by broiler district ranged from 80% (district 5) to 100% (district 8) (Table 5, Figure 1). District alone did not significantly explain the overall variation in the prevalence of ARV (LRT χ2 = 12.98, P = 0.073; Table 4); however, the odds of testing positive for ARV were lower for flocks raised in district 1 (OR = 0.08, P = 0.032), district 3 (OR = 0.09, P = 0.027), district 4 (OR = 0.07, P = 0.028), district 5 (OR = 0.06, P = 0.016), and district 9 (OR = 0.08, P = 0.036) compared to the districts with the highest prevalence (districts 8 and 2). The logistic regression model fit the data (Hosmer-Lemeshow χ2 = 0.00, P = 1.000).
Table 5.
Flock-level avian reovirus prevalence among commercial broiler chicken flocks sampled at processing between July 2010 and January 2012 in Ontario, Canada by broiler district (n = 231 flocks)
| District | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 17/20a (85.0%) | 38/39 (97.4%) | 38/44 (86.4%) | 16/19 (84.2%) | 12/15 (80.0%) | 8/9 (88.9%) | 28/30 (93.3%) | 34/34 (100%) | 18/21 (85.7%) |
The denominator indicates the number of flocks sampled per district.
Figure 1.
Flock-level avian reovirus prevalence among commercial broiler chicken flocks sampled at processing between July 2010 and January 2012 in Ontario, Canada by broiler district (n = 231 flocks). The map was produced using ArcGIS 10.4 (Esri, Redlands, California, USA).
Discussion
Avian reovirus has been found on many poultry farms with chickens suffering from tenosynovitis, malabsorption syndrome, and enteric/respiratory diseases (1). These diseases result in significant economic losses; however, there is insufficient information on the prevalence and distribution of the virus in the commercial broiler population in Canada. This study captured the baseline prevalence of ARV among a large sample of randomly selected broiler flocks at processing in Ontario between July 2010 and January 2012. Although ARV infection was common (90.5%) among broiler flocks in Ontario, this was not synonymous with ARV-associated diseases. Our findings are similar to the reported prevalence in Iran (98.3%) (3), Romania (85.7%) (5), and 4 western provinces in Turkey (70.6% to 77.2%) (4), and higher than the prevalence reported in Nigeria (41.0%) (2). Notwithstanding, there were several aspects of study design/population that differed among these studies and our study, including the classification criteria used to determine flock status (positive/negative), antibody titer thresholds for reovirus seropositivity, the age of broilers at sampling, and vaccination of breeders, which make it difficult to compare findings. Most of these studies used only 1 test (i.e., ELISA) to determine the flocks’ ARV status (3–5). The mean titer threshold used in the Nigerian study was higher than in our study (1351 versus 396); the thresholds used in the Iranian and Romanian studies were not specified. Similar to our study, sampling in the Iranian study was conducted at the time of slaughter, although the age was not specified. In the Romanian study, samples were collected from 35-day-old broilers, which is in line with the average flock age of 38 d in our study. The study conducted in Nigeria sampled blood from 1-day-old to 8-week-old broilers. Furthermore, in contrast to our study, in which all domestic broiler breeder flocks are vaccinated for ARV several times as pullets, including at least 2 live vaccines and 2 killed vaccines, the parent flocks in the Iranian and Nigerian studies were not vaccinated against reovirus.
In our study population, the proportion of flocks that were PCR positive was approximately equal to the proportion that was PCR negative. Further, the distribution of ELISA mean titers was similar among the PCR-positive and PCR-negative flocks. Interpretation of the mean titer and PCR results could indicate when (early versus late) exposure likely occurred. For example, flocks with high mean titers were likely exposed to ARV earlier during the grow-out period, therefore had high antibody titers at the time of slaughter, yet may or may not have been shedding virus at that time. In contrast, PCR-positive flocks with low mean titers were likely exposed to ARV late in the grow-out period, and therefore had not mounted a full immune response at the time of slaughter, yet were shedding virus that could be detected on PCR. Such detail might be important when exploring associations between ARV exposure and production data.
Although several flocks in our study had high mean titers, approximately 3/4 had a mean titer < 1000. This is somewhat comparable to the geometric mean titers of ARV from serological samples from a small study in Romania (7 broiler farms), which ranged between 89 and 775 using IDEXX’s ELISA kit (5). It should be noted that the mean mortality in our study population was consistent with the provincial average; the CFO reported that the average mortality for the province between 2005 and 2009 was 4.0% (range: 3.6% to 6.1%) for all slaughter ages and weights. However, there was no statistically significant association between flock mortality and the flock’s ELISA mean titer and PCR status, suggesting that there was no direct relationship between the antibody titers and mortality in this baseline surveillance study. This finding might be related to a lack of differentiation between pathogenic and non-pathogenic strains. Conversely, because we could not differentiate between natural mortality and culling, mortality in our study might be a surrogate for poor management or heavy culling for ARV-associated diseases or other diseases, as suggested by the widely varied flock-level mortality in several of the low to mid-titer strata.
Randomization resulted in excellent representation of the distribution of broiler production in the province (7). The prevalence of ARV was very high across the province, and although overall, district was not a significant predictor of ARV presence, statistically significant differences in prevalence between districts were identified. The variation in the geographical distribution of ARV could be due to localized differences in risk factors, such as environmental challenges, the density of farms, early mortality associated with E. coli, or subtle differences in management and biosecurity practices among districts. The virus can survive under farm conditions for 12 to 15 wk — it is heat resistant, stable in a wide spectrum of pH (3.0 to 9.0), and resistant to disinfectants commonly used in poultry houses (8). This environmental resistance of the virus is likely one of the reasons for the high prevalence across the province.
To our knowledge, this is the first observational study that has investigated the seasonal variation in ARV prevalence. Avian reovirus was common in all seasons. Meulemanns and Halen (9) found that ARV was active at temperatures up to 50°C, indicating the hardiness of the virus. However, information on the seasonal adaptation of the virus to certain temperatures is unknown. We found that ARV was less prevalent in the summer and autumn compared to the winter and spring. It is possible that factors, such as cleaning and disinfection practices or ventilation, which vary among seasons, might have contributed to the minor seasonal variation in prevalence. In Ontario, the CFO’s On-Farm Food Safety Assurance Program dictates that broiler barns must be disinfected at least once per year (10). Given the climate in Ontario, it is possible that many producers carry out this task in the summer and early autumn when ambient temperatures are more favourable for thorough cleaning and disinfection (9).
Avian reovirus has been associated with several poultry diseases. However, the pathogenesis is unclear and its presentation is likely influenced by unknown factors. Commercial ELISA cannot distinguish pathogenic strains of reovirus from non-pathogenic strains (11); therefore, it is not known what percentage of flocks in our study was exposed to pathogenic strains. Given that ARVs are ubiquitous in domestic fowl, and that the majority of reoviruses isolated from poultry farms are non-pathogenic, it is likely that mainly non-pathogenic strains accounted for the high prevalence of ARV in broiler farms in Ontario. Notwithstanding, because there is considerable variation in virulence between antigenically similar isolates and there can be reassortment of genes when a cell is infected by more than one strain of ARV (12), the high prevalence is of concern because of the potential for the development of new, potentially pathogenic variants that can lead to clinical disease. Genotyping of isolates is warranted to identify pathogenic strains.
Our study findings indicate that ARV is common among commercial Ontario broiler flocks in all seasons and in all broiler districts. Investigation of associations between ARV and aspects of flock management, such as biosecurity and management practices is warranted, as is investigation of associations between ARV and flock production parameters.
Acknowledgments
We are grateful to Dr. Marina Brash, Elise Myers, Thelma Martinez, Heather McFarlane, Stephanie Wong, Veronique Gulde, Christian Sandrock, and Chanelle Taylor, as well as fellow graduate students Dr. Michael Eregae and Hind Kasab-Bachi, for their help during this project. The cooperation of the Chicken Farmers of Ontario, broiler producers, and broiler processing plants was much appreciated.
This project was funded by the Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA)/University of Guelph Partnership, OMAFRA-University of Guelph Agreement through the Animal Health Strategic Investment (AHSI) fund managed by the Animal Health Laboratory of the University of Guelph, the Poultry Industry Council, and the Chicken Farmers of Ontario. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Jones RC. Viral arthritis. In: Saif YM, Fadly AM, Glissen JR, McDougald LR, Nolan LK, Swayne DE, editors. Diseases of Poultry. 12th ed. Hoboken, New Jersey: Wiley-Blackwell; 2008. pp. 309–328. [Google Scholar]
- 2.Owoade A, Ducatez M, Muller C. Seroprevalence of avian influenza virus, infectious bronchitis virus, reovirus, avian pneumovirus, infectious laryngotracheitis virus, and avian leucosis virus in Nigerian poultry. Avian Dis. 2006;50:222–227. doi: 10.1637/7412-071505R.1. [DOI] [PubMed] [Google Scholar]
- 3.Bokaie S, Shojadoost B, Pourbakhsh S, Pourseyyed S, Sharifi L. Sero-prevalence survey on reovirus infection of broiler chickens in Tehran province. Iran J Vet Res. 2008;9:181–183. [Google Scholar]
- 4.Erol N, şengűl SS. Seroprevalence of avian reovirus infections in chickens in western provinces of Turkey. Kafkas Univ Vet Fak Derg. 2012;18:653–656. [Google Scholar]
- 5.Ona P, Bucur I, Fluerasu L, Stancu A. Serological screening for avian reovirosis. Lucrari Stintifice Medicina Veterinara. 2014;47:96–98. [Google Scholar]
- 6.Dohoo I, Martin W, Stryhn H. Veterinary Epidemiologic Research. 2nd ed. Charlottetown, Prince Edward Island: VER Inc; 2009. pp. 33–56. [Google Scholar]
- 7.Eregae ME. PhD dissertation. Guelph, Ontario: University of Guelph; 2014. The epidemiology of chicken anaemia virus, fowl adenovi-rus, and infectious bursal disease virus in Ontario broiler flocks. [Google Scholar]
- 8.Jones RC, Savage CE. The survival of avian reoviruses on materials associated with the poultry house environment. Avian Pathol. 2003;32:419–425. [PubMed] [Google Scholar]
- 9.Meulemanns G, Halen P. Efficacy of some disinfectants against infectious bursal disease virus and avian reovirus. Vet Rec. 1982;111:412–413. doi: 10.1136/vr.111.18.412. [DOI] [PubMed] [Google Scholar]
- 10.Chicken Farmers of Ontario [homepage on the Internet] c2016 On-Farm Food Safety Assurance Program Manual. 2014. [Last accessed May 31, 2017]. Available from: https://www.ontariochicken.ca/About-CFO/Quality-Chicken/On-Farm-Food-Safety-Assurance-Program.aspx.
- 11.Xie Z, Qin C, Xie L, et al. Recombinant protein-based ELISA for detec -tion and differentiation of antibodies against avian reovirus in vaccinated and non-vaccinated chickens. J Virol Methods. 2010;165:108–111. doi: 10.1016/j.jviromet.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 12.McNulty MS, Jones RC, Gough RE. Reoviridae. In: Pattison M, McMullin PF, Bradbury JM, Alexander DJ, editors. Poultry Diseases. 6th ed. Philadelphia, Pennsylvania: Saunders Elsevier; 2008. pp. 382–391. [Google Scholar]