Abstract
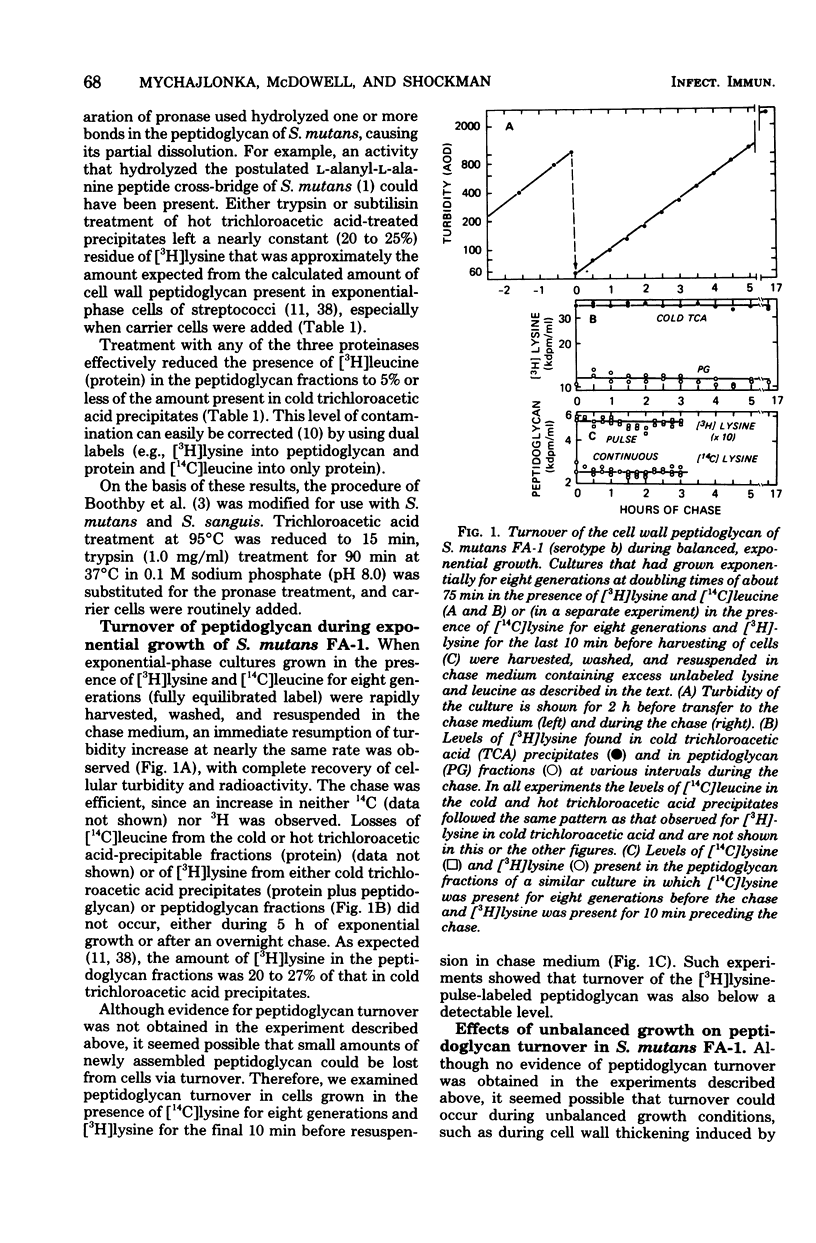
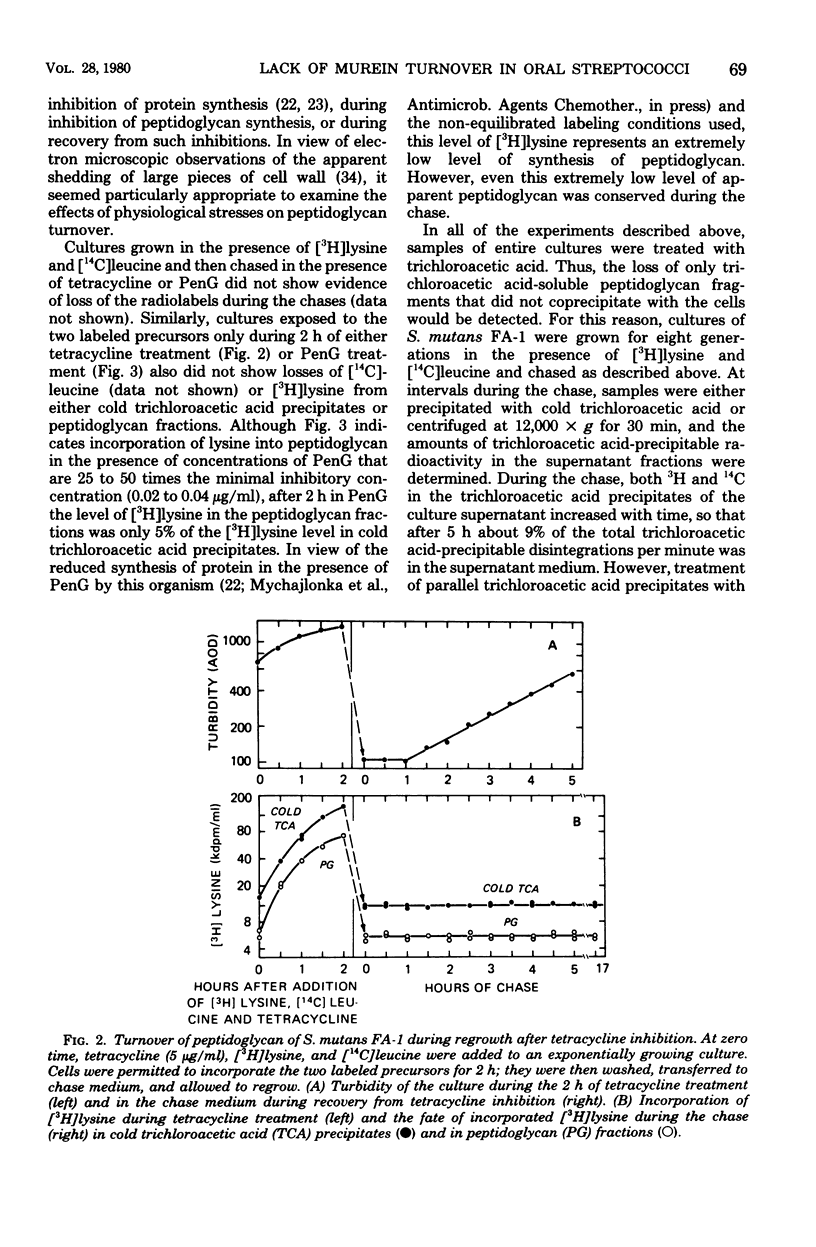
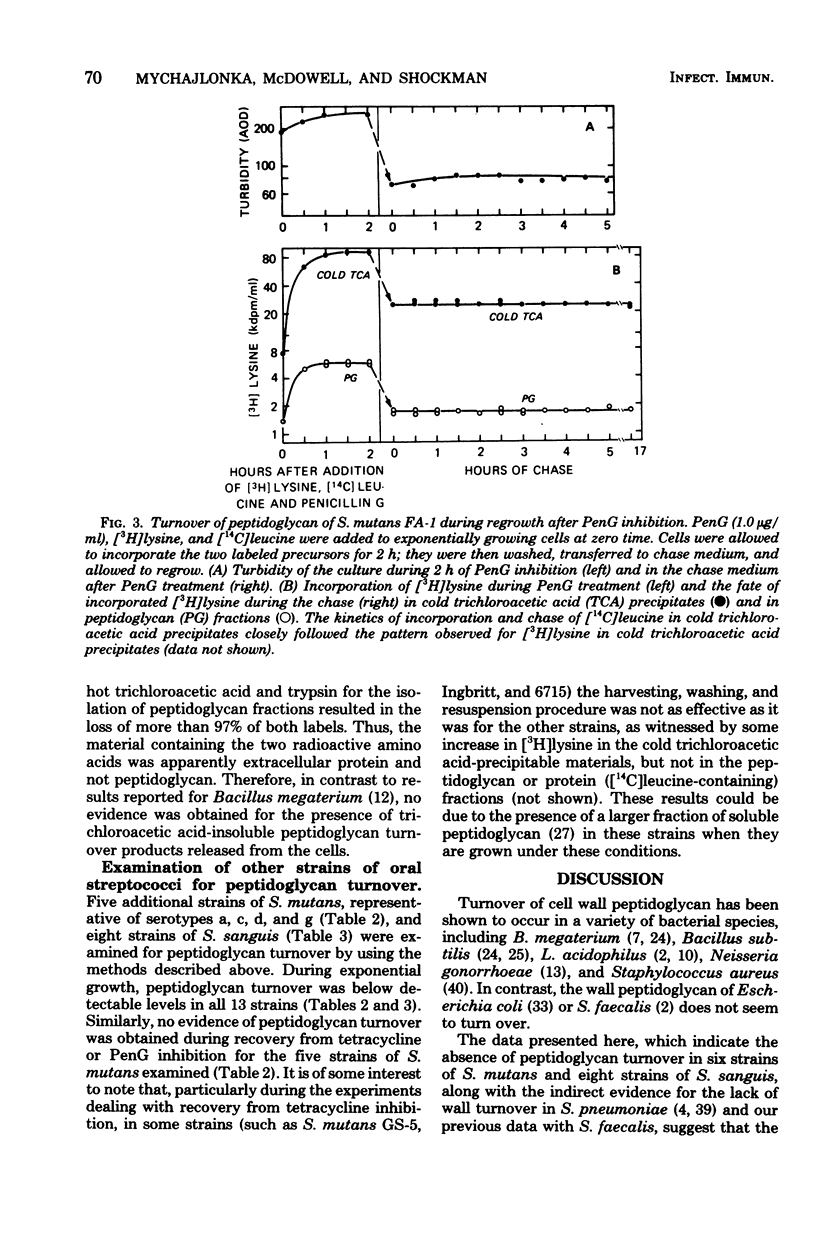
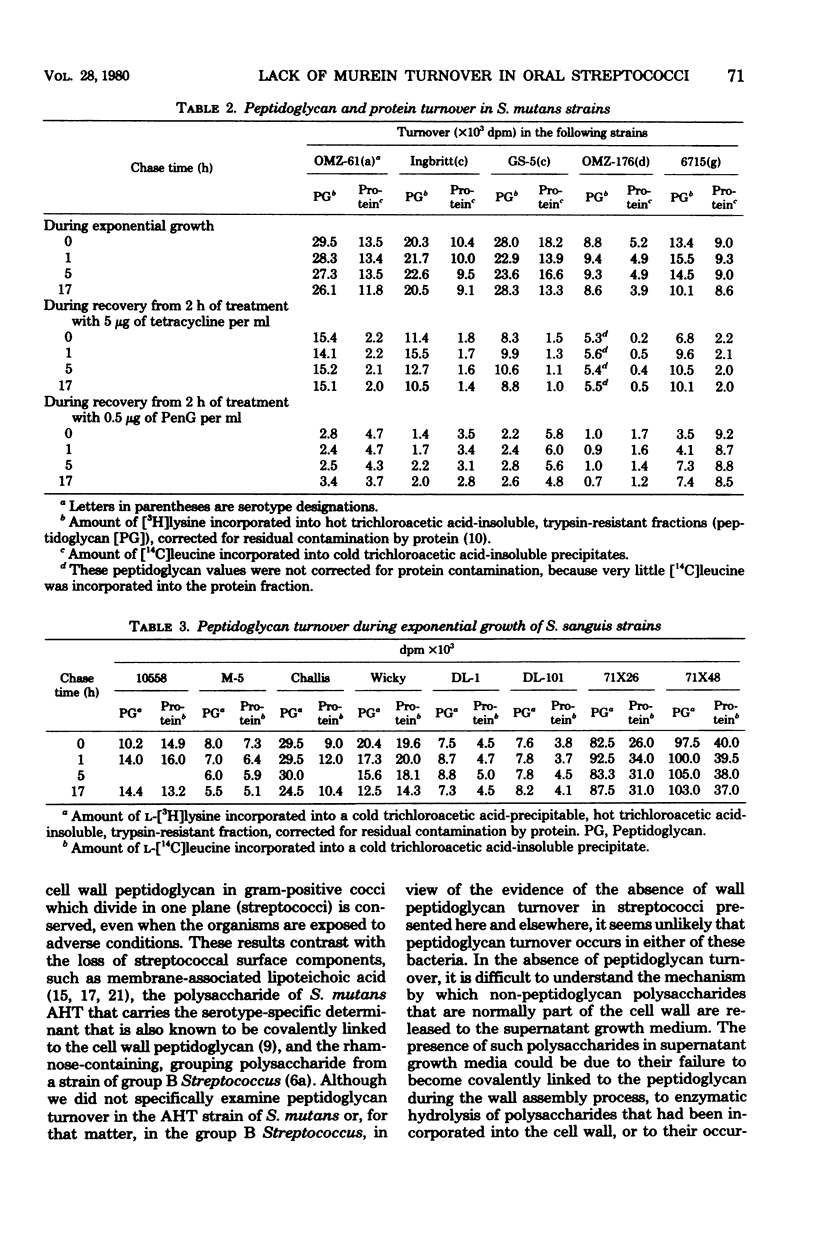
Turnover of the cell wall peptidoglycan fraction of six different strains of Streptococcus mutans and eight different strains of Streptococcus sanguis was examined. Cells were grown in the presence of [3H]lysine and [14C]leucine for at least eight generations and then chased in growth medium lacking the two labels. At intervals during the chase, samples of cultures were removed, and the amounts of the two labeled precursors remaining in the peptidoglycan and protein fractions were quantitated. Similar experiments were done in which the pulse-labeling technique was used. In addition, cells were labeled in the presence of tetracycline or penicillin, chased with growth medium containing no inhibitor, and assayed at intervals during the chase for the amount of [3H]lysine present in peptidoglycan fractions. Studies of cultures of S. mutans strains FA-1, OMZ-61, OMZ-176, 6715, GS-5, and Ingbritt and of S. sanguis strains 10558, M-5, Wicky, DL-101, DL-1, 71X26, and 71X48 maintained in the exponential phase of growth in a chemically defined medium failed to show evidence of loss of insoluble peptidoglycan via turnover. Similarly, for the strains of S. mutans, insoluble peptidoglycan assembled during 2 h of benzylpenicillin or tetracycline treatment was also conserved during recovery from growth inhibition.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bleiweis A. S., Taylor M. C., Deepak J., Brown T. A., Wetherell J. R., Jr Comparative chemical compositions of cell walls of Streptococcus mutans. J Dent Res. 1976 Jan;55:A103–A108. doi: 10.1177/002203457605500102011. [DOI] [PubMed] [Google Scholar]
- Boothby D., Daneo-Moore L., Higgins M. L., Coyette J., Shockman G. D. Turnover of bacterial cell wall peptidoglycans. J Biol Chem. 1973 Mar 25;248(6):2161–2169. [PubMed] [Google Scholar]
- Boothby D., Daneo-Moore L., Shockman G. D. A rapid, guantitative, and selective estimation of radioactively labeled peptidoglycan in gram-positive bacteria. Anal Biochem. 1971 Dec;44(2):645–653. doi: 10.1016/0003-2697(71)90255-7. [DOI] [PubMed] [Google Scholar]
- Briles E. B., Tomasz A. Radioautographic evidence for equatorial wall growth in a gram-positive bacterium. Segregation of choline-3H-labeled teichoic acid. J Cell Biol. 1970 Dec;47(3):786–790. doi: 10.1083/jcb.47.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. A., Bleiweis A. S. Chemical, immunochemical, and structural studies of the cross-reactive antigens of Streptococcus mutans AHT and B13. Infect Immun. 1979 May;24(2):326–336. doi: 10.1128/iai.24.2.326-336.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L. K., Knox K. W., Wicken A. J. Extractability of cell wall polysaccharide from lactobacilli and streptococci by autoclaving and by dilue acid. Infect Immun. 1978 Dec;22(3):842–851. doi: 10.1128/iai.22.3.842-851.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey R. B., Eisenstein T. K., Shockman G. D., Greber T. F., Swenson R. M. Soluble group- and type-specific antigens from type III group B Streptococcus. Infect Immun. 1980 Apr;28(1):195–203. doi: 10.1128/iai.28.1.195-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaloupka J., Krecková P. Turnover of mucopeptide during the life cycle of Bacillus megaterium. Folia Microbiol (Praha) 1971;16(5):372–382. doi: 10.1007/BF02875757. [DOI] [PubMed] [Google Scholar]
- Craig R. A., Riege D. H., Bleiweis A. S. Antigens of Streptococcus mutans: cellular localization of the serotype-specific polysaccharide of strain AHT and release during exponential growth. Infect Immun. 1979 Dec;26(3):1177–1185. doi: 10.1128/iai.26.3.1177-1185.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneo-Moore L., Coyette J., Sayare M., Boothby D., Shockman G. D. Turnover of the cell wall peptidoglycan of Lactobacillus acidophilus. The presence of a fraction immune to turnover. J Biol Chem. 1975 Feb 25;250(4):1348–1353. [PubMed] [Google Scholar]
- Frehel C., Ryter A. Peptidoglycan turnover during growth of a Bacillus megaterium Dap- Lys- mutant. J Bacteriol. 1979 Feb;137(2):947–955. doi: 10.1128/jb.137.2.947-955.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeler B. H., Young F. E. Chemical composition and turnover of peptidoglycan in Neisseria gonorrhoeae. J Bacteriol. 1976 Jun;126(3):1180–1185. doi: 10.1128/jb.126.3.1180-1185.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Study of cycle of cell wall assembly in Streptococcus faecalis by three-dimensional reconstructions of thin sections of cells. J Bacteriol. 1976 Sep;127(3):1346–1358. doi: 10.1128/jb.127.3.1346-1358.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R., Shockman G. D. Synthesis and excretion of glycerol teichoic acid during growth of two streptococcal species. Infect Immun. 1975 Aug;12(2):333–338. doi: 10.1128/iai.12.2.333-338.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keglević D., Ladesić B., Hadzija O., Tomasić J., Valinger Z., Pokorny M., Naumski R. Isolation and study of the composition of a peptidoglycan complex excreted by the biotin-requiring mutant of Brevibacterium divaricatum NRRL-2311 in the presence of penicillin. Eur J Biochem. 1974 Mar 1;42(2):389–400. doi: 10.1111/j.1432-1033.1974.tb03351.x. [DOI] [PubMed] [Google Scholar]
- Kessler R. E., Shockman G. D. Precursor-product relationship of intracellular and extracellular lipoteichoic acids of Streptococcus faecium. J Bacteriol. 1979 Feb;137(2):869–877. doi: 10.1128/jb.137.2.869-877.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Narita T., Stewart-Tull D. E., Shimono T., Watanabe Y. Immunoadjuvant activities of cell walls and their water-soluble fractions prepared from various gram-positive bacteria. Biken J. 1975 Jun;18(2):77–92. [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Shimono T., Narita T., Kato K. Immunoadjuvant activities of cell walls, their water-soluble fractions and peptidoglycan subunits, prepared from various gram-positive bacteria, and of synthetic n-acetylmuramyl peptides. Z Immunitatsforsch Exp Klin Immunol. 1975 Jul;149(2-4):302–319. [PubMed] [Google Scholar]
- Lesher R. J., Bender G. R., Marquis R. E. Bacteriolytic action of fluoride ions. Antimicrob Agents Chemother. 1977 Sep;12(3):339–345. doi: 10.1128/aac.12.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham J. L., Knox K. W., Wicken A. J., Hewett M. J. Formation of extracellular lipoteichoic acid by oral streptococci and lactobacilli. Infect Immun. 1975 Aug;12(2):378–386. doi: 10.1128/iai.12.2.378-386.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly S. J., Daneo-Moore L., Shockman G. D. Factors regulating cell wall thickening and intracellular iodophilic polysaccharide storage in Streptococcus mutans. Infect Immun. 1977 Jun;16(3):967–973. doi: 10.1128/iai.16.3.967-973.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly S. J., Dipersio J. R., Higgins M. L., Shockman G. D. Unbalanced growth and macromolecular synthesis in Streptococcus mutans FA-1. Infect Immun. 1976 Mar;13(3):941–948. doi: 10.1128/iai.13.3.941-948.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck J., Chan L., Glaser L. Turnover of the cell wall of Gram-positive bacteria. J Biol Chem. 1971 Mar 25;246(6):1820–1827. [PubMed] [Google Scholar]
- Mauck J., Glaser L. Turnover of the cell wall of Bacillus subtilis W-23 during logarithmic growth. Biochem Biophys Res Commun. 1970 May 22;39(4):699–706. doi: 10.1016/0006-291x(70)90261-5. [DOI] [PubMed] [Google Scholar]
- Merser C., Sinay P., Adam A. Total synthesis and adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1316–1322. doi: 10.1016/0006-291x(75)90503-3. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Bracha R., Sharon N. Penicillin-induced secretion of soluble, uncross-linked peptidoglycan by Micrococcus luteus cells. Biochemistry. 1974 Nov 19;13(24):5045–5053. doi: 10.1021/bi00721a028. [DOI] [PubMed] [Google Scholar]
- Nauciel C., Fleck J., Mock M., Martin J. P. Activité adjuvante de fractions monomériques de peptidoglycanes bactéiens dans l'hypersensililité de type retardé. C R Acad Sci Hebd Seances Acad Sci D. 1974 Dec 17;277(24):2841–2844. [PubMed] [Google Scholar]
- Pooley H. M. Layered distribution, according to age, within the cell wall of bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1139–1147. doi: 10.1128/jb.125.3.1139-1147.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M. Turnover and spreading of old wall during surface growth of Bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1127–1138. doi: 10.1128/jb.125.3.1127-1138.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Jungkind D., Daneo-Moore L., Shockman G. D. Evidence for the synthesis of soluble peptidoglycan fragments by protoplasts of Streptococcus faecalis. J Bacteriol. 1975 Oct;124(1):398–409. doi: 10.1128/jb.124.1.398-409.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G. S., Shockman G. D., Daneo-Moore L. Balanced macromolecular biosynthesis in "protoplasts" of Streptococcus faecalis. J Bacteriol. 1971 Mar;105(3):710–717. doi: 10.1128/jb.105.3.710-717.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfield L., Pearlman-Kothencz M. Synthesis and assembly of bacterial membrane components. A lipopolysaccharide-phospholipid-protein complex excreted by living bacteria. J Mol Biol. 1969 Sep 28;44(3):477–492. doi: 10.1016/0022-2836(69)90374-x. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Higgins M. L., DaneoMoore L., Mattingly S. J., Diersio J. R., Terleckyj B. Studies of balanced and unblaanced growth of Streptococcus mutans. J Dent Res. 1976 Jan;55:A10–A18. doi: 10.1177/002203457605500101011. [DOI] [PubMed] [Google Scholar]
- TOENNIES G., SHOCKMAN G. D., KOLB J. J. Differential effects of amino acid deficiencies on bacterial cytochemistry. Biochemistry. 1963 Mar-Apr;2:294–296. doi: 10.1021/bi00902a017. [DOI] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Westphal M., Briles E. B., Fletcher P. On the physiological functions of teichoic acids. J Supramol Struct. 1975;3(1):1–16. doi: 10.1002/jss.400030102. [DOI] [PubMed] [Google Scholar]
- Wong W., Young F. E., Chatterjee A. N. Regulation of bacterial cell walls: turnover of cell wall in Staphylococcus aureus. J Bacteriol. 1974 Nov;120(2):837–843. doi: 10.1128/jb.120.2.837-843.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



