Abstract
We systematically reviewed the role of bronchial artery embolization (BAE) in hemoptysis. Literature search was done for studies on BAE published between 1976 and 2016. Twenty-two studies published in English, with sample size of at least 50 patients, reporting indications, technique, efficacy, and follow-up were included in the final analysis. Common indications for BAE included tuberculosis (TB), post-tubercular sequelae, bronchiectasis, and aspergillomas. Most common embolizing agent used was polyvinyl alcohol (size, 300–600 μm) with increasing use of glue in recent years. Overall immediate clinical success rate of BAE, defined as complete cessation of hemoptysis, varied from 70%–99%. However, recurrence rate remains high, ranging from 10%–57%, due to incomplete initial embolization, recanalization of previously embolized arteries, and recruitment of new collaterals. Presence of nonbronchial systemic collaterals, bronchopulmonary shunting, aspergillomas, reactivation TB, and multidrug resistant TB were associated with significantly higher recurrence rates (P < 0.05). Rate of major complications remained negligible and stable over time with median incidence of 0.1% (0%–6.6%). Despite high hemoptysis recurrence rates, BAE continues to be the first-line, minimally invasive treatment of hemoptysis in emergency settings, surgically unfit patients, or in patients with diffuse or bilateral lung disease.
Bronchial artery embolization (BAE) for control of hemoptysis was first described in detail by Remy et al. (1) in 1974. Since its initial descriptions, BAE has evolved in terms of indications, technique, and efficacy. BAE has been used for treatment of both benign and malignant causes of hemoptysis and for all grades of hemoptysis. While a large number of retrospective studies and outcome analyses studies have been published on BAE, especially within the last decade, systematic reviews on BAE are lacking. Though few review articles have been published on BAE (2–4), these reviews have been published before 2012 and do not incorporate data from the studies published within the last 2–3 years. In the last 2–3 years, at least nine retrospective cohort studies with sample populations greater than 50 patients have been published (5–13) and this data has not been systematically reviewed to date. Hence we attempted to perform a systematic review on BAE incorporating the data available from the recently published studies. This systematic review aims to analyze the indications, technique, short-term and long-term efficacy, outcomes, and complications of BAE.
Review method
The structure of this systematic review adheres to the PRISMA guidelines (14). The search methodology employed for this review has been enumerated under the following headings.
Initial eligibility criteria
Studies considered for this review had the following predetermined inclusion criteria: 1) Patients with hemoptysis and undergoing BAE for management; 2) Clinical outcomes, follow-up and complications reported; 3) Full-text publications in English available; 4) Publication date between 1975 and 2016.
Information sources and search strategy
A literature search was performed in May 2016 on PubMed (MEDLINE) for studies which matched the eligibility criteria using the keywords, “bronchial artery embolization” OR “bronchial artery embolisation” OR “transcatheter embolization” OR “hemoptysis”. An additional manual search of EMBASE as well as bibliographies of each included study was done to identify studies not covered by the PubMed search.
Final study selection
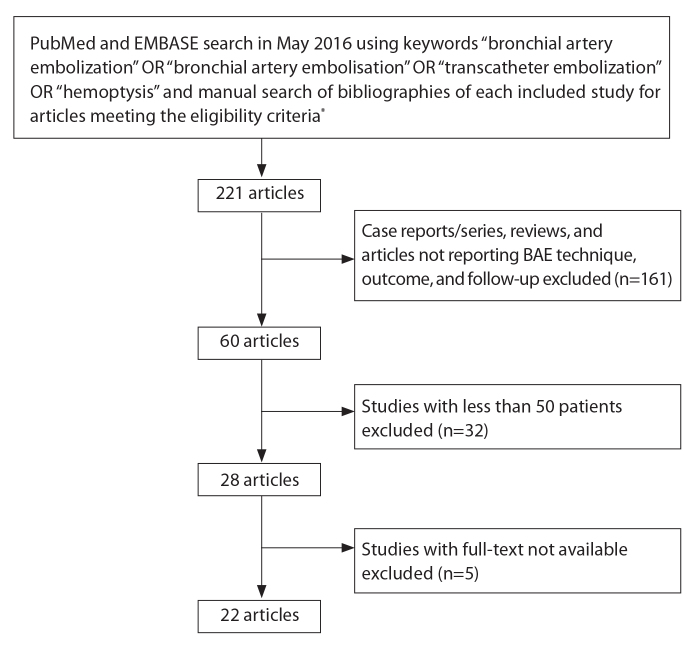
Using these search criteria, initially 221 articles were found. After exclusion of case series and review articles, we narrowed these down to 60 studies reporting the technique, efficacy, and outcomes of BAE in hemoptysis. We further excluded studies with less than 50 patients undergoing BAE, as we felt that the data obtained from these studies were not sufficiently conclusive or representative of the role of BAE in control of hemoptysis. While we did not intend to eliminate specific patient populations such as cystic fibrosis, pneumoconiosis, and malignancies from our search, studies dealing with these selective population groups had less than 50 patients and were thus automatically filtered out. We also excluded five studies whose full-texts were not available despite our best efforts. Finally, there were 22 studies (5–13, 15–27) meeting the selection criteria for this systematic review. The search strategy is schematized in Fig. 1.
Figure 1.
Search strategy. *Eligibility criteria included: 1) Studies on patients with hemoptysis undergoing bronchial artery embolization (BAE) for management; 2) Clinical outcomes, follow-up, and complications reported; 3) Full-text publications in English available; 4) Publication date between 1975 and 2016.
Data extraction and analysis
We examined these studies for the indications of BAE, grades of hemoptysis, technique of embolization, technical success, immediate clinical success, recurrence rate of hemoptysis and complications (Table 1). We also examined if there were any differences in the technique and efficacy of BAE in the studies published before and after the year 2010 (Table 2). We did not attempt a meta-analysis due to the data heterogeneity and the lack of a consistent follow-up time periods in these studies.
Table 1.
Technique, immediate efficacy, recurrence rate, and complications of bronchial artery embolization
| Year | Author | n | Hemoptysis grade | Embolizing agent | Immediate clinical success (%) | Clinical recurrence rate (%)a | Major complication rate (%)b | Levels of evidencec |
|---|---|---|---|---|---|---|---|---|
| 1977 | Remy et al. (15) | 104 | Massive | Gelatin sponge | 84 | 28.6 | 0.9 | 3 |
| 1985 | Uflacker et al. (16) | 64 | Massive | Gelatin sponge, e-amino caproic acid, cellulose sponge, absolute alcohol | 76.6 | 21.4 | 0 | 3 |
| 1987 | Rabkin et al. (17) | 306 | Mild: 60.7% Moderate-massive: 32.3% |
Polyurethrane, human albumin macroaggregates, penicillin solution, 10% hypertonic salt solution | 91.8 | 33.7 | 0 | 3 |
| 1996 | Ramakantan et al. (18) | 140 | Moderate-massive | Gelatin sponge | 73 | 27.1 | 1.4 | 3 |
| 2002 | Goh et al. (19) | 134 | Moderate-massive | PVA, gelatin sponge | 81.6 | 15.5 | 0 | 3 |
| 2002 | Swanson et al. (20) | 54 | Not quantified | PVA, coils, gelatin sponge | 94.4 | 24.1 | 0 | 3 |
| 2007 | van den Heuvel et al. (21) | 75 | Massive | Not specified | 67 | 47 | 4.3 | 3 |
| 2008 | Lee et al. (22) | 70 | Moderate-massive | PVA, coil, gelatin sponge | 99 | 45 | 0 | 3 |
| 2009 | Chan et al. (23) | 167 | Moderate-massive | PVA, NBCA | 95.7 | 45 | 1.2 | 3 |
| 2010 | Chun et al. (24) | 50 | Moderate-massive | PVA | 85 | 28 | 2 | 3 |
| 2011 | Shin et al. (25) | 169 | Mild: 33.7% Moderate: 32% Massive: 24.3% |
PVA, gelatin sponge, coils | 96.4 | 30.6 | 0 | 3 |
| 2011 | Yoo et al. (26) | 106 | Moderate-massive | NBCA | 97.2 | 20 | 0 | 3 |
| 2012 | Anuradha et al. (27) | 58 | Massive | PVA, gelatin sponge, NBCA, coils | 93.1 | 55 | 1.7 | 3 |
| 2013 | Hwang et al. (5) | 72 | Moderate-massive | PVA, gelatin sponge, coils | 93.1 | 40.3 | 0 | 3 |
| 2013 | Woo et al. (6) | 406 | Mild: 29.6% Moderate-massive: 70.4% |
PVA, NBCA | PVA: 92.2 NBCA: 96.9 |
PVA: 29.6 NBCA: 12.8 |
0.2 | 2 |
| 2013 | Agmy, et al. (7) | 348 | Moderate | PVA, microcoils | 95 | 9.8 | 0.6 | 3 |
| 2014 | Pei et al. (8) | 112 | Massive | Gelatin sponge | 86.6 | 24.1 | 0 | 3 |
| 2015 | Fruchter et al. (9) | 52 | Moderate-massive | Embosphere, embozene microspheres, coils | 92 | 57.5 | 6.6 | 3 |
| 2015 | Bhalla et al. (10) | 334 | Mild: 20.9% Moderate: 58.4% Massive: 20.7% |
PVA, gelatin sponge, NBCA | 93.5 | 12.6 | 0 | 3 |
| 2015 | Shao et al. (11) | 344 | Moderate-massive | Coils, gelatin foam | 96 | 39.2 | 0.9 | 3 |
| 2015 | Tom et al. (12) | 69 | Not quantified | PVA, microspheres, microcoil | 82 | 30 | 1 | 3 |
| 2016 | Dabo et al. (13) | 67 | Moderate-massive | PVA, gelatin sponge, spongostat | 98.5 | 37.3 | 0 | 3 |
n, number of patients undergoing bronchial artery embolization; PVA, polyvinyl alcohol; NBCA, N-butyl cyanoacrylate.
Includes all recurrences in immediate period as well as on follow-up.
Major complications included complications increasing hospital stay or morbidity such as neurologic complications (paraparesis/paraplegia/stroke), broncho-esophageal fistuale and esophagus/bowel necrosis. Transient chest or back pain, dysphagia, fever, and self-limiting vascular injuries such as vasospasm, dissection, and perforation were not included under major complications.
Modified from Oxford Centre for Evidence-Based Medicine, http://www.cebm.net/index.aspx?o=5653 (28). For studies on intervention/treatment benefits, Level 2 evidence refers to randomized control trial or observational study with dramatic effect and Level 3 evidence refers to nonrandomized controlled cohort/observational follow-up study.
Table 2.
Comparison of studies published before and after 2010
| Criteria | Published before 2010 (n=9) | Published in 2010 or later (n=13) |
|---|---|---|
| Number of patients, range | 54–306 | 50–406 |
| Flush aortogram | Done in 8/9 studies, not done by Goh et al. (19) | Done in 11/13 studies. Not done by Pei et al. (8) and Fruchter et al. (9) |
| Superselective catheterization | Done in 4/9 studies (44%); all 4 studies published after 2002 | Done in 11/13 studies Not done by Pei et al. (8) and Fruchter et al. (9) |
| Chief embolizing agent | Gelatin sponge alone (n=4) PVA ± gelatin sponge (n=3) |
PVA ± gelatin sponge (n=8) |
| Other/additional embolizing agents | Coils in 4 studies Glue in 1 study Details not available in 2 studies |
Coils and microcoils in 6 studies Glue in 4 studies Embospheres/microspheres in 2 studies Gelatin sponge alone in 1 study |
| PVA sizes (μm), range | 300–600 | 150–1200 (most common 355–500) |
| Immediate clinical success (%), range | 70–99 | 82–98.5 |
| Recurrence rate (%), range | 15.5–47 | 9.8–57.5 |
| Major complication rate (%), range | 0–4.7 | 0–6.6 |
Indications for BAE
The indications for BAE were available in all studies except by Lee et al. (22). These indications have been summarized in Table 3.
Table 3.
Common Indications for BAE
| Study | n | Tuberculosis (active/reactivation TB) | Post-tubercular sequelae | Idiopathic bronchiectasis | Aspergilloma | Lung malignancies (primary/secondary/metastatic/lymphoma) | Lung infections (pyogenic, lung abscess, nontubercular mycobacterial, pneumonia) | Pneumoconiosis/cystic fibrosis, sarcoidosis, vasculitis, vascular abnormalities, othersa | Idiopathic/cryptogenic |
|---|---|---|---|---|---|---|---|---|---|
| Remy et al. (15) | 104 | 35 (33.7) | - | 27 (30) | 19 (18) | 3 (2.9) | 14 (13.5) | 6 (5.7) | - |
| Uflacker et al. (16) | 75 | 71 (94.7) | - | 1 (1.3) | - | 1 (1.3) | 2 (2.7) | - | - |
| Rabkin et al. (17) | 306 | 22 (7.2) | 20 (6.5) | 30 (9.8) | - | 32 (10.5) | 118 (38.6) | 30 (9.8) | 27 (8.8) |
| Ramakantan et al. (18) | 140 | 123 (88) | - | - | 17 (12) | - | - | - | - |
| Goh et al. (19) | 134 | 97 (83.6) | - | - | - | 11 (9.5) | - | 8 (6.9) | 18 (13.4) |
| Swanson et al. (20) | 54 | 2 (3) | - | 9 (17) | 7 (13) | 7 (13) | - | 21 (38) | 8 (15) |
| van den Heuvel et al. (21) | 75 | 8 (10.7) | 60 (80) | - | - | 5 (6.7) | 1 (1.3) | 1 (1.3) | - |
| Chan et al. (23) | 167 | 13 (7.8) | 62 (37.1) | 61 (36.5) | 10 (6.0) | 7 (4.2) | 9 (5.4) | 2 (1.2) | 3 (1.8) |
| Chun et al. (24) | 50 | 6 (12) | 5 (10) | 6 (12) | 8 (16) | 5 (10) | - | 12 (24) | 8 (16) |
| Shin et al. (25) | 169 | 44 (26) | 125 (74) | - | 28b (17.2) | - | - | - | - |
| Yoo et al. (26) | 108 | 18 (16.7) | 31 (28.7) | 34 (31.4) | 6 (5.6) | 8 (7.4) | 4 (3.7) | - | 7 (6.5) |
| Anuradha et al. (27) | 58 | 14 (24) | 44 (76) | - | - | - | - | - | |
| Hwang et al. (5) | 72 | 28 (38.9) | 44 (61.1) | - | 6b (8.3) | - | - | - | |
| Woo et al. (6) | 406 | 49 (12.1) | 100 (24.6) | 117 (28.9) | 29 (7.1) | 19 (4.7) | 32 (7.9) | 139 (34.2) | - |
| Agmy et al. (7) | 348 | 94 (27) | 104 (29.9) | 77 (22.2) | 28 (8.1) | 14 (4.0) | 20 (5.8) | 7 (2.0) | 4 (1.0) |
| Pei et al. (8) | 112 | 68 (60.7) | 44 (39.3) | - | - | - | - | - | - |
| Fruchter et al. (9) | 52 | 0 | 1 (1.9) | 17 (32.7) | - | 16 (30.8) | 2 (3.8) | 14 (27.8) | 2 (3.8) |
| Bhalla et al. (10) | 334 | 21 (6.3) | 248 (74.3) | 39 (11.7) | - | 3 (0.9) | - | - | 23 (6.9) |
| Shao et al. (11) | 344 | 190 (55.2) | - | 99 (28.8) | - | 20 (5.8) | 6 (1.8) | 12 (3.2) | 18 (5.2) |
| Tom et al. (12) | 69 | 3 (4) | - | 3 (4) | - | 8 (11) | 11 (16) | 12 (16) | 7 (10) |
| Dabo et al. (13) | 88 | 4 (4.5) | 31 (35.2) | 35 (38) | 12 (13.6) | 2 (2.3) | - | 4 (4.6) | - |
| Total c | 3265 | 913 (28) | 919 (28) | 555 (17) | 176 (5.4) | 161 (4.9) | 220 (6.7) | 274 (8.4) | 125 (3.8) |
Data are presented as n (%).
Others include connective tissue diseases, primary or secondary pulmonary artery hypertension, hydatid cysts, sequelae of pulmonary emboli, congenital cardiac disease, chronic bronchitis, tracheobronchial fistula, post-radiation fibrosis, fibrosing mediastinitis, and actinomycosis. Vasculitis included Wegener’s granulomatosis and Behcet’s disease. Vascular indications included bronchial and pulmonary aneurysms, broncho-arterial fistula and arteriovenous malformations.
Aspergilloma patients considered part of post-TB sequelae and patients not counted separately by Shin et al. (16) and Hwang et al. (19).
Sum of patients from 21 studies.
The two most common indications for BAE was control of hemoptysis due to active tuberculosis (TB) and post-TB sequelae comprising of fibrosis, bronchiectasis and aspergilloma. Several authors separately reported incidence of aspergilloma/mycetoma as it had prognostic implications (6, 7, 10, 16, 20–22, 26). The incidence of aspergilloma in patients undergoing BAE varied from 5.6%–18% (15, 21, 26). Other indications for BAE included malignancy, lung infections, inflammatory lung disease, pneumoconiosis, cystic fibrosis, sarcoidosis, and cryptogenic hemoptysis. Less common indications for BAE were chronic obstructive pulmonary disease, vascular malformations, Wegener’s disease, Behcet’s disease, hydatid cysts of lung, and congenital cardiac disease.
In contrast to TB/post-TB sequelae, malignances formed a small spectrum of the overall population with an incidence of 1%–12.9% (10, 20). Also, since BAE works on the principle of selective embolization of hypertrophied bronchial arteries and nonbronchial systemic collaterals that get recruited secondary to chronic lung inflammation, bronchial artery abnormalities were less common in malignancies. In a study by Goh et al. (19), about one-third of patients with malignancies had no angiographic abnormalities compared with only 5% of patients with TB with normal bronchial angiography.
Unsurprisingly, there was a selection bias in the indications for BAE as the majority of published data was from Asia wherein patients with active TB and post-TB sequelae formed the majority of those undergoing BAE. In fact, a few studies reported the role of BAE exclusively in patients with active TB and/or post-TB sequelae (9, 18, 21, 25, 27). On the other hand, malignancies, nontubercular mycobacterial infections, lung abscesses, vasculitis, and cryptogenic hemoptysis were more common in studies published from Western population (8, 12). In the study by Tom et al. (12), which reported BAE experience in 69 patients from the United States, sarcoidosis and cystic fibrosis were the most common indications, comprising 36% of patient population while TB and post-TB sequelae formed only 8% of the patient population. Similarly, cystic fibrosis formed 16% of patients in the study by Fruchter et al. (9) compared with only 2% incidence of post-tubercular sequelae.
Grades of hemoptysis
Though hemoptysis was broadly graded as mild, moderate, and severe, there were differences in the definitions of grading in various studies. While some authors have used “major”, “severe”, and “massive” terms interchangeably, others specifically considered massive hemoptysis as life threatening. Furthermore, some authors emphasized that even a small volume of hemoptysis could flood the airspaces in diseased lungs, cause asphyxiation and thus be potentially life-threatening (27). Few studies, instead of grading severity of hemoptysis, described whether BAE was performed in acute massive hemoptysis under emergent conditions or was performed electively for chronic recurrent hemoptysis (13, 22). Also, the amount of hemoptysis was not quantified in two studies (11, 20). These differences in the definition of severity of hemoptysis with respect to both the volume and duration of bleeding and the difference in timing of BAE (emergency/elective) made it difficult to directly compare the result of one study with another. For the purpose of this review, we have broadly defined hemoptysis as mild, moderate, and severe based on consensus definitions obtained from various studies.
Mild hemoptysis referred to hemoptysis of less than 100 mL/day or less than 50 mL/episode. Moderate hemoptysis referred to hemoptysis of 100–300 mL/day or more than three bouts of hemoptysis of more than 100 mL/day in one week. Severe/massive/life-threatening hemoptysis referred to: a) hemoptysis more than 300 mL/day, b) hemoptysis of any volume leading to drop in hemoglobin (drop > 1g/dL) or hematocrit (drop > 5%) or respiratory failure (SPaO2, <60%), or hypotension (systolic blood pressure <90 mm Hg). Yoo et al. (26) and Woo et al. (6) used hemoptysis volume of 240 mL/day as cutoff between moderate and massive hemoptysis, while Chan et al. (23) termed hemoptysis more than 200 mL/day as massive or major hemoptysis. These have been clubbed together as moderate-massive hemoptysis for purposes of this review.
Using these definitions, in 18 out of 22 studies, BAE was performed for moderate to massive hemoptysis. Four authors (6, 10, 17, 25) also performed BAE for mild hemoptysis. The patients with mild hemoptysis formed 20.9%–60.7% of total sample populations (10, 17).
Preprocedure diagnostic workup
Both fiberoptic bronchoscopy (FOB) and CT angiography (CTA) are considered complementary for determining the cause and site of hemoptysis (3). However, preprocedural FOB was performed in only 10 studies, of which two studies were published before 2010 (6–8, 12, 13, 20, 22, 24, 26, 27). Amongst the older studies, Swanson et al. (20) performed FOB in 90.7% of patients; lobar localization of bleeding site was possible in 65% and side localization in 10%, while FOB was unhelpful in 25%. Similarly, in a study by Lee et al. (22), bleeding site lateralization was possible in 71%. In studies published after 2010, FOB bleeding lobe localization varied from 54% to 75% (6, 8, 26). Agmy et al. (7) performed FOB in 82.5% of patients; lobar localization of bleeding site was possible in 74.5%, side localization in 15%, while FOB was not helpful in 10.5%.
Multidetector CT was done in eight studies and in 81%–100% of the sample patients in these studies (6–8, 13, 12, 20, 22, 25). Seven studies performed only contrast-enhanced CT, while Agmy et al. (7) performed CT pulmonary angiography in 300 of 348 patients prior to BAE. CT bronchial angiography was not performed by any of these authors for delineation of bronchial arteries. Woo et al. (6) and Fruchter et al. (9) could localize site of bleeding in 91%, while Shin et al. (25) could identify cause of hemoptysis in 92% but site and lobe localization for bleeding were only 36.2% and 30.5%, respectively. Similarly, in the study by Agmy et al. (7), the cause of hemoptysis could be determined in 83%, while site of bleeding was seen in only 14.3%.
Currently, there are no established recommendations regarding preprocedural FOB and CTA, and performing these procedures prior to BAE is an individualized decision depending on institutional availability. Also there is lack of data regarding the utility of these investigations prior to BAE in terms of decreasing BAE procedure time and improving outcomes of BAE. The American College of Radiology recommends doing a chest radiograph in the initial evaluation of all patients with hemoptysis, since it not only lateralizes the bleeding but also identifies the cause in majority of the cases (29). CTA is the next most appropriate investigation in patients with significant hemoptysis (29). CT is similar to bronchoscopy in localizing the bleeding but fares better in detecting the cause and lays down vascular roadmap for therapeutic interventions. Patients with massive hemoptysis may be directly taken for embolization or surgery without CT (29).
Technique of BAE
The technique of BAE has been described under the following headings.
Route
All authors used the transfemoral route to access the bronchial circulation. Agmy et al. (7) used transaxillary route in six patients, while Bhalla et al. (10) used transbrachial route in one patient in whom the aorta could not be negotiated due to tortuosity and underlying scoliosis. Agmy et al. (7) also performed pulmonary artery angiography in seven patients, in whom preprocedural CT pulmonary angiography showed pulmonary artery aneurysms secondary to Behcet’s disease. In the study by Rabkin et al. (17), pulmonary artery was responsible for 93% of recurrent hemoptysis after initial hemoptysis control was not achieved with BAE.
Flush aortogram
Nearly all authors except three (8, 9, 19) performed prior flush aortograms to identify abnormal bronchial and nonbronchial systemic collaterals. Shin et al. (25) did not perform aortogram in 57 out of 163 patients (35%) and the recurrence rate in the group without aortogram was significantly higher (P = 0.001).
The abnormal findings on angiography necessitating embolization included enlarged or tortuous arteries, hypervascularity, parenchymal blush, active contrast extravasation, bronchopulmonary shunting, artery-artery and artery-vein shunting. After identification of abnormal bronchial arteries or nonsystemic bronchial collaterals, selective catheterization of these vessels were performed prior to embolization.
Superselective catheterization
Superselective catheterization of abnormal arteries using microcatheters was almost routinely performed by all authors after 2010 except Pei et al. (8) and Fruchter et al. (9). Amongst the earlier published studies, superselective embolization was notably performed in four studies (19, 20, 22, 23). Superselective catheterization enabled bypassing anterior spinal arteries and catheterization of smaller, more distal, and torturous arteries; thereby providing better overall hemoptysis control and lesser risk of complications (6, 7, 10, 25).
Culprit bronchial arteries
Bhalla et al. (10) found right intercostobronchial trunk to be the most common culprit artery; while in the other studies right bronchial and common bronchial were the most common culprit arteries (Table 4; Figs. 2, 3).
Table 4.
Incidences of culprit arteries during BAE
| Bronchial arteries | Nonbronchial systemic collaterals | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Right bronchial (%) | Left bronchial (%) | Common bronchial (%) | Right ICBT (%) | Intercostal (%) | Internal mammary branches (%) | Subclavian and its (%) | |
| Agmy et al. (7) | 65 | 23 | 45 | 23 | |||
|
| |||||||
| Anuradha et al. (27) | 84.5 | 53.4 | 35 | 20 | |||
|
| |||||||
| Bhalla et al. (10) | 19 | 23 | 56 | 93 | 11 | 10 | 7 |
|
| |||||||
| Chan et al. (23) | 65 | 52 | 33 | 12 | 13 | ||
|
| |||||||
| Dabo et al. (13) | 46.3 | 20.3 | 32.8 | 50 (break-up not available) | |||
|
| |||||||
| Fruchter et al. (9) | 71 | 21 | 7.7 | Not mentioned | |||
|
| |||||||
| Heuvel et al. (21) | 64 (break-up not available) | 47 (break-up not available) | |||||
|
| |||||||
| Lee et al. (22) | 89 | 11 | 22 | 11 | |||
|
| |||||||
| Ramakantan et al. (18) | 14.1 | 14.9 | 25.4 | 23 | 21.7 | 0.4 | 0.4 |
|
| |||||||
| Shao et al. (11) | 29 | 21 | 18 | 2 | 42 | 17 | |
|
| |||||||
| Tom et al. (12) | 40 | 32 | 28 | 40 (break-up not available) | |||
|
| |||||||
| Yoo et al. (26) | 31.7 | 21.3 | 6 | 27.3 | |||
ICBT, intercostobronchial trunk.
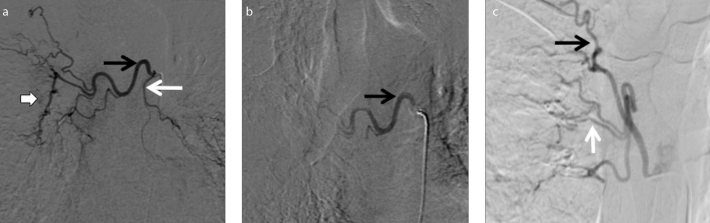
Figure 2. a–c.
Orthotopic bronchial arteries. Preembolization digital subtraction angiography (DSA) image (a) shows selective catheterization of common bronchial artery giving rise to hypertrophied right bronchial artery (black arrow) and left bronchial artery (white arrow) with parenchymal blush (white block arrow). Postembolization DSA image (b) shows contrast stasis in right bronchial artery (black arrow), obliterated left bronchial artery and no residual parenchymal blush suggestive of successful embolization. DSA image in a different patient (c) shows selective run of hypertrophied right intercostobronchial trunk giving rise to right bronchial artery (white arrow) and intercostal artery (black arrow).
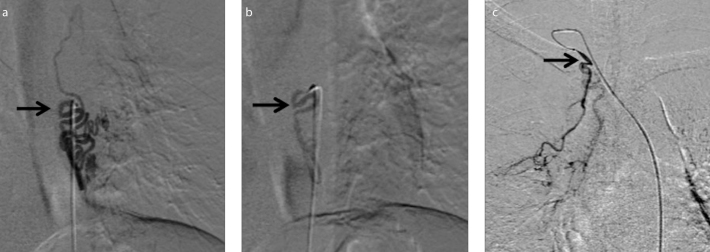
Figure 3. a–c.
Ectopic bronchial arteries. Preembolization DSA image (a) shows selective catheterization of hypertrophied tortuous left bronchial artery, ectopically arising from descending thoracic aorta at D8 level (black arrow). Postembolization DSA image (b) shows contrast stasis and decreased vascularity in left bronchial artery (black arrow) suggestive of successful embolization. DSA image (c) shows ectopic right bronchial artery arising from right subclavian artery (black arrow) in a different patient.
Nonbronchial systemic collaterals
These were encountered and embolized in the first session in about 40%–62% of patients by various authors (6, 7, 9–12, 15, 19–27). This was in contrast to Ramakantan et al. (18) who did not attempt to search for or embolize nonbronchial systemic collaterals in the first session. In general, the search for nonbronchial systemic collaterals reduced rates of recurrence and lead to better overall hemoptysis control (27). The intercostal arteries and internal mammary arteries were the most frequently embolized nonbronchial systemic collaterals (Table 4). Other collaterals included branches of subclavian artery, namely lateral thoracic, costocervical and cervicothoracic arteries and inferior phrenic arteries (Fig. 4).
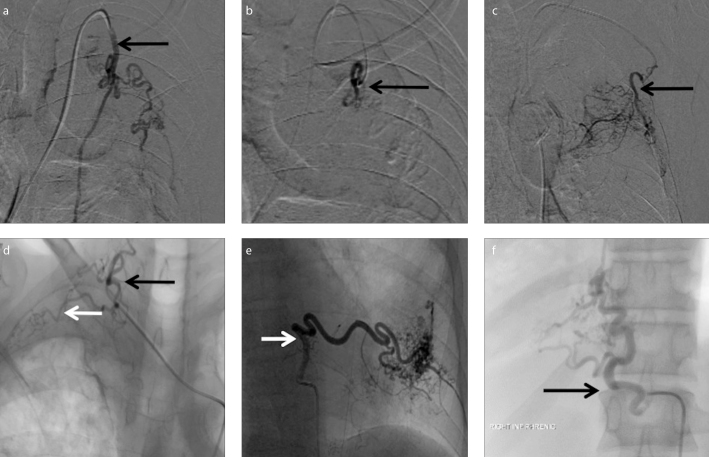
Figure 4. a–f.
Nonbronchial systemic collaterals. Preembolization DSA image (a) shows selective catheterization of hypertrophied left internal mammary artery (arrow) arising from left subclavian artery. Postembolization DSA image (b) shows successful selective embolization of left internal mammary artery branches with decreased vascularity and parenchymal blush (arrow). DSA image (c) of another patient shows selective catheterization of hypertrophied left lateral thoracic artery (arrow) arising from left subclavian artery. DSA image (d) shows hypertrophied right costocervical artery with normal cervical component (black arrow) and abnormal parenchymal blush from the costal component (white arrow) in another patient. DSA image (e) of a different patient shows selective catheterization of hypertrophied left posterior intercostal artery (arrow) with significant parenchymal blush. DSA image (f) of another patient shows hypertrophied right inferior phrenic artery (arrow).
Technical success, i.e., the ability to cannulate and embolize all visualized abnormal arteries varied from 81% to 100% (6, 10, 12, 18, 20, 23). Causes of technical failure included uncooperative patient or patient condition not permitting BAE, artery tortuosity, dissection or vasospasm, ostial narrowing and acute or multiple branching. Nonbronchial systemic collaterals were significantly associated with a higher rate of technical failure and unsuccessful embolization (23). While authors generally embolized a mean of 1.8 to 2.1 arteries per session (7, 23–25), Bhalla et al. (10) embolized a maximum of four arteries per session and Anuradha et al. (27) could embolize a mean of four arteries per session without any complications.
Embolizing agents
The choice of embolizing agents also evolved over the years. While older studies predominantly used gelatin sponge and a motley of other embolizing agents such as dura mater, macroaggregated human albumin, and hypertonic saline. Embolizing agents became more standardized over the years.
The most common preferred embolizing agent was polyvinyl alcohol (PVA; size range, 150–1200 μm) with most commonly used size being 300–500 μm. Studies published after 2010 reported use of wider range of PVA particles (150–1200 μm) compared with the limited 300–600 μm range used earlier. PVA was either used alone or in combination with other embolizing agents. Unlike gelatin sponge, PVA is nonresorbable and thus acts as a permanent occluding agent and is also available in a variety of sizes. The disadvantages of PVA include clumping within the microcatheter leading to more proximal occlusion and catheter blockage. It is also not advisable to use PVA particles less than 300 μm as these can potentially pass through broncho-pulmonary anastomoses which have a mean diameter of 325 μm and cause pulmonary infarcts (3). Three studies (7, 10, 27), all published after 2010, also used PVA particles less than 355 μm for embolization and in all these three studies embolization was performed after superselective catheterization using a 3 F microcatheter.
Few authors also used microspheres in the size range of 100–900 μm and 250–1300 μm (9, 12). Microspheres are hydrophilic and are more uniform in size compared with PVA and thus less prone for clumping within catheters (3).
Liquid embolizing agents such as n-Butyl-2-cyanoacrylate (NBCA) were less preferred earlier as the primary embolizing agent due to the need for greater expertise and more chances of necrosis and other complications. However, Woo et al. (6) recently published a study comparing the safety and efficacy of NBCA versus PVA. In their study, 293 patients embolized with PVA were compared with 113 patients embolized with NBCA. There were no statistically significant differences in the technical and clinical success and complication rates between the groups. NBCA was associated with a better hemoptysis control rate in patients with bronchiectasis. Patients embolized with NBCA also experienced a higher long-term hemoptysis-free survival rate. This was attributed to lower same-vessel recanalization rate with NBCA than with PVA (6).
On the other hand, Shin et al. (25) found no significant difference in the recurrence rates in patients embolized with PVA (n=137) versus 26 patients embolized with gelatins sponge/coils. Swanson et al. (20) also used a variety of embolizing agents and found no association between the embolizing agents used and recurrence. However, the latter two studies had a lesser number of patients in non-PVA group for comparison and this may have contributed to the conflicting results.
In general, the choice of embolizing agent depends chiefly on operator preference and local availability. Coils provide more proximal occlusion compared with PVA, gelatin sponge, and liquid embolizing agents, and thus were used for embolization of pseudoaneurysms, arteriovenous malformations and nonbronchial systemic collaterals in combination with other embolizing agents (7, 9, 11, 13, 20, 22, 25–27).
No data was available regarding procedure duration and radiation doses in any of these studies.
Outcomes of BAE
The outcomes of BAE were described in terms of: a) immediate clinical success, b) hemoptysis recurrence rates, and c) long-term hemoptysis control rates. Immediate clinical success referred to complete cessation or clinically significant reduction in hemoptysis within 24 hours of BAE or within the same admission, while recurrences were defined as clinically significant hemoptysis occurring after discharge, requiring either hospital admission, medical management, or repeat intervention either as bronchoscopy, repeat embolization or surgery. Recurrences were further divided into early recurrences within 2 weeks to 3 months of BAE and late recurrences occurring after 3 months.
The immediate clinical success of BAE varied from 70%–99% in various studies (Table 1). The hemoptysis recurrence rate was as high as 9.8%–57.5% in studies published after 2010 compared with 15.5%–47% recurrence rate in the older studies (Table 1). Thus, there was no significant decrease in hemoptysis recurrence rate despite availability of improved hardware and permanent and more durable embolizing agents. This is not surprising as BAE essentially remains a palliative procedure to manage hemoptysis in patients with diffuse or bilateral lung disease or poor pulmonary reserve who are otherwise unfit to undergo surgery (3, 16).
The median time for recurrent hemoptysis varied between 6 months to 1 year and the recurrence rate increased with time. All the studies with a long-term patient follow-up reported a reduction in cumulative hemoptysis control rate as shown in Table 5. Fruchter et al. (9) observed that patients who achieved complete initial hemoptysis control or were hemoptysis free by 3 years had excellent prognosis and were less likely to rebleed. On the other hand, Goh et al. (19) observed that those who rebleed tend to rebleed, thereby requiring multiple repeat BAE sessions or surgery as a definitive management.
Table 5.
Short-term and long-term cumulative hemoptysis control rate
| Author | Year | Cumulative hemoptysis control rate (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <1 month | 1 month | 1–3 months | 6 months | 1 year | 2 years | 3 years | 5 years | ||
| Chun et al. (15) | 2010 | - | 81.8 | - | - | 69.9 | - | 35.9 | - |
| Shin et al. (7) | 2011 | - | 94.4 | - | - | 76.1 | 62.9 | 51.4 | - |
| Yoo et al. (26) | 2011 | - | 91.4 | - | - | 83.4 | - | 76.7 | 56.8 |
| Anuradha et al. (27) | 2012 | 93.1 | 85.7 | 79.5 | 63.2 | 51 | - | - | - |
| Hwang et al. (5) | 2013 | - | 84.7 | 76.2 | 71.4 | 59.4 | 55.7 | 50.6 | - |
| Pei et al. (8) | 2014 | 86.6 | 84.8 | - | 78.6 | 75.9 | - | - | - |
Early recurrences were attributed to technically inadequate or incomplete embolization due to lack of complete search for all offending vessels or inability to embolize all arteries including nonbronchial systemic vessels in the first session due to extensive collateralization. Late recurrences were attributed to recanalization of previously embolized arteries or recruitment of new arteries, especially nonbronchial systemic arteries, due to underlying disease progression (10, 17, 22, 23, 25). While repeat BAE sessions helped in long-term hemoptysis control (19, 22, 23), surgery remained the definitive treatment for hemoptysis intractable to multiple repeat embolization sessions and maximum medical treatment (9, 18).
Certain angiographic features such as: a) presence of nonbronchial systemic collaterals, b) bronchopulmonary shunting, and c) incomplete initial embolization were significantly associated with higher recurrence rates (5, 12, 13, 22, 23). While a higher recurrence rate has been reported previously in patients with pleural thickening more than 10 mm as it is associated with recruitment of nonbronchial systemic collaterals (30, 31), none of the studies in this review observed a significant association between pleural thickening and recurrence.
The outcomes and long-term efficacy of BAE also varied according to the underlying disease. Recurrence rate was significantly higher in patients with aspergillomas and cavities (5, 15, 16, 21, 25, 26). In the study by van den Heuvel et al. (21), patients with aspergillomas formed 18% of patient population and were associated with an odds ratio of 5.1 for recurrent bleeding as compared to patients without aspergillomas. Similarly in the study by Shin et al. (25), patients with aspergillomas formed 17.2% of patient population and were associated with a significantly higher recurrence rate (P = 0.015).
Active tuberculosis (TB), especially reactivation TB and multidrug resistant (MDR) TB were considered risk factors for recurrence. Lee et al. (22) observed a significantly higher recurrence rate and a shorter recurrence-free time in patients with active TB (P = 0.005). Similarly, in a study by Shin et al. (25), the disease activity showed a statistically significant association; with recurrence being higher in patients with reactivation TB than active TB (P = 0.018). Hwang et al. (5) also observed a higher risk of rebleeding in patients with MDR TB than in patients with non-MDR TB. On the other hand, non-MDR TB was less likely to be associated with repeat episodes of bleeding due to the additive beneficial effect of antitubercular therapy in controlling the underlying disease (5, 9). Other risk factors for poor hemoptysis control included lung malignancy, cystic fibrosis, and idiopathic bronchiectasis (9, 24).
Complications
The most common complications after BAE included transient chest/back pain and dysphagia which were reported in 1.4%–34.5% and 0.7%–30%, respectively (6, 9, 11, 13, 15, 16, 18, 22–27). These figures were lower than those reported previously in older reviews and in studies with less than 50 patients (2, 3). This can be attributable to the greater expertise and experience of the authors of larger studies. Postembolization syndrome comprising of fever, leukocytosis, and pain was another common, self-limiting complication seen in 1.7%–31% (6, 11, 23, 27). Contrast media hypersensitivity, groin puncture hematomas, and femoral artery pseudoaneurysms at puncture site were also reported (5, 6, 9, 11–13, 20, 24–27).
Vascular injuries such as vasospasm, dissection and perforation with wire or catheter were reported in 0.3%–13% (10–13, 20, 22, 23, 25, 27). These vascular complications were usually asymptomatic and self-limiting but contributed to technical failure of BAE. In a study by Uflacker et al. (16), there was transient occlusion of anterior tibial arteries in two patients due to reflux of gelatin sponge from aorta leading to transient claudication of foot.
Neurologic complications due to spinal cord ischemia leading to transient or permanent paraparesis or paraplegia occurred in 0.6%–4.4% (7, 9, 15, 18, 23, 24). This was attributed to inadvertent embolization of spinal arteries arising from bronchial or intercostobronchial arteries. However, in two studies with incidence of paraparesis, spinal arteries were not visualized even on retrospective review of the digital subtraction angiography images (18, 21). While some authors considered visualization of anterior spinal artery as a contraindication for BAE (9, 15), superselective catheterization enabled safer embolization in most studies. The neurologic complication rates were similar for studies done both before and after 2010 with Fruchter et al. (9) reporting a 4.4% incidence of neurologic complications, comparable to the 2.7%–4.7% incidence in studies published before 2010 (9, 21). Though Fruchter et al. (9) published their study in 2014, the BAE procedures were done between 1999 and 2012, with a majority of procedures done before 2010. The relatively high incidence of neurologic complications could be attributed to lack of performing superselective embolization. This could have led to reflux of embolic material into anterior spinal artery and subsequent neurologic complications.
Transient ischemia/stroke and cortical blindness were described in 0.6%–2% (7, 23, 24, 27) and was attributed to manipulation of subclavian arteries or due to embolizing agents crossing bronchopulmonary anastomoses. Remy et al. (15) and Fruchter et al. (9) also encountered isolated cases of bowel necrosis and tracheal fistula due to inadvertent nontarget embolization, while Ramakantan et al. (18) also described 9 patients with transient referred pain to ipsilateral orbit during embolization attributed to stimulation of autonomic nerve fibers.
Conclusion
The key points and recommendations for BAE based on this review have been summarized in Table 6.
Table 6.
Essential take-home points on bronchial artery embolization
| • Most common indications for BAE include tuberculosis, post-tubercular sequelae, bronchiectasis, and aspergilloma |
| • Performed for moderate to massive hemoptysis not controlled by medical/conservative treatment or causing hemodynamic instability |
| • Currently no standard recommendations available on use of FOB or CTA prior to BAE. |
| • Flush aortogram should be performed prior to embolization to identify all abnormal arteries |
| • Superselective catheterization is recommended to ensure more distal embolization and to decrease complication rates |
| • Technical success of BAE varies from 81%–100% |
| • Agent of choice is PVA particles and particles should be chosen so as not to cross broncho-pulmonary anastomoses, which are about 325 μ in size. Glue (N-butyl cyanoacrylate) has been increasingly used in recent years |
| • Immediate clinical success of BAE varies from 70%–99% |
| • Recurrence rate varies from 12%–57% with no decrease in recurrence rates with time |
| • Recurrence attributable to a) incomplete embolization of all abnormal arteries b) recanalization of previously embolized arteries and c) recruitment of new collaterals due to underlying disease progression |
| • Presence of nonbronchial systemic collaterals, bronchopulmonary shunting, aspergillomas, reactivation, and multidrug resistant TB are associated with higher recurrence rates |
| • It is recommended to actively search for and embolize as many nonbronchial systemic collaterals in the first BAE procedure itself to decrease recurrence rates |
| • The median incidence of major complication is 0.1% (0%–6.6%) |
BAE, bronchial artery embolization; FOB, fiberoptic bronchoscopy; CTA, computed tomography angiography; PVA, polyvinyl alcohol; TB, tuberculosis.
The potential limitations of the review include strict inclusion criteria wherein we eliminated studies with less than 50 patients and hence did not analyze the efficacy and outcome of BAE in specific patient populations such as cystic fibrosis and pneumoconiosis. Also this review chiefly talks about embolization of bronchial arteries and bronchial collaterals for control of hemoptysis and does not discuss embolization in causes of hemoptysis secondary to pulmonary artery circulation or in pulmonary arteriovenous malformations.
In conclusion, BAE is a safe, universally accepted procedure for control of hemoptysis of varying etiologies. BAE can be safely performed under both emergent and elective settings. Although there have been technical refinements leading to improved technical and immediate clinical success rates of BAE, hemoptysis recurrence rates remain high. While recurrences can be successfully managed with multiple repeat sessions, BAE essentially remains a palliative procedure for management of hemoptysis in patients who are unfit to undergo more definitive treatments such as surgery.
Main points.
Bronchial artery embolization (BAE) is commonly performed for moderate-massive hemoptysis not controlled by conservative measures. Common underlying diseases include tuberculosis, bronchiectasis, and aspergillomas.
The main difference in studies published before and after 2010 is more frequent use of superselective catheterization in the recent studies (thus decreasing complications) and use of polyvinyl alcohol particles/glue instead of gelatin sponge.
Technical success and immediate clinical success of BAE is high; however, recurrence of hemoptysis may occur.
Recurrence may be attributable to incomplete embolization, recanalization of previously embolized arteries, and recruitment of new collaterals due to underlying disease progression.
Presence of nonbronchial systemic collaterals, bronchopulmonary shunting, aspergillomas, reactivation, and multidrug resistant TB are associated with higher recurrence rates, and hence concurrent treatment of underlying disease, if active, is important.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Remy J, Voisin C, Dupuis C. Traitement des hemoptysies par embolization de la circulation systemique. Ann Radiol. 1974;17:5–16. [PubMed] [Google Scholar]
- 2.Chun JY, Morgan R, Belli AM. Radiological management of hemoptysis: a comprehensive review of diagnostic imaging and bronchial arterial embolization. Cardiovasc Intervent Radiol. 2010;33:240–250. doi: 10.1007/s00270-009-9788-z. https://doi.org/10.1007/s00270-009-9788-z. [DOI] [PubMed] [Google Scholar]
- 3.Sopko DR, Smith TP. Bronchial artery embolization for haemoptysis. Semin Interv Radiol. 2011;28:48–62. doi: 10.1055/s-0031-1273940. https://doi.org/10.1055/s-0031-1273940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorenz J, Sheth D, Patel J. Bronchial artery embolization. Semin Interv Radiol. 2012;29:155–160. doi: 10.1055/s-0032-1326923. https://doi.org/10.1055/s-0032-1326923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang HG, Lee HS, Choi JS, Seo KH, Kim YH, Na JO. Risk factors influencing rebleeding after bronchial artery embolization on the management of hemoptysis associated with pulmonary tuberculosis. Tuberc Respir Dis (Seoul) 2013;74:111–119. doi: 10.4046/trd.2013.74.3.111. https://doi.org/10.4046/trd.2013.74.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woo S, Yoon CJ, Chung JW, et al. Bronchial artery embolization to control hemoptysis: comparison of N-butyl-2-cyanoacrylate and polyvinyl alcohol particles. Radiology. 2013;269:594–602. doi: 10.1148/radiol.13130046. https://doi.org/10.1148/radiol.13130046. [DOI] [PubMed] [Google Scholar]
- 7.Agmy GM, Wafy SM, Mohamed SAA, Gad YA, Mustafa H, Abd El-Aziz AES. Bronchial and nonbronchial systemic artery embolization in management of hemoptysis: experience with 348 patients. Int Sch Res Not. 2013;26:e263259. https://doi.org/10.1155/2013/263259. [Google Scholar]
- 8.Pei R, Zhou Y, Wang G, et al. Outcomes of bronchial artery embolization for life-threatening hemoptysis secondary to tuberculosis. PLoS ONE. 2014;9:e115956. doi: 10.1371/journal.pone.0115956. https://doi.org/10.1371/journal.pone.0115956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fruchter O, Schneer S, Rusanov V, Belenky A, Kramer MR. Bronchial artery embolization for massive hemoptysis: long-term follow-up. Asian Cardiovasc Thorac Ann. 2015;23:55–60. doi: 10.1177/0218492314544310. https://doi.org/10.1177/0218492314544310. [DOI] [PubMed] [Google Scholar]
- 10.Bhalla A, Kandasamy D, Veedu P, Mohan A, Gamanagatti S. A retrospective analysis of 334 cases of hemoptysis treated by bronchial artery embolization. Oman Med J. 2015;30:119–128. doi: 10.5001/omj.2015.26. https://doi.org/10.5001/omj.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shao H, Wu J, Wu Q, Sun X, Li L, Xing Z, Sun H. Bronchial artery embolization for hemoptysis: a retrospective observational study of 344 patients. Chin Med J (Engl) 2015;12:58–62. doi: 10.4103/0366-6999.147811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tom LM, Palevsky HI, Holsclaw DS, et al. Recurrent bleeding, survival, and longitudinal pulmonary function following bronchial artery embolization for hemoptysis in a U.S. adult population. J Vasc Interv Radiol. 2015;26:1806–1813. doi: 10.1016/j.jvir.2015.08.019. https://doi.org/10.1016/j.jvir.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Dabo H, Gomes R, Marinho A, Madureira M, Paquete J, Morgado P. Bronchial artery embolisation in management of hemoptysis - A retrospective analysis in a tertiary university hospital. Rev Port Pneumol (2006) 2016;22:34–38. doi: 10.1016/j.rppnen.2015.09.001. https://doi.org/10.1016/j.rppnen.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Stewart LA, Clarke M, Rovers M, et al. Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA. 2015;313:1657–1665. doi: 10.1001/jama.2015.3656. https://doi.org/10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- 15.Rémy J, Arnaud A, Fardou H, Giraud R, Voisin C. Treatment of hemoptysis by embolization of bronchial arteries. Radiology. 1977;122:33–37. doi: 10.1148/122.1.33. https://doi.org/10.1148/122.1.33. [DOI] [PubMed] [Google Scholar]
- 16.Uflacker R, Kaemmerer A, Picon PD, et al. Bronchial artery embolization in the management of hemoptysis: technical aspects and long-term results. Radiology. 1985;157:637–644. doi: 10.1148/radiology.157.3.4059552. https://doi.org/10.1148/radiology.157.3.4059552. [DOI] [PubMed] [Google Scholar]
- 17.Rabkin JE, Astafjev VI, Gothman LN, Grigorjev YG. Transcatheter embolization in the management of pulmonary hemorrhage. Radiology. 1987;163:361–365. doi: 10.1148/radiology.163.2.3562815. https://doi.org/10.1148/radiology.163.2.3562815. [DOI] [PubMed] [Google Scholar]
- 18.Ramakantan R, Bandekar VG, Gandhi MS, Aulakh BG, Deshmukh HL. Massive hemoptysis due to pulmonary tuberculosis: control with bronchial artery embolization. Radiology. 1996;200:691–694. doi: 10.1148/radiology.200.3.8756916. https://doi.org/10.1148/radiology.200.3.8756916. [DOI] [PubMed] [Google Scholar]
- 19.Yu-Tang Goh P, Lin M, Teo N, En Shen Wong D. Embolization for hemoptysis: a six-year review. Cardiovasc Intervent Radiol. 2002;25:17–25. doi: 10.1007/s00270-001-0047-1. https://doi.org/10.1007/s00270-001-0047-1. [DOI] [PubMed] [Google Scholar]
- 20.Swanson KL, Johnson CM, Prakash UBS, McKusick MA, Andrews JC, Stanson AW. Bronchial artery embolization: experience with 54 patients. Chest. 2002;121:789–795. doi: 10.1378/chest.121.3.789. https://doi.org/10.1378/chest.121.3.789. [DOI] [PubMed] [Google Scholar]
- 21.van den Heuvel MM, Els Z, Koegelenberg CF, Naidu KM, Bolliger CT, Diacon AH. Risk factors for recurrence of haemoptysis following bronchial artery embolisation for life-threatening haemoptysis. Int J Tuberc Lung Dis. 2007;11:909–914. [PubMed] [Google Scholar]
- 22.Lee S, Chan JWM, Chan SCH, et al. Bronchial artery embolisation can be equally safe and effective in the management of chronic recurrent haemoptysis. Hong Kong Med J. 2008;14:14–20. [PubMed] [Google Scholar]
- 23.Chan VL, So LKY, Lam JYM, et al. Major haemoptysis in Hong Kong: aetiologies, angiographic findings and outcomes of bronchial artery embolisation. Int J Tuberc Lung Dis. 2009;13:1167–1173. [PubMed] [Google Scholar]
- 24.Chun JY, Belli AM. Immediate and long-term outcomes of bronchial and non-bronchial systemic artery embolisation for the management of haemoptysis. Eur Radiol. 2010;20:558–565. doi: 10.1007/s00330-009-1591-3. https://doi.org/10.1007/s00330-009-1591-3. [DOI] [PubMed] [Google Scholar]
- 25.Shin BS, Jeon GS, Lee SA, Park MH. Bronchial artery embolisation for the management of haemoptysis in patients with pulmonary tuberculosis. Int J Tuberc Lung Dis. 2011;15:1093–1098. doi: 10.5588/ijtld.10.0659. https://doi.org/10.5588/ijtld.10.0659. [DOI] [PubMed] [Google Scholar]
- 26.Yoo DH, Yoon CJ, Kang SG, Burke CT, Lee JH, Lee CT. Bronchial and nonbronchial systemic artery embolization in patients with major hemoptysis: safety and efficacy of N-butyl cyanoacrylate. AJR Am J Roentgenol. 2011;196:W199–204. doi: 10.2214/AJR.10.4763. https://doi.org/10.2214/AJR.10.4763. [DOI] [PubMed] [Google Scholar]
- 27.Anuradha C, Shyamkumar NK, Vinu M, Babu NRSS, Christopher DJ. Outcomes of bronchial artery embolization for life-threatening hemoptysis due to tuberculosis and post-tuberculosis sequelae. Diagn Interv Radiol. 2012;18:96–101. doi: 10.4261/1305-3825.DIR.3876-11.2. [DOI] [PubMed] [Google Scholar]
- 28.Home - CEBM [Internet] [cited 2016 Aug 21]. Available from: http://www.cebm.net/
- 29.Ketai LH, Kirsch J, Kanne JP, et al. ACR Appropriateness Criteria® Hemoptysis. J Thorac Imaging. 2014;29:W19–22. doi: 10.1097/RTI.0000000000000084. https://doi.org/10.1097/RTI.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 30.Uflacker R, Kaemmerer A, Neves C, Picon PD. Management of massive hemoptysis by bronchial artery embolization. Radiology. 1983;146:627–634. doi: 10.1148/radiology.146.3.6828674. https://doi.org/10.1148/radiology.146.3.6828674. [DOI] [PubMed] [Google Scholar]
- 31.Tamura S, Kodama T, Otsuka N, et al. Embolotherapy for persistent hemoptysis: the significance of pleural thickening. Cardiovasc Intervent Radiol. 1993;16:85–88. doi: 10.1007/BF02602984. https://doi.org/10.1007/BF02602984. [DOI] [PubMed] [Google Scholar]