Abstract
PURPOSE
We aimed to determine dual-energy computed tomography (DECT) characteristics of colorectal cancer and investigate effectiveness of DECT method in differentiating tumor from stool in patients with colorectal cancer.
METHODS
Fifty consecutive patients with colorectal tumors were enrolled. Staging was performed by DECT (80–140 kV) using dual-source CT after rectal air insufflation and without bowel preparation. Both visual and quantitative analyses were performed at 80 kV and 140 kV, on iodine map and virtual noncontrast (VNC) images.
RESULTS
All colorectal tumors had homogeneous pattern on iodine map. Stools demonstrated heterogeneous pattern in 86% (43/50) and homogeneous pattern in 14% (7/50) on iodine maps and were less visible on VNC images. Median density of tumors was 54 HU (18–100 HU) on iodine map and 28 HU (11–56 HU) on VNC images. Median density of stool was 36.5 HU (8–165 HU) on iodine map and −135.5 HU (−438 HU to −13 HU) on VNC images. The density of stools was significantly lower than tumors on both iodine map and VNC images (P < 0.001). The cutoff point of density measurement on VNC images was −1 HU with area under the curve of 1 and a sensitivity and specificity of 100%.
CONCLUSION
Density or visual analysis of iodine map and VNC DECT images allow accurate differentiation of tumor from stool.
Computed tomography (CT) colonography is a noninvasive method for colorectal carcinoma screening and was shown to be highly sensitive for colorectal cancer and polyp detection in previous studies (1–4). The major problem for diagnostic and screening CT colonography is differentiation of tumor and polyps from stool. Therefore, several methods are used for this purpose such as prone and supine imaging (looking for movement of stool), use of laxatives (to clear stool from colon), barium tagging (to mark stools with barium) and density measurements (5). However, some patients can refuse or cannot tolerate bowel preparation leading to cancellation of CT colonography examinations. For patients who do not tolerate bowel preparation, laxative-free or reduced preparation protocols have been developed.
Dual-energy computed tomography (DECT) is becoming increasingly used for body computed tomography (CT) applications (6–8). The major advantage of DECT is its ability to obtain iodine map and virtual noncontrast (VNC) images in a single CT study by acquiring two datasets with different kilovolts. On contrast-enhanced single-energy CT images, the density of a measured area is equal to basal density of the area plus the density increment due to iodine uptake. DECT allows measurement of basal density and the degree of iodine uptake on VNC and iodine map images, respectively, without acquiring unenhanced CT images (9). Pilot studies reported utility of DECT principle for diagnostic CT colonography and electronic cleansing after barium tagging for CT colonography (5, 10, 11). Recent studies showed that colorectal cancer can be detected without bowel preparation by DECT (12) and DECT can be used as an accurate staging method for patients with colorectal cancer (13).
The current study aims to determine DECT characteristics of colorectal cancer and to investigate the effectiveness of DECT method in differentiating tumor from stool in patients who were diagnosed with cancer by optical colonoscopy and biopsy.
Methods
This prospective study was approved by the Institutional Review Board of our university and all patients signed the informed consent. Data of the patients were collected from hospital information system.
Study population
The study enrolled 50 consecutive patients (35 male and 15 female patients) meeting the inclusion criteria, who underwent DECT for staging of colorectal tumors at our department from June 2011 to November 2012. Inclusion criteria of the study were diagnosis of colon tumor detected on colonoscopy and/or clinical history and physical examination, body weight ≤118 kg and waist circumference ≤90 cm (mean body weight was 75.9±12.9 kg). Exclusion criteria were having a waist circumference over 90 cm, a body weight over 118 kg, and a previous colon cancer surgery.
Dual-energy CT acquisition
Images were obtained using DECT method by the first generation of dual-source CT (Definition, Siemens Medical Systems). No bowel preparation or diet was given and patients were scanned at least five days after biopsy. Patients started drinking 1 L water without any contrast one hour before the examination. Images were obtained at supine position after rectal air insufflation. Iodinated contrast agent (350 mg/mL, 120 mL) was administered by power injector at a rate of 3 mL/s. CT acquisition started at 70 seconds after the injection. Technical parameters were as follows: detector configuration, 2×2×32; tube voltage, 140kV/80kV, slice thickness, 1.5 mm, and pitch, 1. Axial images (1.5 mm and 5 mm) were transferred to a workstation (Leonardo, Siemens Medical Systems). 80 kV and 140 kV images reconstructed by dual-energy kernel were loaded to dual-energy software application and iodine map, and VNC and mixed images were obtained. Dose-length product values were recorded to estimate the radiation dose.
Image analysis
VNC, iodine map and mixed DECT images were recorded. Mixed DECT images provided 80 kV and 140 kV density values on single image. Visual analyses of these images were performed by two radiologists in consensus (with 4 and 16 years of CT experience). Homogeneous pattern on iodine map is defined as smooth appearance without dark areas; heterogeneous pattern is defined as mottled appearance with alternating dark and bright areas (Fig. 1). Density measurements were made on tumor and stool (n=50, 100%), polyps (n=8, 16%), hepatic metastasis (n=6, 12%) and extra pathologies such as renal cell carcinoma (n=2, 4%), peritoneal involvement (n=2, 4%) and lymphadenopathy (n=7, 14%), at 80 kV and 140 kV, on iodine map and VNC images. Density measurements were made on these images by a radiologist using a circular region-of-interest of approximately 10 mm2. In addition to density measurements, distension level of six colon segments (cecum, ascending colon, transverse colon, descending colon, sigmoid, and rectum) were scored as good, moderate, or poor.
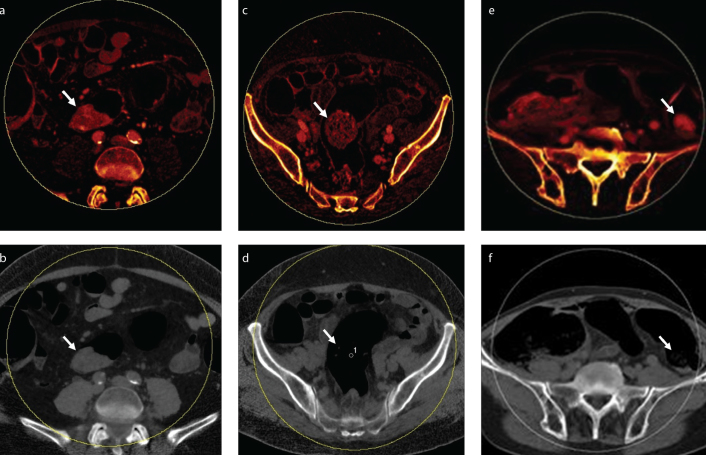
Figure 1. a–f.
Axial dual-energy computed tomography (DECT) images show characteristics of tumor and stool. Tumors had homogeneous pattern on iodine map (a) and appeared similar in size on virtual noncontrast (VNC) image (b). Stool had heterogeneous pattern on iodine map (c) and was less visible on VNC image (d) in most of the patients. In minority of the patients, stool appeared homogeneous on iodine map (e), but it was less visible on VNC image (f).
Optical colonoscopy
Video endoscopes (Olympus Optical Co.) were used for all procedures. The patients were prescribed polyethylene glycol lavage bowel preparation or equivalent, and the examiners cleaned the colon during instrument insertion and withdrawal as much as possible. Colorectal carcinomas and polyps were identified mainly during withdrawal. The method of retrieval was at the endoscopist’s discretion: retrieval net or tripod.
Histologic assessment
Pathologic evaluations were performed by a gastrointestinal pathologist. All collected specimens were fixed in 10% formalin within one hour of the removal and then fixed for a minimum of four hours. Then the fixed specimens were cut into 2 mm slices according to standard pathology laboratory protocols.
Statistical analysis
Data of all study participants were statistically analyzed. Mann-Whitney U test was used for independent group comparisons, depending on the distributional properties of the data. Receiver operating characteristic (ROC) curves were used to describe and compare the performance of diagnostics value of DECT. Cutoff ranges were calculated around the optimal cutoff to maximize sensitivity and specificity based on the Youden index to differentiate tumor from stool. The area under the corresponding curves (AUC) was calculated. The comparison of density measurement values in subgroups of differentiation degree of adenocarcinoma and tumor localization were performed with Kruskal-Wallis or one-way ANOVA tests. The relation between density measurements and tumor length and thickness was examined with Spearman’s correlation coefficient. A P value < 0.05 was considered as statistically significant. All analyses were performed by using IBM SPSS Statistics for Windows, Version 20.0.
Results
A total of 50 patients (35 men and 15 women) with histopathology-proven colorectal tumors (colon adenocarcinoma, n=48, and malignant melanoma n=2) were included in the study. The mean age of the study population was 59.5±13.3 years (range, 27–85 years). Mean body mass index of the patients was 26±4.3 kg/m2. Location of the tumors were cecum (n=3, 6%), ascending colon (n=2, 4%), hepatic flexure (n=2, 4%), transverse colon (n=5, 10%), splenic flexure (n=2, 4%), descending colon (n=2, 4%), sigmoid colon (n=6, 12%), rectosigmoid colon (n=9, 18%), and rectum (n=19, 38%). Median length and thickness of the tumors was 5 cm (2–12 cm) and 2 cm (0.5–5 cm), respectively.
Mean dose-length product was 511±121 mGy·cm and mean estimated dose was 8.2 mSv. Distension levels of colon segments are given in Table 1. Moderate and good distension level was observed in 77% of all patients. According to the visual analyses, all tumors had homogeneous pattern on iodine map. Stools demonstrated heterogeneous pattern in 86% (43/50) and homogeneous pattern in 14% (7/50) of the patients on iodine map. Stools with homogeneous (pseudolesions) and heterogeneous pattern were not visualized on VNC images, but all tumors were visualized on VNC images similar to their appearance on the iodine maps (Fig. 1).
Table 1.
The distention levels of colon segments
| Good, n (%) | Moderate, n (%) | Poor, n (%) | |
|---|---|---|---|
| Cecum | 25 (50) | 16 (32) | 9 (18) |
| Ascending colon | 25 (50) | 21 (42) | 4 (8) |
| Transverse colon | 19 (38) | 27 (54) | 4 (8) |
| Descending colon | 6 (12) | 33 (66) | 11 (22) |
| Sigmoid colon | 6 (12) | 24 (48) | 20 (40) |
| Rectum | 15 (30) | 16 (32) | 19 (38) |
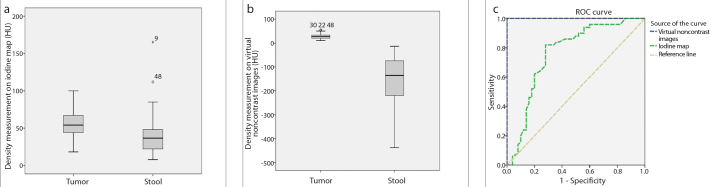
Median density of colon tumors was 54 HU (18–100 HU) on iodine maps and 28 HU (11–56 HU) on VNC images (Fig. 2). Median density of stool was 36.5 HU (8–165 HU) on iodine maps and −135.5 HU (−438 HU to −13 HU) on VNC images (Fig. 3). Densities of colon tumors and stool were significantly different on both iodine maps and VNC images (P < 0.001; Fig. 4, Table 2).
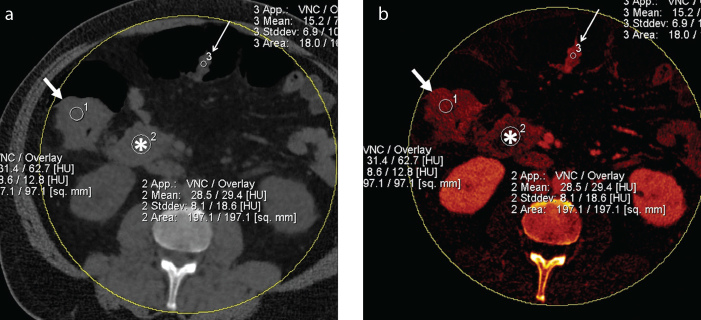
Figure 2. a, b.
Axial mixed (a) and iodine map (b) DECT images of a patient with ascending colon adenocarcinoma (thick arrow) showed homogeneous pattern on iodine map. Density on iodine map due to iodine uptake is 62.7 HU (overlay) and basal density of tumor on VNC image is 31.4 HU. Note mesenteric lymphadenopathy (asterisk) and polyp (thin arrow) in the transverse colon, which appeared similar to tumor.
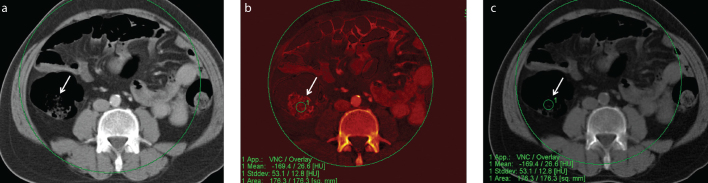
Figure 3. a–c.
Axial mixed (a), iodine map (b) and VNC (c) DECT images of a patient with stool in ascending colon (arrow) showed heterogeneous pattern on iodine map, which was less visible on VNC image. Density on iodine map was 26.6 HU (overlay) and basal density was −169.4 HU.
Figure 4. a–c.
The boxplots show the comparison of density measurements on iodine map (a) and VNC images (b) between tumor and stool. Graph shows the receiver operating curves of density measurements on iodine map and VNC images for differentiation of tumor from stool (c).
Table 2.
The density characteristics of tumor and stool in the study population
| Tumor | Stool | P | |
|---|---|---|---|
| Iodine map (HU) | 54 (18–100) | 36.5 (8–165) | <0.001 |
| VNC images (HU) | 28 (11–56) | −135.5 (−438 to −13) | <0.001 |
Data are presented as median (range).
HU, Hounsfield Unit; VNC, virtual noncontrast.
The cutoff point of density measurement on iodine map was 43.5 HU (area under the curve, 0.76; 95% confidence interval [CI], 0.66–0.86, P <0.001) to differentiate tumor from stool, with a sensitivity of 82% and a specificity of 72%. The cutoff point of density measurement on VNC images was −1 HU (area under the curve, 1.00; 95% CI, 1.00–1.00, P < 0.001) to differentiate tumor from stool, with a sensitivity of 100% and a specificity of 100% (Fig. 4c).
A total of eight polyps were identified in six patients and mean density of polyps were 70±8 HU, 16±5 HU, on iodine maps and VNC images, respectively. Four of these eight polyps were not detected with optical colonoscopy. Local serosal involvement was detected in 8% of patients (4/50). Lymphadenopathy was detected in nearby mesocolon in 30% of patients (15/50). Also, synchronous renal tumor was found in 4% of patients (2/50). DECT measurement values of these pathologies are given in Table 3.
Table 3.
Dual-energy measurements of polyps, peritoneal involvement, and paraaortic-paracaval lymphadenopathies
| Polyp (n=8, 16%) | Liver metastasis (n=6, 12%) | Peritoneal involvement (n=2, 4%) | Lymphadenopathy (n=7, 14%) | |
|---|---|---|---|---|
| Iodine map (HU) | 70±8 | 26±7 | 73±11 | 38±14 |
| VNC images (HU) | 16±5 | 15±10 | 30±4 | 30±5 |
Data are presented as mean±SD.
HU, Hounsfield Unit; VNC, virtual noncontrast; SD, standard deviation.
Adenocarcinoma was diagnosed in tumor biopsy specimens in 96% of patients (48/50). Of all diagnosed cases, 75% (36/48) were moderately differentiated adenocarcinoma, 17% (8/48) were well-differentiated adenocarcinoma and 8% (4/48) were poorly differentiated adenocarcinoma. Malignant melanoma was found in 4% of biopsy specimens (2/50). No significant difference was observed in density measurements among subgroups of differentiation degree of adenocarcinoma (P = 0.261 for iodine map; P = 0.921 for VNC images) and tumor localization (P = 0.976 for iodine map; P = 0.876 for VNC images). There was no correlation between density measurements and tumor length (P = 0.512, rs=−0.095 for iodine map; P = 0.228, rs=0.174 for VNC images) and thickness (P = 0.459, rs=−0.107 for iodine map; P = 0.050, rs=0.278 for VNC images).
Anterior resection was performed in 20% of patients (10/50). Low anterior resection, right hemicolectomy and left hemicolectomy were preferred in 34% (17/50), 20% (10/50), and 2% (1/50) of patients, respectively, and loop colostomy and loop ileostomy were performed in 4% of patients (2/50), since they were inoperable. Of all patients, 8% (4/50) underwent subtotal colectomy and %12 (6/50) did not undergo surgery.
Discussion
In this study, DECT characteristics accurately differentiated colorectal tumor from stool both by visual analysis and by density measurements on iodine map and VNC images. According to the visual analyses, we observed a homogeneous pattern in all tumors on iodine map; but same pattern was also seen in stools on iodine maps in a minority of the patients. In these cases, we were able to differentiate tumor and stool by looking at VNC images. Stools were less visible, but tumors were similar in size on VNC images. There were overlaps between density measurements of tumor and stool on iodine maps by quantitative analyses, but no overlap was observed on VNC images. Polyps, metastases, and renal cell carcinomas were also detected with this technique.
Laxative use and having to scan the patient in supine and prone positions are the two major disadvantages of the clinical use of screening CT colonography technique recommended by the American Cancer Association, which may prompt some patients to decline the use of CT for screening (14–18). Previously, Fini et al. (19) showed that noncathartic CT colonography is an effective screening method in relatives of patients with colorectal cancer. In the present study, we could differentiate tumors from stools by DECT without laxative use. This protocol may be offered to patients over 50 years of age, who will undergo abdominal CT for an indication other than screening, and it can lead to screening of more patients. This technique may also be useful for detecting polyps since they have similar enhancement characteristics with colon tumor, without use of bowel laxatives.
For single-energy CT scanning density measurements, unenhanced image must be obtained to detect the degree of iodine uptake caused by injection of contrast. DECT allows simultaneous calculation of basal density of a lesion on VNC images and iodine uptake on iodine maps in a single CT acquisition. Also pattern analysis on iodine map and VNC images can be incorporated, which can help in diagnosis and detection of colon tumors. Computer-aided diagnosis algorithms, which are used for virtual CT colonography, detect lesions primarily based on shape and density characteristics. But with DECT colonography procedure, density characteristics of tumors can be measured and these values can help to create new algorithms based on enhancement characteristics by computer-aided diagnosis (11). Schaeffer et al. (13) reported detection of colon carcinomas in 95% and synchronous lesions in 71% of their patients with use of 25 HU enhancement threshold by DECT technique and did not report density values on VNC images. However, in our study, density characteristics on VNC images and visual analysis of VNC and iodine map images enabled distinction of tumor from stool, in all patients. Better performance of VNC images in differentiation of stool and mass might be due to fat content.
Recently, Boellaard et al. (12) investigated the feasibility of colorectal cancer detection by DECT without bowel preparation or bowel distension. They detected all colorectal cancers during the unblinded reading and 90%–96.7% of them during the blinded reading. Similar to their study, no bowel preparation was given to the patients in our study. However, distention level of the colon segments, which is a critical issue in image analysis, was moderate or good in majority of the colon segments, which enhanced the visibility of the lesions.
The main disadvantages of DECT technique are the necessity of dual-energy software and hardware in the CT device, field-of-view limitation (with dual-source CT scanners) and slightly increased radiation exposure (6, 7, 10). However, CT machines with dual-energy properties are increasingly used by many centers. Field-of-view limitation is considerably solved with new generation dual-source DECT scanners and fast kVp switching methods, respectively, and it is possible to scan more obese patients now (6, 7). Apart from dual-source DECT and kVp switching, there are layered detectors that have recently become commercially available as well (20). Regarding radiation dose, newer DECT scanners allow dose neutral image acquisition; also, lack of unenhanced CT with this protocol can allow substantial dose reduction for diagnostic DECT colonography (6, 9). Furthermore, Lambert et al. (21) showed that the diagnostic performance of sub-milliSievert ultra-low dose CT colonography is suitable to be used with both hybrid and iterative model reconstruction techniques.
While the feasibility of detection of tumors and polyps is demonstrated by this study, further studies are required to assess the effectiveness of the technique. There can be two practical implications of this study. First, evaluation of iodine map and VNC images by visual analysis can enable quick check for tumors, and second, incorporation of VNC density analysis for computer-aided diagnosis algorithms can allow distinction of tumor from stool. This may be important in the follow-up of the colorectal cancer patients, which may obviate use of frequent optical colonoscopy in detection of recurrence and metachronous tumors (22).
Our study has some limitations. First, our population is comprised of patients with known tumors. Further studies are needed to demonstrate efficacy of this technique in patient populations with low tumor prevalence. Second, we did not include patients with a waist circumference above 90 cm, because of limited field of view (26 cm) of our CT scanner for DECT application. Third, although we observed polyps in addition to primary colonic tumors, we did not intend to test the effectiveness of DECT for polyp detection. Fourth, the size of the cohort is relatively small. We did not find a correlation between density measurements and tumor sizes. However, we did not measure the size of the stools, which could affect DECT characteristics.
In conclusion, DECT characteristics can allow differentiation of tumors from stool by visual analysis or by density measurements on iodine map and VNC images. It is also possible to detect polyps and second primary tumors in patients with colon cancer by DECT.
Main points.
Dual-energy computed tomography (DECT) characteristics of colorectal cancer provide differentiation of tumor from stool by both visual and quantitative analyses.
The density of stool was significantly lower than tumor on both iodine map and virtual noncontrast (VNC) images (P < 0.001).
Quantitative analyses on iodine map differentiated tumor from stool with an area under the curve of 0.76, a sensitivity of 82%, and a specificity of 72%; whereas on VNC images tumor was differentiated with an area under the curve of 1 and a sensitivity and specificity of 100%.
It is possible to differentiate tumor from stool according to the characteristics of the lesion on DECT imaging, with no requirement for bowel preparation or scanning the patient in two positions.
DECT can also help to detect polyps and second primary tumors in patients with colon cancer.
Acknowledgements
Musturay Karcaaltincaba has been supported for this project by the Turkish Academy of Sciences (TUBA), in the framework of the Young Scientist Award Program (EA-TUBA-GEBIP/2011).
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Atkin W, Dadswell E, Wooldrage K, et al. SIGGAR investigators. Computed tomographic colonography versus colonoscopy for investigation of patients with symptoms suggestive of colorectal cancer (SIGGAR): a multicentre randomized trial. Lancet. 2013;381:1194–1202. doi: 10.1016/S0140-6736(12)62186-2. https://doi.org/10.1016/S0140-6736(12)62186-2. [DOI] [PubMed] [Google Scholar]
- 2.Regge D, Laudi C, Galatola G, et al. Diagnostic accuracy of computed tomographic colonography for the detection of advanced neoplasia in individuals at increased risk of colorectal cancer. JAMA. 2009;301:2453–2461. doi: 10.1001/jama.2009.832. https://doi.org/10.1001/jama.2009.832. [DOI] [PubMed] [Google Scholar]
- 3.Pickhardt PJ, Kim DH, Meiners RJ, et al. Colorectal and extracolonic cancers detected at screening CT colonography in 10,286 asymptomatic adults. Radiology. 2010;255:83–88. doi: 10.1148/radiol.09090939. https://doi.org/10.1148/radiol.09090939. [DOI] [PubMed] [Google Scholar]
- 4.Pickhardt PJ, Hassan C, Halligan S, Marmo R. Colorectal cancer: CT colonography and colonoscopy for detection--systematic review and meta-analysis. Radiology. 2011;259:393–405. doi: 10.1148/radiol.11101887. https://doi.org/10.1148/radiol.11101887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eliahou R, Azraq Y, Carmi R, Mahgerefteh SY, Sosna J. Dual-energy based spectral electronic cleansing in non-cathartic computed tomography colonography: an emerging novel technique. Semin Ultrasound CT MR. 2010;31:309–314. doi: 10.1053/j.sult.2010.05.005. https://doi.org/10.1053/j.sult.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Marin D, Boll DT, Mileto A, Nelson RC. State of the art: dual-energy CT of the abdomen. Radiology. 2014;271:327–342. doi: 10.1148/radiol.14131480. https://doi.org/10.1148/radiol.14131480. [DOI] [PubMed] [Google Scholar]
- 7.McCollough CH, Leng S, Yu L, Fletcher JG. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology. 2015;276:637–653. doi: 10.1148/radiol.2015142631. https://doi.org/10.1148/radiol.2015142631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vrtiska TJ, Takahashi N, Fletcher JG, Hartman RP, Yu L, Kawashima A. Genitourinary applications of dual energy CT. AJR Am J Roentgenol. 2010;194:1434–1442. doi: 10.2214/AJR.10.4404. https://doi.org/10.2214/AJR.10.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karcaaltıncaba M, Aktaş A. Dual-energy CT revisited with multidetector CT: review of principles and clinical applications. Diagn Interv Radiol. 2011;17:181–194. doi: 10.4261/1305-3825.DIR.3860-10.0. [DOI] [PubMed] [Google Scholar]
- 10.Karcaaltincaba M, Karaosmanoglu D, Akata D, Sentürk S, Ozmen M, Alibek S. Dual energy virtual CT colonoscopy with dual source computed tomography: initial experience. Rofo. 2009;181:859–862. doi: 10.1055/s-0028-1109569. https://doi.org/10.1055/s-0028-1109569. [DOI] [PubMed] [Google Scholar]
- 11.Cai W, Kim SH, Lee JG, Yoshida H. Informatics in radiology: dual-energy electronic cleansing for fecal-tagging CT colonography. Radiographics. 2013;33:891–912. doi: 10.1148/rg.333125039. https://doi.org/10.1148/rg.333125039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boellaard TN, Henneman OD, Streekstra GJ, et al. The feasibility of colorectal cancer detection using dual-energy computed tomography with iodine mapping. Clin Radiol. 2013;68:799–806. doi: 10.1016/j.crad.2013.03.005. https://doi.org/10.1016/j.crad.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Schaeffer B, Johnson TR, Mang T, Kreis ME, Reiser MF, Graser A. Dual-energy CT colonography for preoperative “one-stop” staging in patients with colonic neoplasia. Acad Radiol. 2014;21:1567–1572. doi: 10.1016/j.acra.2014.07.019. https://doi.org/10.1016/j.acra.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Levin B, Lieberman DA, McFarland B, et al. American Cancer Society Colorectal Cancer Advisory Group; US Multi-Society Task Force; American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–160. doi: 10.3322/CA.2007.0018. https://doi.org/10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 15.Hara AK, Kuo MD, Blevins M, et al. National CT colonography trial (ACRIN 6664): comparison of three full-laxative bowel preparations in more than 2500 average-risk patients. AJR Am J Roentgenol. 2011;196:1076–1082. doi: 10.2214/AJR.10.4334. https://doi.org/10.2214/AJR.10.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson CD, Herman BA, Chen MH, et al. The National CT Colonography Trial: assessment of accuracy in participants 65 years of age and older. Radiology. 2012;263:401–408. doi: 10.1148/radiol.12102177. https://doi.org/10.1148/radiol.12102177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine MS, Yee J. History, evolution, and current status of radiologic imaging tests for colorectal cancer screening. Radiology. 2014;273:S160–180. doi: 10.1148/radiol.14140531. https://doi.org/10.1148/radiol.14140531. [DOI] [PubMed] [Google Scholar]
- 18.Trilisky I, Wroblewski K, Vannier MW, Horne JM, Dachman AH. CT colonography with computer-aided detection: recognizing the causes of false-positive reader results. Radiographics. 2014;34:1885–1905. doi: 10.1148/rg.347130053. https://doi.org/10.1148/rg.347130053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fini L, Laghi L, Hassan C, et al. Noncathartic CT colonography to screen for colorectal neoplasia in subjects with a family history of colorectal cancer. Radiology. 2014;270:784–790. doi: 10.1148/radiol.13130373. https://doi.org/10.1148/radiol.13130373. [DOI] [PubMed] [Google Scholar]
- 20.Shefer E, Altman A, Behling R, et al. State of the art of CT detectors and sources: A Literature Review. Curr Radiol Rep. 2013;1:76–91. https://doi.org/10.1007/s40134-012-0006-4. [Google Scholar]
- 21.Lambert L, Ourednicek P, Briza J, et al. Sub-milliSievert ultralow-dose CT colonography with iterative model reconstruction technique. Peer J. 2016;4:e1883. doi: 10.7717/peerj.1883. https://doi.org/10.7717/peerj.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HJ, Park SH, Pickhardt PJ, et al. CT colonography for combined colonic and extracolonic surveillance after curative resection of colorectal cancer. Radiology. 2010;257:697–704. doi: 10.1148/radiol.10100385. https://doi.org/10.1148/radiol.10100385. [DOI] [PubMed] [Google Scholar]