Abstract
PURPOSE
We aimed to perform intraindividual comparison of computed tomography (CT) parameters, image quality, and radiation exposure between standard CT angiography (CTA) and high-pitch dual source (DS)-CTA, in subjects undergoing serial CTA of thoracoabdominal aorta.
METHODS
Eighteen subjects with thoracoabdominal CTA by standard technique and high-pitch DS-CTA technique within 6 months of each other were retrieved for intraindividual comparison of image quality in thoracic and abdominal aorta. Quantitative analysis was performed by comparison of mean aortic attenuation, noise, signal-to-noise ratio (SNR), and contrast-to-noise ratio (CNR). Qualitative analysis was performed by visual assessment of motion artifacts and diagnostic confidence. Radiation exposure was quantified by effective dose. Image quality was apportioned to radiation exposure by means of figure of merit.
RESULTS
Mean aortic attenuation and noise were higher in high-pitch DS-CTA of thoracoabdominal aorta, whereas SNR and CNR were similar in thoracic aorta and significantly lower in high-pitch DS-CTA of abdominal aorta (P = 0.024 and P = 0.016). High-pitch DS-CTA was significantly better in the first segment of thoracic aorta. Effective dose was reduced by 72% in high-pitch DS-CTA.
CONCLUSION
High-pitch DS-CTA without electrocardiography-gating is an effective technique for imaging aorta with very low radiation exposure and with significant reduction of motion artifacts in ascending aorta; however, the overall quality of high-pitch DS-CTA in abdominal aorta is lower than standard CTA.
Computed tomography angiography (CTA) of the aorta is the reference method to investigate aortic disease and to follow up patients after surgical or endovascular aortic repair, either thoracic or abdominal. Thoracic aorta usually demands electrocardiography (ECG)-gated CTA to avoid motion artifacts related to cardiac cycle, notably for imaging aortic root and ascending segment (1). However, ECG-gating results in significantly increased radiation exposure, notably in case of low-pitch acquisition for retrospective reconstruction. Furthermore, ECG-gating technique is also related to significant increase of imaging time and volume of contrast agent.
Dual-source (DS) hardware is a significant technological advance because it allows acquisition with exceptionally high-pitch and fast rotation speed, with substantial reduction of acquisition time and time resolution. For instance, DS-CTA with ECG-gating allows whole coronary imaging (scan length, 12–14 cm) in a single heart cycle with significant reduction in radiation dose and volume of contrast agent (2). Furthermore, DS-CTA with ECG-gating provides high quality images with minor motion artifacts also in imaging of whole thoracic aorta (scan length 20–30 cm) (3). Karlo et al. (4) demonstrated that high-pitch DS-CTA without ECG-gating provides diagnostic image quality of the aortic valve-aortic root complex similar to ECG-gated technique. Despite these advantages in acquisition of short vascular segments (e.g., coronary or thoracic aorta), the role of high-pitch DS-CTA in imaging the whole thoracoabdominal aorta still remains a challenge and a topic of debate (5–8) because the 50–70 cm scan length requires a scan time ≥1 s, which is prone to motion artifacts in the evaluation of aortic root and ascending aorta.
The purpose of this study was to conduct intraindividual comparison of CT parameters, image quality, and radiation exposure between standard CTA and high-pitch DS-CTA (both without ECG-gating), in subjects undergoing serial CTA of thoracoabdominal aorta.
Methods
Study population
A retrospective query was operated on the radiology information system of the University Hospital of Parma, for thoracoabdominal CTA between October 2014 and March 2015. We selected subjects who underwent both standard CTA (single source) and high-pitch DS-CTA technique within 6 months of each other. The clinical enquiry for thoracoabdominal CTA included: a) follow-up after thoracic vascular surgery or endovascular repair, b) follow-up of untreated ascending aorta aneurysm or untreated dissection, c) suspected acute aortic syndrome. Exclusion criteria for CTA were severe allergy to iodine contrast agent and pregnancy. The Institutional Review Board waived informed consent for retrospective evaluation of CTA.
CT technique
Standard CTA and high-pitch DS-CTA were both performed without ECG-gating, by a dual-source 128-section CT system (Somatom Definition Flash, Siemens Healthcare). Standard CTA was performed by single source technique as follows: voltage 120–140 kV, current 200–250 mAs, pitch 1.0, collimation 128×0.6 mm, and rotation time 0.5 ms. High-pitch DS-CTA was performed by dual-source technique as follows: voltage 100 kV, current 120–140 mAs, pitch 3.0, collimation 2×128×0.6 mm, and rotation time 0.28 s. Image reconstruction was performed by vascular kernel (B20f) on filtered back projection. Scans were performed in cranio-caudal direction during deep inspiratory breath-hold, with scan volume from supra-aortic vessels to the common iliac arteries.
Contrast injection was performed by double-syringe electronic injector (Medrad Stellant, Bayer Ag) with 90 mL of Iomeprol at a concentration of 400 mg iodine/mL (Iomeron 400, Bracco), followed by 40 mL saline chaser; injection rate was 3 mL/s.
Bolus tracking technique was used to automatically start the scan with a region of interest (ROI) positioned in descending aorta at the level of diaphragm, trigger threshold 140 HU and scan delay 15 s. All CT scans were reconstructed with slice thickness 1.0 mm, slice increment 0.7 mm, vascular image reconstruction algorithm (B36), and dedicated vascular window setting (WL 300–WW 700).
A radiologist (with 10-year experience in cardiovascular CT imaging) reviewed the datasets on a standard clinical reporting workstation (BARCO visualization system) connected to the local picture and archiving and communication system (PACS, EBIT, Esaote Group), and evaluated the images according to quantitative and qualitative scores.
Quantitative image analysis
Quantitative image analysis was performed on axial CT images at five predefined anatomical levels: 1) ascending aorta in proximity of the aortic valve; 2) aortic arch at the origin of brachiocephalic trunk; 3) thoracic descending aorta at the level of pulmonary trunk; 4) aorta at the level of diaphragm; 5) abdominal aorta at the level of the renal arteries. At each level, two circular ROIs were drawn in the aortic lumen and in the adjacent muscle; the following parameters were recorded: a) mean aortic attenuation; b) image noise, defined as the standard deviation (SD) of the CT attenuation value of the muscle; c) signal-to-noise ratio (SNR), calculated as the mean attenuation of the artery divided by the image noise:
d) contrast-to-noise ratio (CNR), calculated as the difference between the mean attenuation of the artery and the mean attenuation of the muscle, divided by image noise:
Qualitative image analysis
Multiplanar reconstructions with orthogonal plane to major axis of the aorta were obtained at five predefined target anatomical structures: a) aortic valve, b) origin of coronary arteries, c) ascending aorta, d) origin of supra-aortic vessels, e) descending mediothoracic aorta at the level of pulmonary artery bifurcation. Motion artifacts and diagnostic confidence were rated according to qualitative scores by three-point scales of anatomical detail of vessel wall, lumen caliper, and depiction of parietal thrombus (4). Motion artifacts were scored as follows: 1) poor quality, with severe blurring or motion (e.g., doubling of target anatomical structures); 2) moderate quality, with minor motion (blurring or virtually thickened aortic wall) or step artifacts (discontinuity in the aortic wall); 3) excellent quality, without motion or step artifacts. Particular attention was paid to motion artifacts appearing as double contour of the ascending aorta because of the potential mimicking of type A dissection. Diagnostic confidence was scored as follows: 1) low confidence, no anatomical structures assessable; 2) moderate confidence, part of anatomical structures assessable; 3) high confidence, all anatomical structures assessable.
A second radiologist with 10-year experience in cardiovascular CT imaging performed qualitative analysis to assess interobserver variability.
Radiation exposure
Scan range, volume CT dose index (CTDIvol, reported in mGy) and dose length product (DLP, reported in mGy × cm) were recorded from the patient protocol. Furthermore, estimated effective dose (ED) was calculated for each CT examination by multiplying the DLP by normalized ED conversion coefficient. Because a combination of chest, abdominal, and pelvic acquisitions was performed, the mean of these region-specific conversion coefficients (k = 0.017 mSv/mGy × cm) was used (9).
To evaluate image quality at a given radiation exposure, the CNR was normalized to ED according to the formula of the figure of merit (FOM) (10, 11).
The FOM quantity enabled the assessment of CNR change independent of the tube current–time product and the ED.
Statistical analysis
Continuous variables were expressed as median and interquartile range (IQR), while categorical variables as frequencies or percentages. For continuous parameters, the intraindividual differences were tested by the Wilcoxon test. Interobserver agreement was calculated by the Cohen weighted κ, with strength of agreement expressed by κ value as follows: <0.2 poor, 0.2–0.4 fair, 0.4–0.6 moderate, 0.6–0.8 good, and 0.8–1.0 excellent. Furthermore, anatomical grouping of more levels was operated for compartmental comparison of the median of quantitative parameters, as follow: a) thoracic aorta, including from aortic valve to descending aorta (levels 1, 2, and 3 of quantitative analysis); b) abdominal aorta, including diaphragmatic aorta and aorta at the level of renal arteries origin (levels 4 and 5 of quantitative analysis). Categorical variables were tested by Chi-square test. P value < 0.05 was considered statistically significant.
Results
Eighteen subjects (12 men, 6 women; mean age, 61±15 years; age range, 30–84 years) fulfilled the inclusion criteria. Biometrics were as follows: mean weight 78.1±13.5 kg (range, 47–97 kg), mean height 172±10 cm (range, 153–191 cm), mean body mass index 22.5±3.1 kg/m2 (range, 14.5–26.8 kg/m2). The mean time interval between standard CTA and high-pitch DS-CTA was 104±41 days (range, 14–178 days); there was no significant change of weight (P = 0.398) or body mass index (P = 0.433) between imagings. The majority of the patients were referred for CT angiography of the thoracoabdominal aorta for follow-up after surgical aortic repair (n=6) or grafting (n=6). Other indications were follow-up of ascending aorta aneurysm (n=4), nontreated dissection (n=1) and suspected aortic syndrome (n=1). Aortic dissection was seen in 7/18 subjects. All CT examinations were considered diagnostic for their specific clinical indication.
Quantitative parameters of standard CTA and high-pitch DS-CTA are summarized in Table 1. Aortic enhancement was constantly higher in DS-CTA resulting in higher density of the lumen with advantage in assessing the internal surface of vessel wall. However, DS-CTA appeared always noisier than standard CTA, with increasing difference from ascending aorta (P = 0.038) to lower abdominal level (P < 0.0001). SNR and CNR were always lower in high-pitch DS-CTA than in standard CTA, with nonsignificant difference in thoracic aorta (comparison of data at levels 1, 2, and 3: P = 0.081 and P = 0.097, respectively) and significant difference in abdominal aorta (comparison of data at levels 4 and 5: P = 0.024 and P = 0.018, respectively). Table 2 shows results of comparison between standard CTA and high-pitch DS-CTA according to anatomical compartmental grouping.
Table 1.
Comparison of quantitative image analysis by Wilcoxon test
| Standard CTA | DS-CTA | P | |||
|---|---|---|---|---|---|
|
|
|
||||
| Median | IQR | Median | IQR | ||
| Ascending aorta | |||||
| Mean aortic density | 288 | 260–365 | 360 | 325–412 | 0.043 |
| Noise | 16 | 14–21 | 27 | 23–31 | 0.038 |
| SNR | 17 | 15–24 | 15 | 13–17 | 0.073 |
| CNR | 14 | 13–21 | 13 | 11–14 | 0.091 |
|
| |||||
| Aortic arch | |||||
| Mean aortic density | 284 | 259–391 | 405 | 338–448 | 0.018 |
| Noise | 15 | 13–18 | 25 | 20–29 | 0.031 |
| SNR | 21 | 17–24 | 17 | 12–20 | 0.113 |
| CNR | 17 | 13–25 | 14 | 11–19 | 0.087 |
|
| |||||
| Descending aorta | |||||
| Mean aortic density | 281 | 244–401 | 381 | 314–415 | 0.054 |
| Noise | 14 | 12–17 | 23 | 19–29 | 0.034 |
| SNR | 20 | 15–31 | 15 | 12–22 | 0.076 |
| CNR | 16 | 12–27 | 13 | 10–18 | 0.129 |
|
| |||||
| Diaphragmatic aorta | |||||
| Mean aortic density | 279 | 252–372 | 371 | 324–427 | 0.016 |
| Noise | 16 | 14–23 | 31 | 27–34 | <0.0001 |
| SNR | 19 | 13–25 | 13 | 10–16 | 0.0008 |
| CNR | 15 | 11–21 | 11 | 9–14 | 0.002 |
|
| |||||
| Origin of renal arteries | |||||
| Mean aortic density | 274 | 257–385 | 350 | 323–425 | 0.027 |
| Noise | 17 | 15–24 | 33 | 25–36 | <0.0001 |
| SNR | 18 | 12–22 | 13 | 9–17 | 0.0004 |
| CNR | 14 | 11–19 | 11 | 8–14 | 0.0007 |
P < 0.05 is deemed statistically significant.
Quantitative parameters are reported as median and IQR for standard CTA and DS-CTA at the level of 1) ascending aorta above the aortic valve, 2) aortic arch at the origin of the brachiocephalic trunk, 3) thoracic descending aorta at the level of pulmonary trunk, 4) thoracic descending aorta at the diaphragm, 5) abdominal aorta at the level of the renal arteries.
CTA, computed tomography angiography; DS-CTA, dual-source computed tomography angiography; IQR, interquartile range; SNR, signal-to-noise ratio; CNR, contrast-to-noise ratio.
Table 2.
Wilcoxon test according to compartmental anatomical grouping of more levels
| Standard CTA | DS-CTA | P | |||
|---|---|---|---|---|---|
|
|
|
||||
| Median | IQR | Median | IQR | ||
| Thoracic aorta (levels 1, 2, and 3) | |||||
| Mean aortic density | 285 | 256–354 | 384 | 322–428 | 0.020 |
| Noise | 16 | 13–19 | 25 | 20–30 | 0.033 |
| SNR | 20 | 16–27 | 16 | 12–20 | 0.081 |
| CNR | 16 | 13–25 | 13 | 11–17 | 0.097 |
|
| |||||
| Abdominal aorta (levels 4 and 5) | |||||
| Mean aortic density | 277 | 255–378 | 360 | 323–426 | 0.016 |
| Noise | 16 | 14–24 | 32 | 26–36 | 0.0002 |
| SNR | 19 | 13–26 | 13 | 10–16 | 0.024 |
| CNR | 15 | 11–21 | 11 | 9–14 | 0.016 |
CTA, computed tomography angiography; DS-CTA, dual-source computed tomography angiography; IQR, interquartile range; SNR, signal-to-noise ratio; CNR, contrast-to-noise ratio.
Qualitative assessment of standard CTA and high-pitch DS-CTA was significantly different in the first segment of thoracic aorta, whereas they performed similar from aortic arch to descending aorta (Fig. 1). In particular, motion artifacts were near absent in high-pitch DS-CTA at the level of aortic valve and origin of coronary arteries, whereas standard CTA reading was conditioned by severe motion artifacts (P = 0.0001, at both levels) (Figs. 2, 3). Motion artifacts were still significantly lower in DS-CTA at the level of ascending aorta (P = 0.003). Also, diagnostic confidence was significantly higher for DS-CTA at level of aortic valve (P = 0.020) and origin of coronary arteries (P = 0.028), but it was similar in ascending aorta (P = 0.104). Standard CTA and high-pitch DS-CTA performed similar at the level of aortic arch (P = 0.868 and P > 0.99, for motion artifacts and diagnostic confidence, respectively) and in descending aorta (P = 0.868 and P = 0.467, for motion artifacts and diagnostic confidence, respectively).
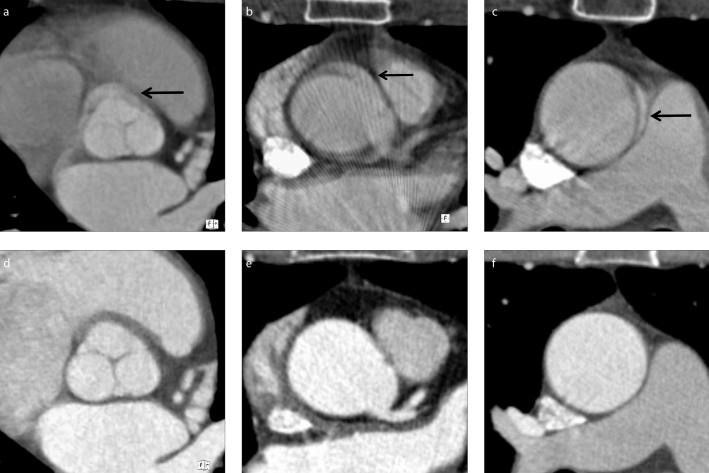
Figure 1. a–f.
Intraindividual comparison of motion artifacts between standard computed tomography angiography (CTA, a–c) and dual source CTA (DS-CTA, d–f) at different levels of thoracic aorta. Motion artifacts in standard CTA appear more evident at the level of aortic valve (arrow in a, compared with no artifact in d), origin of coronary arteries (arrow in b, compared with no artifact in e), and ascending aorta. Of note, the presence of double contours in ascending aorta mimicking type A dissections in standard CTA (arrow in c, compared with no artifact in f) would be a clinically significant artifact in suspected acute aortic syndrome.
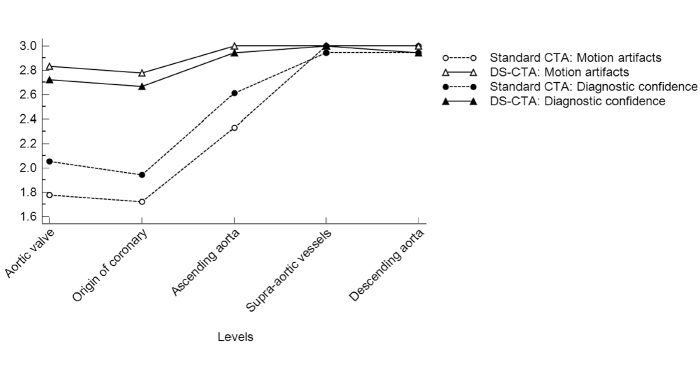
Figure 2.
Motion artifacts and diagnostic confidence in different anatomical structures of thoracic aorta. High-pitch DS-CTA had better image quality (higher median scores) in the segment from aortic valve to ascending aorta because of higher temporal resolution compared with standard CTA (lower median scores). From aortic arch further, motion artifacts and diagnostic confidence are similar between standard CTA and high-pitch DS-CTA.
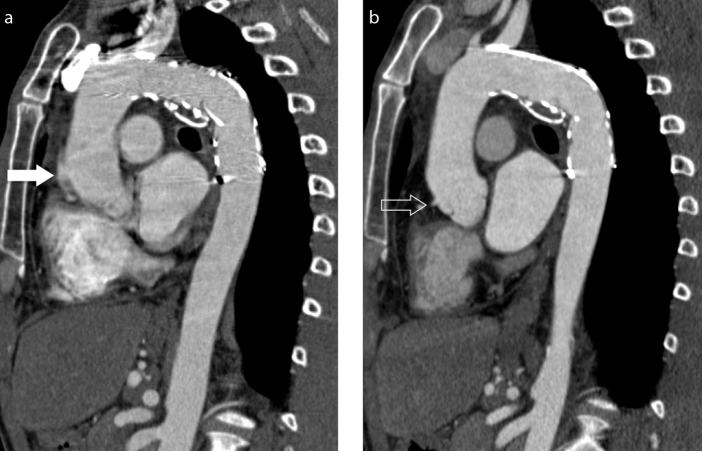
Figure 3. a, b.
Motion artifacts at the level of aortic root. Oblique coronal reconstruction (also called “candy cane” multiplanar reconstruction) shows motion artifact mimicking aortic dissection (arrow) on standard CTA image (a), which are significantly reduced on high-pitch DS-CTA image (b) where sinuses and the right coronary origin are seen (open arrow).
The interobserver agreement for qualitative assessment ranged between good and excellent for motion artifacts and diagnostic confidence on high-pitch DS-CTA images, while it ranged between fair and excellent for motion artifacts and diagnostic confidence on standard CTA images (Table 3). In particular, the second radiologist expressed an overall lower diagnostic confidence at the level of aortic valve and origin of coronary arteries on standard CT images. Conversely, excellent agreement was observed for motion artifacts and diagnostic confidence on both imaging datasets.
Table 3.
Interobserver agreement for qualitative assessment of CTA images
| Standard CTA | DS-CTA | |||
|---|---|---|---|---|
|
|
|
|||
| MA | DC | MA | DC | |
| Aortic valve | 0.471 | 0.312 | 0.769 | 0.640 |
|
| ||||
| Origin of coronary arteries | 0.452 | 0.312 | 0.625 | 0.625 |
|
| ||||
| Ascending aorta | 0.357 | 0.348 | 1.0 | 0.654 |
|
| ||||
| Origin of supra-aortic vessels | 0.625 | 0.625 | 1.0 | 1.0 |
|
| ||||
| Descending mediothoracic aorta at the level of pulmonary artery bifurcation | 1.0 | 1.0 | 1.0 | 1.0 |
CTA, computed tomography angiography; DS-CTA, dual-source computed tomography angiography; MA, motion artifacts; DC, diagnostic confidence.
Similar scan length was used for standard CTA and high-pitch DS-CTA of each subject (P = 0.284), but radiation exposure was significantly lower in high-pitch DS-CTA (Table 4). In particular, high-pitch DS-CTA technique brought a median reduction of ED equal to 72%. The reduction of radiation exposure was associated with decay in image quality in high-pitch DS-CTA, as observed by quantitative analysis (see above). However, the combined evaluation of image quality and dose by FOM showed that high-pitch DS-CTA was still superior to standard CTA for the evaluation of thoracic aorta (P = 0.001; detailed comparison of each level is reported in Table 3). Conversely, FOM was similar between the two techniques in abdominal aorta (P = 0.068; detailed comparison of each level is reported in Table 3).
Table 4.
Comparison of radiation exposure between standard CTA and DS-CTA by Wilcoxon test
| Standard CTA | DS-CTA | P | |||
|---|---|---|---|---|---|
|
|
|
||||
| Median | IQR | Median | IQR | ||
| Scan length (cm) | 64.7 | 60.8–66.9 | 61.3 | 59.7–66.5 | 0.284 |
|
| |||||
| CTDI (mGr) | 14.0 | 13.4–18.6 | 4.0 | 4.0–4.1 | <0.0001 |
|
| |||||
| DLP (mGr × cm) | 989 | 835–1191 | 276 | 264–291 | <0.0001 |
|
| |||||
| ED (mSv) | 16.8 | 14.2–20.3 | 4.7 | 4.5–4.9 | <0.0001 |
|
| |||||
| FOM | |||||
| Thoracic aorta (all segments) | 14 | 9–37 | 38 | 26–61 | 0.001 |
| Ascending aorta | 13 | 9–29 | 35 | 26–45 | 0.016 |
| Aortic arch | 23 | 9–29 | 41 | 26–70 | 0.010 |
| Descending aorta | 15 | 11–46 | 39 | 24–71 | 0.054 |
| Abdominal aorta (all segments) | 12 | 6–30 | 27 | 16–41 | 0.068 |
| Diaphragmatic aorta | 12 | 6–32 | 27 | 16–38 | 0.092 |
| Origin of renal arteries | 17 | 15–24 | 33 | 25–36 | <0.0001 |
CTA, computed tomography angiography; DS-CTA, dual-source computed tomography angiography; IQR, interquartile range; CTDI, computed tomography dose index; DLP, dose length product; ED, effective dose; FOM, figure of merit.
Discussion
In our study, comparison between standard CTA and high-pitch DS-CTA showed that reduction in motion artifacts and radiation dose is feasible by DS-CTA with the drawback of increased noise, which appears trivial in thoracic aorta. In abdominal aorta, the substantial increase in image noise seen in high-pitch DS-CTA makes standard CTA preferable.
Reduction of motion artifacts in thoracic aorta is feasible by ECG-gated techniques, with the drawback of increase in radiation exposure and examination time. Radiation exposure can be reduced by prospective technique; however, positioning ECG electrodes still remains a complex and time-consuming procedure. Karlo et al. (4) reported that high-pitch DS-CTA without ECG-gating of the aortic valve–aortic root complex is equivalent to ECG-gated one. Furthermore, Nagakawa et al. (12) showed that this technique is especially useful in emergency settings, with the potential of motion-free imaging also in case of tachycardia and scant collaboration (e.g., no compliance to breath-hold directions). Our study is consistent with these data in thoracic aorta, especially regarding the aortic valve, origin of coronary arteries, and ascending aorta. Our data add to those of Beeres et al. (8) who reported substantial equivalence in image quality of high-pitch DS-CTA with or without ECG-gating. Christensen et al. (13) reported significant reduction in motion artifacts by high-pitch technique without significant loss in vascular attenuation. Our data are in line with this report. In particular, we observed increased aortic attenuation in DS-CTA images, which were acquired with lower tube voltage. This advantage is readily explained by the application of 100 kVp, which is closer to the K-edge of iodine and enables better interaction than higher potentials, such as those used for standard CTA (14). The increased aortic attenuation might contribute to increased diagnostic confidence (e.g., depiction and characterization of parietal thrombus) along with reduction of motion artifacts achieved by the higher temporal resolution (15). Lower kVp can also be used to increase the aortic attenuation, with further reduction in radiation dose and even reduction in volume of contrast agent compared with standard CTA. In particular, Zhang et al. (7) reported good image quality by iterative reconstruction in non-ECG-gated high-pitch DS-CTA at 70 kVp. We observed worse noise, SNR, and CNR in high-pitch DS-CTA images compared with standard CTA. SNR and CNR decreased significantly in more distal tracts of aorta, especially in the abdominal tract where they were significantly lower for high-pitch DS-CTA. This observation is in line with a report from Apfaltrer et al. (16). In particular, it cannot be overemphasized that the benefit derived from improvement of temporal resolution was greater than noise increase in thoracic aorta and, therefore, resulted in much greater image quality, especially from aortic valve to ascending aorta. For example, double contour of the ascending aorta mimicking type A dissection is often seen in standard CTA but absent in high-pitch DS-CTA. Conversely, more distal levels of thoracic aorta have less motion artifact; therefore, increasing temporal resolution is associated with worsening of image quality without improvement of diagnostic confidence. Furthermore, high body mass in abdomen relates with lower signal reaching scanner detectors. This implies dramatic increase in noise and reduction of SNR and CNR, which make high-pitch DS-CTA more difficult to interpret than standard CTA. In particular, significant decrease in SNR and CNR could be a limitation in patients studied for the first time because of possible overlooking of pathologic findings in the abdominal aorta. Sahani et al. (17) reported similar image quality for standard CTA and DS-CTA in abdominopelvic vessels; however, they did not explore quantitative metrics. Moreover, Russo et al. (18) reported quantitative parameters of 128-slice scanner with high-speed rotation time for imaging thoracoabdominal aorta. In particular, they showed minor motion artifacts and good quality of images also in the abdominal aorta. Of note, they used iterative reconstruction, which has potential of reducing image noise. We think iterative reconstruction could be applied to high-pitch DS-CTA in an attempt to reduce noise; however, the paucity of signal using high-technique in the abdomen could reduce contrast resolution. Therefore, one must be cautious of using high-pitch DS-CTA in patients scanned for the first time with suspicion of acute aortic syndrome.
Several authors reported significant radiation dose reduction by high-pitch DS-CTA compared with standard CTA (4, 16), which is confirmed in our study by the 72% reduction in ED. Dose reduction appears particularly important in the setting of long-term follow-up where high-pitch DS-CTA could be effectively employed instead of standard CTA. Indeed, a moderate increase in image noise may be acceptable as long as diagnostic information is not compromised, as required by the as low as reasonably achievable (ALARA) principle. Feasibility of extra-low dose CTA by high-pitch DS-CTA was reported on both phantom and patient by Mileto et al. (6) who reported linear correlation of dose with both phantom size and pitch, showing that higher pitch should be granted for lower weight patients who frequently are younger (e.g., long-term control of post-traumatic aortic disease). Furthermore, the use of fast acquisition would allow reduction of contrast agent, which is again of paramount importance for long-term follow-up. Possibility of reducing volume of contrast agent has been recently reported with promising results (18–20).
This study has several limitations. First of all, our study is limited by its retrospective nature and relatively small number of patients. Second, although reader was blinded to the acquisition mode, protocols might have been occasionally recognized by their peculiarities, such as different size of field of view. Also, the noise was calculated on a single ROI, while averaging of multiple ROIs could have been superior. Finally, the image quality was assessed only on the plane orthogonal to the vessel. Future studies should consider assessing motion artifacts also on other multiplanar reformats.
In conclusion, high-pitch DS-CTA without ECG-gating abuts motion artifacts in ascending aorta with minor detrimental effects on noise, SNR, and CNR. For this reason, DS-CTA should be preferred to standard CTA in assessing thoracic aorta. Furthermore, DS-CTA reduces radiation exposure by 72%; therefore, it may be recommended for long-term surveillance of aortic disease. However, quantitative parameters of DS-CTA are significantly worse than standard CTA for abdominal aorta; therefore, the latter technique should be preferred for imaging diseases of the abdominal aorta.
Main points.
Motion artifacts are a major diagnostic issue in vascular imaging, notably in thoracic aorta.
High-pitch dual source computed tomography angiography (DS-CTA) grants extremely low acquisition time compared with single source CTA, with significant reduction of motion artifacts.
Radiation dose of high-pitch DS-CTA is significantly lower than single source CTA, which makes the former technique preferable for long-term follow-up.
Image quality of high-pitch DS-CTA is comparable to single source CTA in thoracic aorta, whereas it is poorer in abdominal aorta.
References
- 1.Schernthaner RE, Stadler A, Beitzke D, et al. Dose modulated retrospective ECG-gated versus non-gated 64-row CT angiography of the aorta at the same radiation dose: comparison of motion artifacts, diagnostic confidence and signal-to-noise-ratios. Eur J Radiol. 2012;81:e585–590. doi: 10.1016/j.ejrad.2011.06.053. https://doi.org/10.1016/j.ejrad.2011.06.053. [DOI] [PubMed] [Google Scholar]
- 2.Flohr TG, Leng S, Yu L, et al. Dual-source spiral CT with pitch up to 3.2 and 75 ms temporal resolution: image reconstruction and assessment of image quality. Med Phys. 2009;36:5641–5653. doi: 10.1118/1.3259739. https://doi.org/10.1118/1.3259739. [DOI] [PubMed] [Google Scholar]
- 3.Bolen MA, Popovic ZB, Tandon N, Flamm SD, Schoenhagen P, Halliburton SS. Image quality, contrast enhancement, and radiation dose of ECG-triggered high-pitch CT versus non-ECG-triggered standard-pitch CT of the thoracoabdominal aorta. AJR Am J Roentgenol. 2012;198:931–938. doi: 10.2214/AJR.11.6921. https://doi.org/10.2214/AJR.11.6921. [DOI] [PubMed] [Google Scholar]
- 4.Karlo C, Leschka S, Goetti RP, et al. High-pitch dual-source CT angiography of the aortic valve-aortic root complex without ECG-synchronization. Eur Radiol. 2011;21:205–212. doi: 10.1007/s00330-010-1907-3. https://doi.org/10.1007/s00330-010-1907-3. [DOI] [PubMed] [Google Scholar]
- 5.Bischoff B, Meinel FG, Reiser M, Becker HC. Novel single-source high-pitch protocol for CT angiography of the aorta: comparison to high-pitch dual-source protocol in the context of TAVI planning. Int J Cardiovasc Imaging. 2013;29:1159–1165. doi: 10.1007/s10554-013-0182-1. https://doi.org/10.1007/s10554-013-0182-1. [DOI] [PubMed] [Google Scholar]
- 6.Mileto A, Ramirez-Giraldo JC, Nelson RC, et al. High-pitch dual-source MDCT for imaging of the thoracoabdominal aorta: relationships among radiation dose, noise, pitch, and body size in a phantom experiment and clinical study. AJR Am J Roentgenol. 2015;205:834–839. doi: 10.2214/AJR.15.14334. https://doi.org/10.2214/AJR.15.14334. [DOI] [PubMed] [Google Scholar]
- 7.Zhang LJ, Li X, Schoepf UJ, et al. Non-electrocardiogram-triggered 70-kvp high-pitch computed tomography angiography of the whole aorta with iterative reconstruction: initial results. J Comput Assist Tomogr. 2016;40:109–117. doi: 10.1097/RCT.0000000000000329. https://doi.org/10.1097/RCT.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 8.Beeres M, Wichmann JL, Frellesen C, et al. ECG-gated versus non-ECG-gated high-pitch dual-source CT for whole body CT angiography (CTA) Academic Radiol. 2016;23:163–167. doi: 10.1016/j.acra.2015.09.003. https://doi.org/10.1016/j.acra.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Menzel H, Schibilla H, Teunen D, editors. European guidelines on quality criteria for computed tomography. Luxembourg: European Commission; 2000. Publication No. EUR 16262 EN. [DOI] [PubMed] [Google Scholar]
- 10.Samei E, Dobbins JT, 3rd, Lo JY, Tornai MP. A framework for optimising the radiographic technique in digital X-ray imaging. Radiat Prot Dosimetry. 2005;114:220–229. doi: 10.1093/rpd/nch562. https://doi.org/10.1093/rpd/nch562. [DOI] [PubMed] [Google Scholar]
- 11.Schindera ST, Nelson RC, Mukundan S, Jr, et al. Hypervascular liver tumors: low tube voltage, high tube current multi-detector row CT for enhanced detection--phantom study. Radiology. 2008;246:125–132. doi: 10.1148/radiol.2461070307. https://doi.org/10.1148/radiol.2461070307. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa J, Tasaki O, Watanabe Y, et al. Reduction of thoracic aorta motion artifact with high-pitch 128-slice dual-source computed tomographic angiography: a historical control study. J Comput Assist Tomogr. 2013;37:755–759. doi: 10.1097/RCT.0b013e31829c3f76. https://doi.org/10.1097/RCT.0b013e31829c3f76. [DOI] [PubMed] [Google Scholar]
- 13.Christensen JD, Seaman DM, Lungren MP, Hurwitz LM, Boll DT. Assessment of vascular contrast and wall motion of the aortic root and ascending aorta on MDCT angiography: dual-source high-pitch vs. non-gated single-source acquisition schemes. Eur Radiol. 2014;24:990–997. doi: 10.1007/s00330-014-3120-2. https://doi.org/10.1007/s00330-014-3120-2. [DOI] [PubMed] [Google Scholar]
- 14.Holmquist F, Nyman U. Eighty-peak kilovoltage 16-channel multidetector computed tomography and reduced contrast-medium doses tailored to body weight to diagnose pulmonary embolism in azotaemic patients. Eur Radiol. 2006;16:1165–1176. doi: 10.1007/s00330-005-0117-x. https://doi.org/10.1007/s00330-005-0117-x. [DOI] [PubMed] [Google Scholar]
- 15.Beeres M, Schell B, Mastragelopoulos A, et al. High-pitch dual-source CT angiography of the whole aorta without ECG synchronisation: initial experience. Eur Radiol. 2012;22:129–137. doi: 10.1007/s00330-011-2257-5. https://doi.org/10.1007/s00330-011-2257-5. [DOI] [PubMed] [Google Scholar]
- 16.Apfaltrer P, Hanna EL, Schoepf UJ, et al. Radiation dose and image quality at high-pitch CT angiography of the aorta: intraindividual and interindividual comparisons with conventional CT angiography. AJR Am J Roentgenol. 2012;199:1402–1409. doi: 10.2214/AJR.12.8652. https://doi.org/10.2214/AJR.12.8652. [DOI] [PubMed] [Google Scholar]
- 17.Sahani D, Saini S, D’Souza RV, et al. Comparison between low (3:1) and high (6:1) pitch for routine abdominal/pelvic imaging with multislice computed tomography. J Comput Assist Tomogr. 2003;27:105–109. doi: 10.1097/00004728-200303000-00001. https://doi.org/10.1097/00004728-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Russo V, Garattoni M, Buia F, Attina D, Lovato L, Zompatori M. 128-slice CT angiography of the aorta without ECG-gating: efficacy of faster gantry rotation time and iterative reconstruction in terms of image quality and radiation dose. Eur Radiol. 2016;26:359–369. doi: 10.1007/s00330-015-3848-3. [DOI] [PubMed] [Google Scholar]
- 19.Geyer LL, De Cecco CN, Schoepf UJ, et al. Low-volume contrast medium protocol for comprehensive cardiac and aortoiliac CT assessment in the context of transcatheter aortic valve replacement. Academic Radiol. 2015;22:1138–1146. doi: 10.1016/j.acra.2015.03.018. https://doi.org/10.1016/j.acra.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Wuest W, Anders K, Schuhbaeck A, et al. Dual source multidetector CT-angiography before transcatheter aortic valve implantation (TAVI) using a high-pitch spiral acquisition mode. Eur Radiol. 2012;22:51–58. doi: 10.1007/s00330-011-2233-0. https://doi.org/10.1007/s00330-011-2233-0. [DOI] [PubMed] [Google Scholar]