Abstract
Human diabetic patients may have increased lactate levels compared to non-diabetics. Despite the use of lactate levels in critical care assessment, information is lacking for diabetic dogs. Therefore, this prospective cross-sectional clinical study aimed to determine lactate concentrations in 75 diabetic dogs [25 newly diagnosed non-ketotic diabetics, 25 under insulin treatment, and 25 in diabetic ketoacidosis (DKA)], compared to 25 non-diabetic dogs. Lactate levels (mmol/L) were not different among groups (P = 0.20); median and 25th to 75th percentile were 2.23 and P25–75 = 1.46 to 2.83 for controls, 1.69 and P25–75 = 1.09 to 2.40 for newly diagnosed non-ketotic diabetics, 2.27 and P25–75 = 1.44 to 2.90 for dogs under insulin treatment for at least 30 days, and 2.40 and P25–75 = 1.58 to 3.01 for dogs in DKA. Longitudinal studies assessing both isomers (L- and D-lactate) are needed to better elucidate the role of lactate in the pathophysiology of diabetes acid-base status in dogs.
Résumé
La concentration de lactate de sang chez les chiens diabétiques. Des patients humains avec diabète peuvent présenter augmentation des niveaux de lactate, quand comparés aux non diabetiques. Bien que est utilisé d’évaluer les patients dans un état critique, cette information manque pour les chiens diabétiques. Par conséquent, cette étude clinique s’agit d’une prospective transversale en vue de detérminer les concentrations du lactate en 75 chiens diabétiques [25 au moment du diagnostic, 25 sous traitement à l’insuline et 25 dans l’acido-cétose diabétique (ACD)], par rapport aux 25 chiens non diabétiques. Les niveaux de L-lactate ne différaient pas entre les groupes (P = 0,20). Les valeurs médianes et les centiles 25 % et 75 % étaient de 2,23 mmol/L (P25–75 = 1,46 à 2,83) pour les contrôles, 1,69 mmol/L (P25–75 = 1,09 à 2,40) au diagnostic, 2,27 mmol/L (P25–75 = 1,44 à 2,90) sous traitement à l’insuline pendant au moins 30 jours, et 2,40 mmol/L (P25–75 = 1,58 à 3,01) dans ACD. Des études longitudinales évaluant les deux isomères (L et D-lactate) sont nécessaires pour élucider son rôle dans la physiopathologie et le déséquilibre acide-base chez les chiens diabétiques.
(Traduit par Ana Carolina Possas Viana)
Introduction
Lactate, a product of glucose anaerobic metabolism, is considered a diagnostic and prognostic biomarker for shock in human and veterinary medicine, and can be considered as a marker for detecting hypoperfusion, although with some limitations (1). Poor tissue perfusion/oxygenation is strongly correlated with morbidity and mortality (2–6).
The imbalance between lactate production, breakdown, and clearance, will be reflected in circulating lactate concentration (7). Hyperlactatemia, a persistent increase in blood lactate concentration, can arise from impaired tissue oxygenation (hypoxic) and also from non-hypoxic processes, and may result in lactic acidosis (7–9). The most common identified form is Type A lactic acidosis, caused by hypoxic conditions, whereas Type B is secondary to non-hypoxic causes. Type B lactic acidosis is further divided into B1, B2, and B3 types. Type B1 is related to systemic diseases, and has been identified in human patients with diabetes mellitus (DM), malignancy, bowel disease, pancreatitis, infection, renal failure, hepatic failure, and other conditions. Type B2 includes causes related to some drugs (as metformin, beta-adrenergic agents, salicylates, sulfasalazine, and toxins) and Type B3 is related to inborn defects of metabolism such as some enzymes deficiencies (7–9). This classification is considered over-simplified by some authors, as in critical illness there is overlap of factors that influence lactate dynamics (9).
Lactate concentration is significantly higher in type II diabetic human patients than in controls, and it is higher in patients with severe glucose intolerance than in patients with mild glucose intolerance (10). It has also been reported that lactate concentration is consistently higher in human patients with stable (non-complicated) type I DM, than in normal individuals (11).
Diabetic ketoacidosis (DKA) is considered the main endocrine emergency in small animals (12), characterized by fluid, electrolyte, and acid-base imbalance, and is frequently associated with comorbidities and high mortality rates (13,14). Lactic acidosis has been reported in human diabetic patients (11) and may occur in dogs with DKA, contributing to metabolic acidosis (1). It is important to differentiate between lactic acidosis and ketoacidosis in acidemic diabetic patients, as a different treatment is required for each condition (7,15). The motivation for this study was the lack of information in the veterinary literature regarding lactate levels in diabetic dogs, as in humans it has been determined that non-ketotic diabetic patients have higher lactate levels compared with non-diabetics. Therefore, the aim of this study was to determine if newly diagnosed diabetics and known diabetics undergoing treatment for a minimum of 30 d have higher blood lactate concentrations than normal individuals. An additional goal was to determine if lactate concentrations differ among non-ketotic diabetics compared with patients with DKA. As a secondary objective, we aimed to determine whether lactate levels were associated with an increased likelihood of negative outcomes for DKA dogs (death or euthanasia due to non-improvement of DKA symptoms).
Materials and methods
A prospective cross-sectional clinical study was conducted following ethical principles of animal research, approved by the Institution Animal Use & Ethics Committee (Protocol: 2542/2012). Client-owned dogs presented to the Veterinary Teaching Hospital of the School of Veterinary Medicine and Animal Science, University of São Paulo, Brazil (94 dogs) or to a private Veterinary Hospital (6 dogs) between January 2011 and August 2013, were recruited consecutively.
Dogs were included if they had a diagnosis of diabetes mellitus and had been under treatment with insulin for more than 30 d, if they were newly diagnosed non-ketotic diabetics and if they were diagnosed with DKA and were under treatment or newly diagnosed. The exclusion criterion for all the groups was the presence of any of the following comorbidities: acute hepatic failure, renal failure, heart failure, advanced respiratory disease, anticonvulsive therapy, sepsis, septic and cardiogenic shock, as these conditions may alter lactate levels. Dogs with DKA had similar exclusion criteria, with the exception of comorbidities that were linked to decompensation, which are indicated in the results section.
The dogs were assigned to 4 groups: 25 healthy controls and 75 diabetics, as follows: 25 newly diagnosed non-ketotic diabetics, 25 diabetics under insulin therapy, and 25 in DKA.
All dogs underwent a complete physical examination, hemogram [complete blood (cell) count (CBC)] (Horiba ABX; model ABC Vet, Montpellier, France), serum biochemical profile, (Labmax 240; Tokyo Boeki Machinery, Hatchobori, Chuo-ku, Tokyo, Japan), blood glucose, urinalysis, urine culture, and blood lactate concentration. Venous blood gas analysis was used to assess acid-base status and electrolytes. Ketosis was determined by whole blood β-hydroxybutyrate (β-OHB) concentration at admission, using the same glucometer with specific β-hydroxybutyrate strips (Abbott Diabetes Care, Witney, Oxon, England, UK). Considering that lactate concentration may vary depending on venous sampling site (2,16), blood samples were always obtained from the jugular vein.
Blood glucose was measured in a portable glucometer (Optium Xceed; Abbott Diabetes Care, Alameda, California, USA) and lactate concentrations, in a point-of-care blood analyzer (iSTAT; Abbott Point of Care, Abbott Laboratories, Abbott Park, Illinois, USA). Both were determined from whole blood, immediately after collection.
Blood glucose values obtained in the glucometer were compared to values obtained in the same sample, and sent to a clinical pathology laboratory for analysis. Considering that the average values were within the 95% confidence interval (CI), and this is not considered clinically relevant as it does not change the diagnosis, we decided to use the portable glucometer values, as this is the method mostly used in clinical practice (data not shown).
Venous blood gas analysis on the point-of-care blood analyzer (iSTAT; Abbott Point of Care) included pH, tCO2, pCO2, pO2, base excess (BE), HCO3, SO2, anion gap (AG), concentration of sodium (Na+), potassium (K+), cloride (Cl−), and ionized calcium (iCa2+).
Urine samples for analysis were obtained by cystocentesis. Urinary specific gravity was measured in a portable refractometer. Urine culture was performed in all dogs to determine the presence of urinary tract infection and to conduct antimicrobial susceptibility tests for treatment.
Diabetic dogs were allocated to the different groups based on blood glucose tests, blood gas analysis, and β-OHB concentration. For the diagnosis of DKA, and considering that there is no consensus in the veterinary literature for bicarbonate levels and blood pH (12,17,18), criteria recommended in human medicine were used: acidemia (blood pH < 7.3) or acidosis (HCO3− < 15 mmol/L) associated ketonemia and hyperglycemia (blood glucose > 13.9 mmol/L) (19), with or without ketonuria. The criteria were similar to those used by Durocher et al (20).
Statistical methods
Results are presented as median and percentiles (P25–75) for each group. The Shapiro-Wilk normality test was used to determine whether data followed Gaussian distribution. The Kruskal-Wallis test was used for comparison of groups for non-parametric quantitative variables, followed by Dunn’s test. Spearman correlation was applied to assess relations between lactate levels and glycemia, blood urea nitrogen (BUN), creatinine, anion gap, and β-hydroxybutyrate, as well as correlation between pH and lactate, BUN and creatinine. A Chi-squared test was used to compare all groups for gender distribution. Fisher’s exact test was applied to evaluate association between increased lactate levels and mortality risk. A Mann-Whitney test was used as a post-hoc subgroup analysis to compare DKA dogs that were already under treatment with DKA dogs that were newly diagnosed for analyzed variables (blood glucose, lactate, pH, anion gap, creatinine, BUN).
Spearman correlation was used to assess the relation between the measured variables of interest, including the whole study population. Fisher’s exact test was used to assess, as a secondary aim, whether lactate was associated with a negative outcome in DKA patients (death or euthanasia due to non-improvement of DKA symptoms).
Statistical analysis was performed using GraphPad Prism version 5.0 (San Diego, California, USA). Values of P < 0.05 were considered significant.
Results
Groups were equivalent with respect to gender and weight distribution. Diabetic groups were older than the control group (P < 0.0001) (Table 1).
Table 1.
Study population characteristics for gender, age, and weight by group distribution
| Variables | Control n = 25 |
At diagnosis n = 25 |
Under treatment n = 25 |
DKA n = 25 |
Statistic P-value |
|---|---|---|---|---|---|
| Gender/Condition, n (%) | |||||
| M 20 (55.9) | 8 (66.7) | 5 (83.3) | 4 (50) | 3 (37.5) | |
| CM 14 (41.2) | 4 (33.3) | 1 (16.7) | 4 (50) | 5 (62.5) | χ2 |
| F 30 (45.5) | 7 (53.8) | 11 (57.9) | 4 (23.5) | 8 (47.1) | 0.2372 |
| SF 36 (54.5) | 6 (46.2) | 8 (42.1) | 13 (76.5) | 9 (52.9) | |
| Age (months) | |||||
| Med | 53a | 118b | 120b | 120b | KW |
| (P25-P75) | (24–75.5) | (101.5–132) | (90–144) | (96–143) | < 0.0001 |
| Weight (kg) | |||||
| Med | 18 | 9.8 | 8.9 | 14 | KW |
| (P25-P75) | (12.45–32.7) | (6.2–20.5) | (5.3–20.8) | (7.9–27.25) | 0.071 |
DKA — diabetic ketoacidosis; M — male; CM — castrated male; F — female; SF — spayed female; χ2 — Chi-squared; KW — Kruskal-Wallis; Med — median; P25 — 25th percentile; P75 — 75th percentile.
Different letters between groups show differences by Dunn’s test (P < 0.05).
Mixed breed dogs were predominant (27%), followed by miniature poodle (13%), Labrador (8%), and dachshund (5%). Other breeds (bichon frise, bull terrier, bulldog, cocker spaniel, Dalmatian, Brazilian terrier, golden retriever, Siberian husky, lhasa apso, Maltese, German shepherd, miniature pinscher, pit bull, pug, Rottweiler, miniature schnauzer, shar-pei, and shih tzu) were less than 5% each.
Factors that may have contributed to DKA were urinary tract infection (n = 13), hyperadrenocorticism (n = 8), corticosteroid use (n = 7), mammary tumor, diestrus, obesity (n = 2 each), pancreatitis, abscess, and estrus (n = 1 each). Among the dogs with DKA, 15 were newly diagnosed cases of DM.
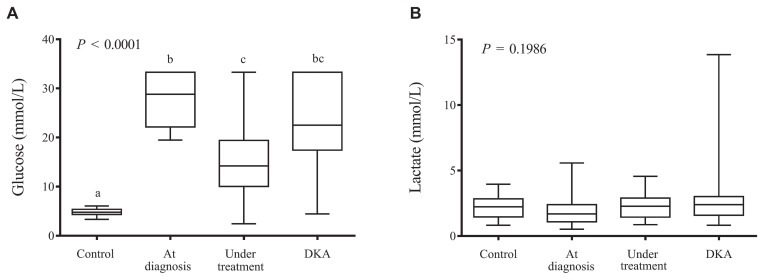
By definition, dogs in diabetic groups had higher glycemic values than controls. Blood glucose levels were significantly higher in dogs with newly diagnosed DM and those with DKA compared with those under treatment (P < 0.0001) (Figure 1A). The post-hoc subgroup analysis between dogs with DKA that were already under treatment and dogs with DKA that had been newly diagnosed showed that glycemia was higher in newly diagnosed DKA cases (23.8 versus 19.6 mmol/L; P = 0.045). None of the other measured variables differed.
Figure 1.
Box-and-whisker plots representing (A) glucose (mmol/L) and (B) lactate (mmol/L) concentration. Median and interquartile range (box), and minimum and maximum values (whiskers) in study groups (control, at diagnosis, under insulin treatment, DKA). Note that diabetic dogs under treatment show lower blood glucose values than those at diagnosis and DKA, but higher than controls (P < 0.05; Kruskal-Wallis/Dunn). Lactate concentrations were similar among groups.
Lactate concentrations were similar among the groups (Figure 1B; Table 2). Anion gap was higher in the DKA group compared with control and under treatment groups (P < 0.0001) (Table 2). There was no correlation between lactate and glycemia (r = 0.055; P = 0.62), and no correlation between lactate and anion gap (r = 0.149; P = 0.17). Correlations between variables are shown in Table 3. There was no association between lactate levels and outcome.
Table 2.
Comparison of measured variables among groups
| Variables | Control n = 25 |
At diagnosis n = 25 |
Under treatment n = 25 |
DKA n = 25 |
P-value |
|---|---|---|---|---|---|
| Lactate (mmol/L) | |||||
| Med | 2.23 | 1.69 | 2.27 | 2.29 | 0.2466 |
| (P25-P75) | (1.46–2.23) | (1.09–2.40) | (1.44–2.90) | (1.48–2.86) | |
| Glucose (mmol/L) | |||||
| Med | 4.77a | 28.81b | 14.21c | 22.51b,c | < 0.0001 |
| (P25-P75) | (4.33–5.38) | (22.15–33.31) | (10.02–19.40) | (17.43–33.31) | |
| Anion gap (mmol/L) | |||||
| Med | 14.0a | 14.5a,b | 13.0a | 17.0b | < 0.0001 |
| (P25-P75) | (13.0–15.5) | (12.2–16.0) | (10.0–14.0) | (16.0–21.0) | |
| Sodium (Na) (mmol/L) | |||||
| Med | 147.0a | 140.0b | 142.0b | 140.0b | < 0.0001 |
| (P25-P75) | (146.5–148.5) | (135.5–141.0) | (140.0–143.0) | (133.0–153.0) | |
| Potassium (K) (mmol/L) | |||||
| Med | 4.00a | 4.80b | 4.50b | 4.20a,b | < 0.001 |
| (P25-P75) | (3.65–4.30) | (4.40–5.05) | (4.30–4.80) | (3.00–5.09) | |
| Chloride (Cl) (mmol/L) | |||||
| Med | 117.0a | 108.0b | 114.0a,b | 110.0b | < 0.0001 |
| (P25-P75) | (115.0–118.5) | (102.9–114.0) | (110.0–115.8) | (107.3–126.0) | |
| Ionized calcium (iCa) (mmol/L) | |||||
| Med | 1.38a | 1.27b,c | 1.35a,b | 1.23c | < 0.0001 |
| (P25-P75) | (1.35–1.41) | (1.18–1.35) | (1.28–1.40) | (1.15–1.30) | |
| Beta-OHB (mmol/L) | |||||
| Med | 0.20a | 1.00b | 0.30a | 4.15b | < 0.0001 |
| (P25-P75) | (0.20–0.30) | (0.65–1.75) | (0.20–0.40) | (1.45–5.77) | |
| Urea nitrogen (BUN) (mmol/L) | |||||
| Med | 12.14a | 12.85a | 13.92a,b | 31.42b | < 0.001 |
| (P25-P75) | (9.64–14.28) | (8.92–16.78) | (10.12–18.74) | (9.99–71.76) | |
| Creatinine (mmol/L) | |||||
| Med | 0.08a | 0.07a,b | 0.06b | 0.11a | 0.0016 |
| (P25-P75) | (0.07–0.084) | (0.05–0.10) | (0.04–0.07) | (0.06–0.30) | |
DKA — diabetic ketoacidosis; Beta-OHB — beta-hydroxybutyrate; Med — median; P25 — 25th percentile; P75 — 75th percentile.
Different letters between groups show differences by Dunn’s test (P < 0.05).
Table 3.
Spearman’s Rank-Order Correlation between the measured variables of interest, including the whole study population (n = 100)
| Glycemia | BUN | Creatinine | pH | β-OHB | Anion gap | HCO3− | |
|---|---|---|---|---|---|---|---|
| Lactate | r = 0.05 | r = 0.09 | r = 0.02 | r = −0.16 | r = 0.01 | r = 0.15 | r = −0.12 |
| P = 0.62 | P = 0.37 | P = 0.83 | P = 0.11 | P = 0.91 | P = 0.17 | P = 0.23 | |
| Glucose | r = 0.25 | r = 0.005 | r = −0.18 | r = 0.66 | r = 0.07 | r = −0.10 | |
| P = 0.02 | P = 0.96 | P = 0.07 | P < 0.0001 | P = 0.53 | P = 0.32 | ||
| BUN | r = 0.48 | r = −0.20 | r = 0.23 | r = −0.36 | r = −0.06 | ||
| P < 0.0001 | P = 0.048 | P = 0.02 | P = 0.0007 | P = 0.54 | |||
| Creatinine | r = −0.18 | r = 0.17 | r = −0.11 | r = −0.14 | |||
| P = 0.08 | P = 0.09 | P = 0.30 | P = 0.17 | ||||
| pH | r = −0.47 | r = −0.37 | r = 0.68 | ||||
| P < 0.0001 | P = 0.0005 | P < 0.0001 | |||||
| β-OHB | r = 0.30 | r = −0.45 | |||||
| P = 0.005 | P < 0.0001 | ||||||
| Anion gap | r = −0.61 | ||||||
| P < 0.0001 |
BUN — blood urea nitrogen; β-OHB — beta-hydroxybutyrate; HCO3− — bicarbonate. Significant correlations are indicated in bold font within the table.
Discussion
Lactate concentration in diabetic dogs, newly diagnosed and under treatment, as well as in dogs with DKA was not significantly different from controls (Figure 1B), in this population. The lack of significant difference on lactate concentration among the groups may have been due to the study design, i.e., cross-sectional; in the study on non-insulin-dependent diabetic humans, the patients were followed for 24 h with sequential lactate measurements. In that study, non-obese patients with moderate-to-severe glucose intolerance had higher lactate levels compared to patients with normal glucose tolerance (10).
Our results also differed from a study that evaluated acid-base abnormalities and found higher lactate concentration in diabetic dogs compared to controls, with median values of 2.0 and 1.0 mmol/L, respectively; although all results were within normal ranges for dogs (20).
One possibility for not finding increased lactate levels in dogs with DKA, other than study design, may be that none of the dogs were in hypovolemic shock and, therefore, did not have the most common cause of hyperlactatemia (type A lactic acidosis). In DM, inadequate oxygen utilization by the tissues may also play a role in lactate increase rather than deficient oxygen supply (21). Considering the exclusion criteria, none of the dogs had systemic diseases, other than DM, that could account for type B1 lactic acidosis (7–9), except in a few of the DKA cases, which may not constitute enough power to detect a difference.
The positive correlation between lactate and glucose concentration noted by other authors (10,22) was not observed in this study. This finding may also be related to the fact that we had no dogs in critical condition, due to the adopted exclusion criteria. Lactate blood concentration was not correlated with any of the measured variables for this study population. In human patients with DKA, a significant but weak correlation between β-OHB and glucose levels was observed on admission (23). In the present study, there was a moderate correlation between glycemia and β-OHB. This correlation was stronger than that reported in humans, likely because a larger range of glycemia and β-OHB blood levels was taken into account in the present study (the study population included not only DKA, but nonketotic diabetics that were newly diagnosed, non-ketotic diabetics under insulin treatment that were clinically stable, and healthy dogs). This association is understandable based on the pathophysiology of DKA. An absolute or relative insulin deficiency or insulin resistance facilitated by a comorbidity and secondary increase in cortisol, epinephrine, and glucagon leads to increased breakdown of triglycerides due to cellular starvation, with consequent production of β-OHB, derived from Acetyl-Coenzyme A (24,25).
Glycemia levels were positively correlated with BUN, but not with creatinine in this cohort. This is probably related to pre-renal azotemia secondary to increased diuresis related to glucosuria (osmotic diuresis), but there are other possibilities.
Blood urea nitrogen levels can rise in situations of increased production of urea, by excessive protein catabolic rate, decrease in glomerular filtration rate, and increase in tubular urea absorption, influenced by antidiuretic hormone (ADH) (26–28). The clinical syndrome of pre-renal or functional renal failure, also referred to as pre-renal azotemia, is characterized by a disproportionate increase in BUN in relation to creatinine (high BUN to creatinine ratio) and a low urine volume, in the presence of an intact renal parenchyma, and related to renal hypoperfusion (27,28). There are, however, situations in which urine flow may be high (28), as with corticosteroid use and osmotic diuresis (as happens in DM). In a simplistic way, disproportionate BUN increase is often attributed only to a decreased glomerular filtration rate (27,29). However, the antidiuretic hormone released in response to hypovolemia and/or hypotension, also plays a role as it increases reabsorption of both water and urea in the distal renal tubules (30), based on that fact that urea can “back-diffuse” into papillary interstitium under the influence of ADH (28). Both hypovolemia and increased plasma osmolarity contribute to ADH secretion; therefore, hypovolemia was probably not the only factor involved, since DKA patients may have increased plasma osmolarity because of hyperglycemia. Other causes for volume depletion are extra-renal fluid loss (vomiting, diarrhea).
Diabetic ketoacidosis patients may have all these contributions to the enhanced blood urea concentration, as pre-renal factors. Besides those, gastrointestinal (GI) bleeding cannot be ruled out, although none of our patients had overt GI blood loss. We did not assess the amount of protein intake in these dogs, and the DKA dogs were anorexic. In dogs with DKA, there is an increased protein catabolic state (26), which may also contribute to increased plasma BUN.
The negative weak correlation between BUN and pH may be explained by a metabolic acidemia related to azotemia. The moderate negative correlation between β-OHB and blood pH is in agreement with the ketoacidosis state.
Hypoperfusion and ketosis are not the exclusive reasons to explain the acidic state in DKA, as changes in glucose metabolism and reduction of lactate removal from circulation may play a role (1,6), which deserves more investigation regarding DM pathophysiology and its complications.
The equipment routinely used for lactate measurement assesses L-lactate isomer, the predominant portion derived from pyruvic acid breakdown in anaerobic conditions. D-lactate is produced from methylglyoxal (MG) and is increased in diabetic humans and animals, possibly caused by increased production of its precursor in these subjects (31,32). The handheld analyzer (i-STAT) used herein for measurement of lactate, measures only the L-lactate isomer. This may be the reason why we could not find differences among groups, especially between control and diabetics. The studies that report differences in lactate concentration do not specify whether L- or D-lactate had been measured (10,20).
An experimental study showed that plasma, liver, and skeletal muscle from diabetic rats had increased D-lactate compared with that of control rats, while L-lactate was not increased in plasma, but was elevated in the liver and decreased in muscle. In this same study, excretion of D-lactate was higher than L-lactate and diabetic rats excreted more D-lactate than L-lactate compared with controls (33). A positive correlation was observed between MG and D-lactate in humans, but not with L-lactate (31).
In humans with DKA, D-lactate is associated with metabolic acidosis and increased anion gap (31,34). A contribution of L-lactate to the increased AG in DKA was not found in dogs in one study (20). In order to determine whether diabetic dogs and dogs with DKA also have increased D-lactate, specific methods must be used, as the ones routinely used measure only L-lactate.
Consistently elevated lactate has been reported in stable human patients with Type I DM compared with non-diabetics, and 10% to 15% of DKA patients have elevated lactate concentrations (> 5 mmol/L) (11). A similar percentage (~12%) was observed in another study in humans (23). In the present study, 16% of dogs in DKA had a lactate concentration > 5 mmol/L, even though the comparison of groups did not show a significant difference for lactate values. Lactate levels were not associated with outcome in the DKA group; however, this was a secondary analysis and may be underpowered to answer this question.
In conclusion, the most common clinically measured lactate isomer, L-lactate, plasma concentration was not different in diabetic dogs, compared with healthy dogs. Dogs with DKA may present with a higher lactate concentration depending on volemia status, related to tissue perfusion; however, this association was not observed herein, probably because the patients were not severely hypovolemic. Longitudinal studies that assess both isomers (L- and D-lactate) and include more severely ill diabetic dogs are needed to better elucidate the role of these isomers in the pathophysiology of diabetes acid-base status in dogs.
Acknowledgments
The authors thank the entire Veterinary Hospital of the School of Veterinary Medicine and Animal Science, University of São Paulo (USP). We also acknowledge the institutional support, the Department of Internal Medicine (VCM) FMVZ-USP, and a financial grant by FAPESP (Process n. 2011-21116-1) and CAPES. We also thank Ms. Maria Amelia Ahren Beraldo for reviewing the French translation of the abstract. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Allen SE, Holm JL. Lactate: Physiology and clinical utility. J Vet Emerg Crit Care. 2008;18:123–132. [Google Scholar]
- 2.Hughes D, Rozanski ER, Shofer FS, Laster LL, Drobatz KJ. Effect of sampling site, repeated sampling, pH, and PCO2 on plasma lactate concentration in healthy dogs. Am J Vet Res. 1999;60:521–524. [PubMed] [Google Scholar]
- 3.Nemec A, Pecar J, Seliskar A, Kompan L, Butinar J. Assessment of acid-base status and plasma lactate concentrations in arterial, mixed venous and portal blood from dogs during experimental hepatic blood inflow occlusion. Am J Vet Res. 2003;64:599–608. doi: 10.2460/ajvr.2003.64.599. [DOI] [PubMed] [Google Scholar]
- 4.Thorneloe C, Bédard C, Boysen S. Evaluation of a hand-held lactate analyzer in dogs. Can Vet J. 2007;48:283–288. [PMC free article] [PubMed] [Google Scholar]
- 5.Tas O, De Rooster H, Baert E, Doom MH, Duchateau L. The accuracy of the Lactate Pro hand-held analyser to determine blood lactate in healthy dogs. J Small Anim Pract. 2008;49:504–508. doi: 10.1111/j.1748-5827.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- 6.Zacher LA, Berg J, Shaw SP, Kudej RK. Association between outcome and changes in plasma lactate concentration during presurgical treatment in dogs with gastric dilatation-volvulus: 64 cases (2002–2008) J Am Vet Med Assoc. 2010;236:892–897. doi: 10.2460/javma.236.8.892. [DOI] [PubMed] [Google Scholar]
- 7.Kovacic JP. Lactic acidosis. In: Silvesrtain DC, Hopper K, editors. Small Animal Critical Care Medicine. St Louis, Missouri: Saunders-Elsevier; 2009. [Google Scholar]
- 8.Gunnerson KJ, Harvey CE. Lactic Acidosis. [Last accessed June 14, 2017]. Updated: Apr 25, 2016. Available from: http://emedicine.medscape.com/article/167027-overview#a5.
- 9.Phypers B, Pierce JM. Lactate physiology in health and disease. Cont Educ Anaesth Crit Care Pain. 2006;6:128–132. [Google Scholar]
- 10.Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YD. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes. 1988;37:1020–1024. doi: 10.2337/diab.37.8.1020. [DOI] [PubMed] [Google Scholar]
- 11.Becker KL. Principles and Practice of Endocrinology and Metabolism. 3rd ed. Philadelphia, Pennsylvania: Lippincott William & Wilkins; 2001. Disorders of fuel metabolism; pp. 1448–1449. [Google Scholar]
- 12.Macintire DK. Treatment of diabetic ketoacidosis in dogs by continuous low-dose intravenous infusion of insulin. J Am Vet Med Assoc. 1993;202:1266–1272. [PubMed] [Google Scholar]
- 13.Nichols R, Crenshaw KL. Complications and concurrent disease associated with diabetic ketoacidosis and other severe forms of diabetes mellitus. Diabetes Mellitus. 1995;25:617–650. doi: 10.1016/s0195-5616(95)50057-9. [DOI] [PubMed] [Google Scholar]
- 14.Boysen SR. Fluid and electrolyte therapy in endocrine disorders: Diabetes mellitus and hypoadrenocorticism. Vet Clin North Am Small Anim Pract. 2008;38:699–717. doi: 10.1016/j.cvsm.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Castagnino J, Reussi RR, Mercado J, Torreno M, Tajer C, Charask A. Niveles elevados de lactato medidos a la cabecera del paciente se asocian a mayor riesgo de muerte em cuidados intensivos. Revista de La Associación Médica Argentina. 1998;111:10–16. [Google Scholar]
- 16.Ferasin L, Nguyenba TP. Comparison of canine capillary and jugular venous blood lactate concentrations determined by use of an enzymatic-amperometric bedside system. Am J Vet Res. 2008;69:208–211. doi: 10.2460/ajvr.69.2.208. [DOI] [PubMed] [Google Scholar]
- 17.Bruyette DS. Diabetic ketoacidosis. Semin Vet Med Surg (Small Anim) 1997;12:239–247. doi: 10.1016/s1096-2867(97)80016-3. [DOI] [PubMed] [Google Scholar]
- 18.Feldman EC, Nelson RW. Canine and Feline Endocrinology and Reproduction. 3rd ed. Philadelphia, Pennsylvania: WB Saunders; 2004. Canine diabetes mellitus; pp. 580–615. [Google Scholar]
- 19.American Diabetes Association. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 20.Durocher LL, Hinchcliff KW, Dibartola SP, Johnson SE. Acid-base and hormonal abnormalities in dogs with naturally occurring diabetes mellitus. J Am Vet Med Assoc. 2008;232:1310–1320. doi: 10.2460/javma.232.9.1310. [DOI] [PubMed] [Google Scholar]
- 21.Blomkalns AL. Lactate — A Marker for Sepsis and Trauma. [Last accessed June 14, 2017];Emergency Medicine Cardiac Research and Education Group. 2006 2:1–6. Available from: http://www.emcreg.org/pdf/monographs/lactate06.pdf. [Google Scholar]
- 22.Cox K, Cocchi M, Salciccioli J, Carney E, Howell M, Donnino MW. Prevalence and significance of lactic acidosis in diabetic ketoacidosis. J Crit Care. 2012;27:132–137. doi: 10.1016/j.jcrc.2011.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheikh-Ali M, Karon BS, Basu A, et al. Can serum β-hydroxybutyrate be used to diagnose diabetic ketoacidosis? Diabetes Care. 2008;31:643–647. doi: 10.2337/dc07-1683. [DOI] [PubMed] [Google Scholar]
- 24.Stojanovic V, Ihle S. Role of beta-hydroxybutyric acid in diabetic ketoacidosis: A review. Can Vet J. 2011;52:426–430. [PMC free article] [PubMed] [Google Scholar]
- 25.Henderson DW, Schlesinger DP. Use of a point-of-care beta-hydroxybutyrate sensor for detection of ketonemia in dogs. Can Vet J. 2010;51:1000–1002. [PMC free article] [PubMed] [Google Scholar]
- 26.Adrogué HJ. Fluid-electrolyte and acid-base disorders complicating diabetes mellitus. In: Schrier RW, editor. Diseases of the Kidney and Urinary Tract. 8th ed. Philadelphia, Pennsylvania: Lippincott Williams & Wilkins; 2007. pp. 2353–2378. [Google Scholar]
- 27.Feinfeld DA, Bargouthi H, Niaz Q, Carvounis CP. Massive and disproportionate elevation of blood urea nitrogen in acute azotemia. Int Urol Nephrol. 2002;34:143–145. doi: 10.1023/a:1021346401701. [DOI] [PubMed] [Google Scholar]
- 28.Blantz RC. Nephrology forum: Pathophysiology of pre-renal azotemia. Kidney Int. 1998;53:512–523. doi: 10.1046/j.1523-1755.2003_t01-1-00784.x. [DOI] [PubMed] [Google Scholar]
- 29.Morgan DB, Carver ME, Payne RB. Plasma creatinine and urea:creatinine ratio in patients with raised plasma urea. Brit Med J. 1977;2:929–932. doi: 10.1136/bmj.2.6092.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badr KF, Ichikawa I. Prerenal failure: A deleterious shift from renal compensation to decompensation. N Engl J Med. 1988;319:623–629. doi: 10.1056/NEJM198809083191007. [DOI] [PubMed] [Google Scholar]
- 31.Lu J, Zello GA, Randell E, Adeli K, Krahn J, Meng QH. Closing the anion gap: Contribution of D-lactate to diabetic ketoacidosis. Clin Chim Acta. 2012;412:286–291. doi: 10.1016/j.cca.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 32.Tsutsui H, Mochizuki T, Maeda T, et al. Simultaneous determination of DL-lactic acid and DL-3-hydroxybutyric acid enantiomers in saliva of diabetes mellitus patients by high-throughput LC-ESI-MS/MS. Anal Bioanal Chem. 2012;414:1925–1934. doi: 10.1007/s00216-012-6320-0. [DOI] [PubMed] [Google Scholar]
- 33.Kondoh Y, Kawase M, Kawakami Y, Ohmori S. Concentrations of D-lactate and its related metabolic intermediates in liver, blood, and muscle of diabetic and starved rats. Res Exp Med (Berl) 1992;192:407–414. doi: 10.1007/BF02576298. [DOI] [PubMed] [Google Scholar]
- 34.Forni LG, Mckinnon W, Lord GA, Treacher DF, Peron JM, Hilton PJ. Circulating anions usually associated with the Krebs cycle in patients with metabolic acidosis. Crit Care. 2005;9:591–595. doi: 10.1186/cc3806. [DOI] [PMC free article] [PubMed] [Google Scholar]