Abstract
Transjugular intrahepatic portosystemic shunt (TIPS) insertion is commonly performed for refractory ascites or variceal bleeding. However, TIPS dysfunction can be seen in both early and late settings, with shunt thrombosis a particular problem. Treatment of shunt dysfunction commonly involves angioplasty and re-lining, with or without embolectomy, mechanical thrombectomy, or thrombolysis. Ultrasound-assisted thrombolysis has been shown to be successful for treatment of pulmonary embolism, deep vein thrombosis, and peripheral arterial thromboembolism, but has not been described before for TIPS occlusion. Ultrasound is theorized to lead to a shortened duration of thrombolysis due to thinning of the fibrin clot and exposing plasminogen receptor sites. In this technical report, we describe the first published use of ultrasound-assisted thrombolysis in the declotting of an occluded TIPS. We found that the use of ultrasound-assisted thrombolysis allowed a relatively short duration of thrombolytic therapy, with removal of thrombus extending into the portal vein, facilitating stent re-lining. No complications were observed, in particular no bleeding complications. The TIPS remains patent at 8 months postprocedure.
Transjugular intrahepatic portosystemic shunt (TIPS) insertion has been proven to be of benefit in secondary prophylaxis of variceal bleeding, and the treatment of refractory ascites in the setting of portal hypertension.
Although the use of covered stent-grafts has significantly reduced shunt dysfunction, which is usually due to stenosis from pseudo-intimal hyperplasia or shunt thrombosis and occlusion, this still occurs in 24% within two years (1). Stenosis is typically treated with angioplasty with or without stent-graft relining. Thrombosis can be either an acute or chronic event, with early thrombosis often due to technical factors at the time of insertion (2). Recanalization of the occluded TIPS is often attempted with angioplasty and re-lining, but if this is unsuccessful or not indicated, more advanced techniques have been described; suction embolectomy, thrombolysis, and mechanical thrombectomy.
The EKOS endovascular system (EKOS Corp.) is a device utilizing a multi-side-hole catheter with an integral core ultrasound emitter. Thrombolytic agent is administered with simultaneous localized ultrasound pulsing through the catheter, which is theorized to thin the localized fibrin clot and expose plasminogen receptor sites (3). By doing so treatment times can be reduced (4, 5) and there may be improved degree of lysis (3). Clinical success has been demonstrated in the treatment of pulmonary embolism (4), deep vein thrombosis (6), and peripheral arterial thromboembolism (5).
In this report, we describe the first published use of ultrasound-assisted thrombolysis for TIPS occlusion.
Technique
A 47-year-old woman with decompensated cirrhosis (secondary to alcohol) underwent TIPS for recurrent paracentesis. There was no past history of variceal bleeding, and the patient refused diagnostic gastroscopy. The initial TIPS was performed between the right hepatic vein and a right portal vein branch, with implantation of a 10 mm × 6 cm × 2 cm Viatorr (Gore Medical) stent-graft (Fig. 1).
Figure 1.
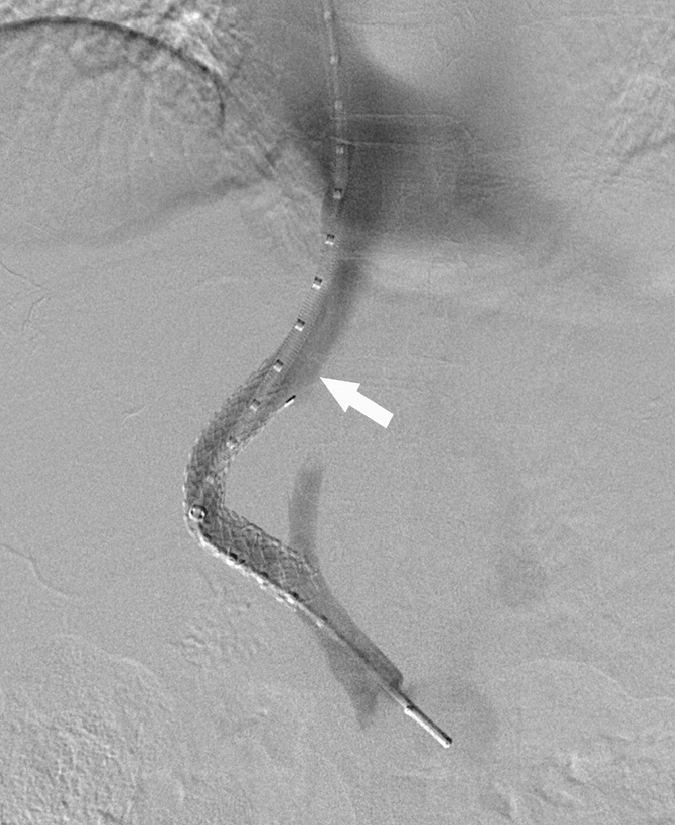
Digital subtraction angiogram of the initial transjugular intrahepatic portosystemic shunt (TIPS) procedure, with good flow seen through the stent at the time of procedure completion. Note how the TIPS stent is short of the hepatic vein confluence with inferior vena cava (arrow).
Seven weeks post TIPS, an ultrasound performed due to reaccumulation of ascites, described reduced flow through the stent-graft. Ten days later, diagnostic venogram found the TIPS to be completely occluded. A contemporaneous CT found thrombus extending from the TIPS into the portal vein.
The decision to revise the TIPS was taken 16 weeks following the initial procedure, and at least two months since the occlusion had formed.
Right internal jugular access was gained, and 10 F TIPS sheath was inserted (Cook Medical), with coaxial 7 F sheath. Multiple catheters and hydrophilic wires were used to try and gain access to the TIPS, but were unsuccessful. Using biplane screening, a Rosch-Uchida needle (Cook Medical) was used to puncture the TIPS at the hepatic venous end (Fig. 2), successfully gaining wire access on the first pass. Venography confirmed TIPS occlusion with portal vein thrombus (Fig. 3), and also demonstrated a large coronary vein varix; this was embolized with an Amplatzer type II plug (St. Jude Medical).
Figure 2.
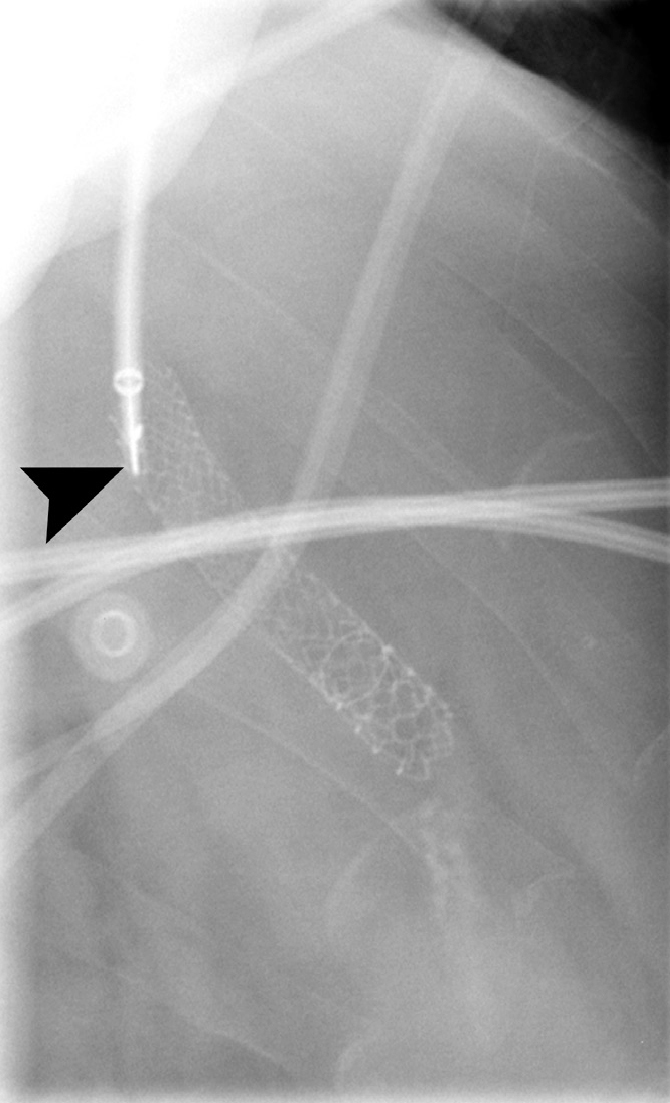
Digital subtraction angiogram in a lateral projection. Note the Rosch-Uchida needle (arrowhead) about to puncture the fibrous occlusion of the TIPS.
Figure 3.
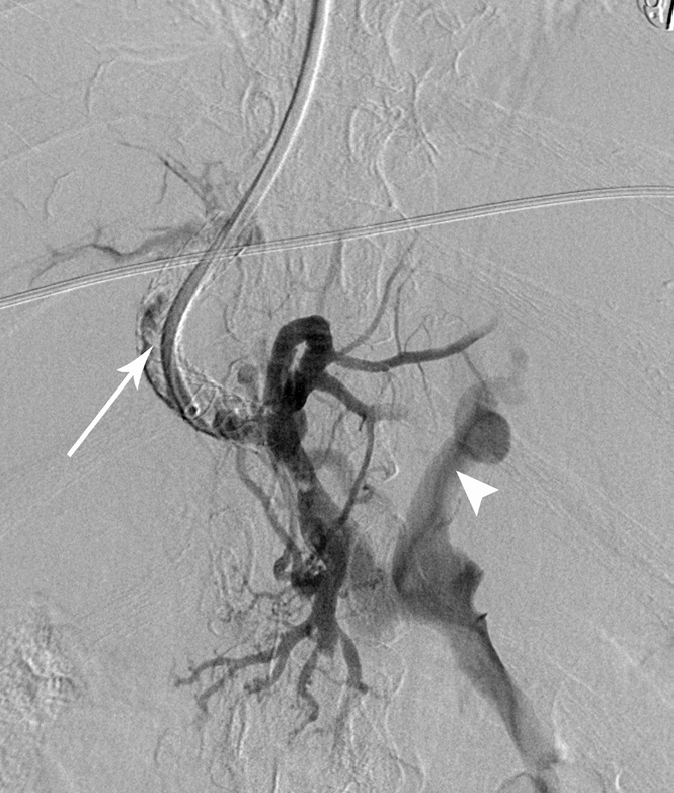
Digital subtraction angiogram of the TIPS following traversal of the occlusion. Heavy thrombus load is seen within the TIPS stent (arrow), with filling of a large collateral vessel (arrowhead).
An EKOS multi-side-hole lysis catheter (EKOS Corp.) was then positioned across the TIPS, and following a 5 mg bolus of alteplase, thrombolysis commenced at a rate of 1 mg/h alteplase.
Five and a half hours postprocedure the EKOS ultrasound generator reported a technical issue. Repeat venogram found a kink in the catheter at the junction with the 7 F sheath, presumably caused by cardiac motion. With difficulty, the catheter was replaced and repositioned, although venogram at this point demonstrated significant resolution of thrombus (Fig. 4). Thrombolysis was continued at 1 mg/h.
Figure 4.
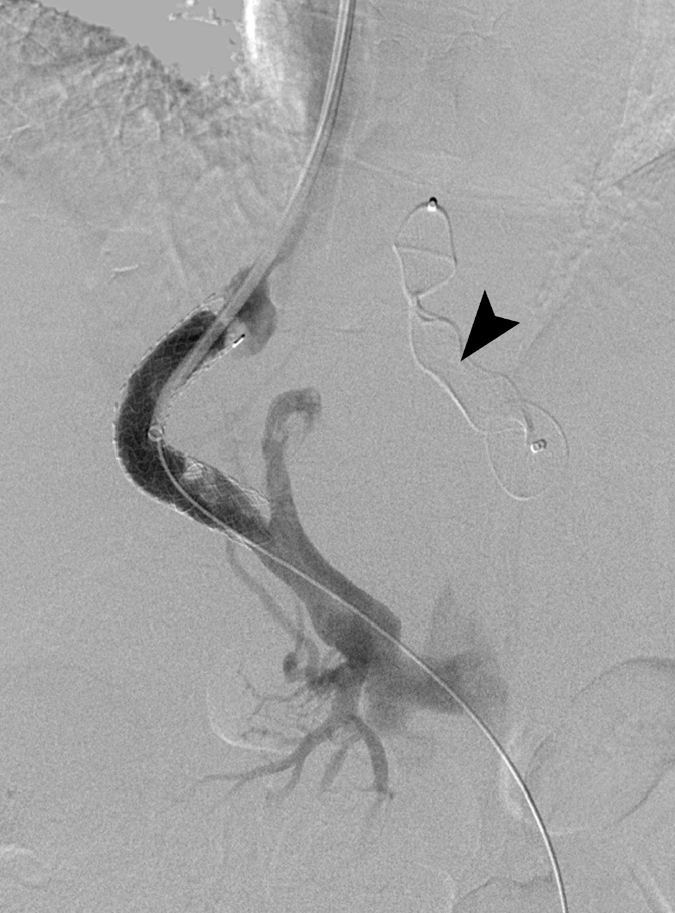
Digital subtraction angiogram following 5.5 hours thrombolysis with the EKOS catheter. Significant improvement in thrombus burden is seen. Also note the embolization of the large collateral with an Amplatzer plug (arrowhead).
After a further 8 hours thrombolysis check venogram showed satisfactory resolution of thrombus. The TIPS was then re-lined with a new 10 mm × 8 cm × 2 cm Viatorr stent-graft, with distal landing zone closer to the hepatocaval junction (Fig. 5). No complications were encountered, in particular no bleeding complications. Follow-up CT 3 days later showed a patent TIPS and portal vein. The TIPS functioned as an eventual bridge to transplant 8 months later.
Figure 5.
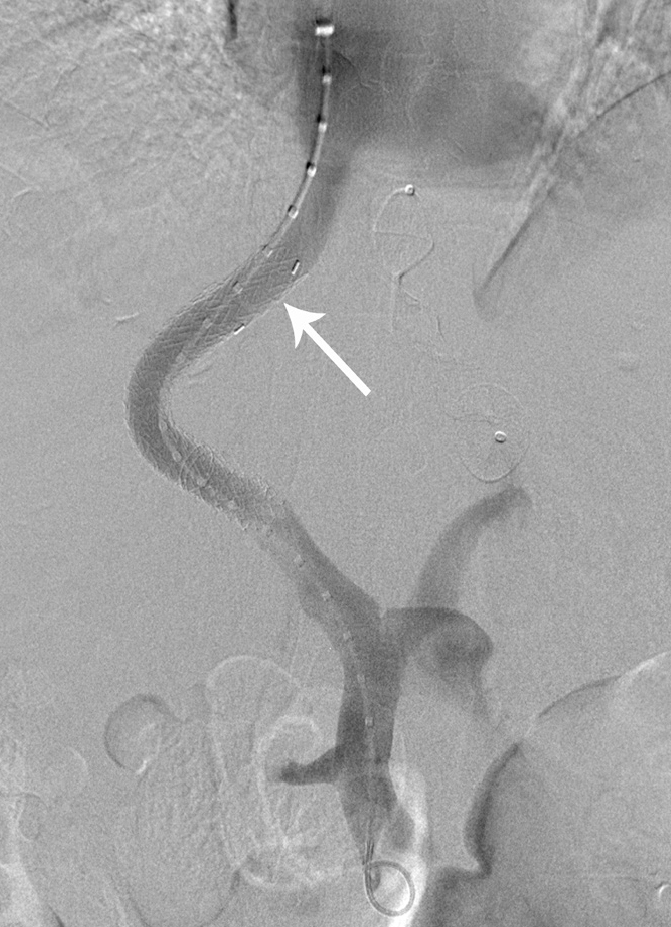
Final digital subtraction angiogram, following TIPS re-lining with good flow through the stent. Note the closer position of the TIPS stent to the hepatocaval confluence (arrow).
Discussion
TIPS dysfunction is important to recognize and treat, as it can lead to recurrent variceal bleeding or ascites accumulation (2). To our knowledge, this is the first published use of ultrasound-assisted thrombolysis for the treatment of TIPS occlusion.
Successful thrombolysis of occluded TIPS has been previously described, using conventional catheters for delivery. However, this approach is recognized to place the patient at risk of bleeding complications. This is particularly important in patients who have TIPS for the secondary prophylaxis of variceal bleeding, or have deranged clotting parameters caused by hepatic dysfunction. In the case described, the TIPS was performed for refractory ascites, with no previous history of variceal bleeding complications, therefore the benefit was felt to outweigh risks.
The advantage of ultrasound-assisted thrombolysis is that it is proven to require shorter infusion time than conventional catheter-directed thrombolysis in treatment of both pulmonary embolism (4) and peripheral arterial thromboembolism (5), with fewer treatment-related hemorrhagic complications (4). Ultrasound accelerates the thrombolytic process through separation of the fibrin strands, which increases the number of plasminogen receptor sites accessible to the fibrinolytic agent (3). In the context of TIPS thrombosis this shortened therapy time minimizes the risk of complications secondary to administration of thrombolysis.
It is likely that the shunt failed acutely due to inadequate extension of the initial stent-graft into the hepatic vein. The most important predictor of long-term shunt patency is placement within the hepatic vein, with extension to the hepatocaval confluence shown to the optimal position. This is an important technical factor to consider when sizing TIPS stents, and furthermore when sizing stent-grafts the addition of 1 cm to catheter measured tract length compensates for the curved course of the TIPS through the liver.
A further technical point to note was the use of the Rosch-Uchida needle to access the occluded hepatic venous end of the TIPS, necessitated due to the inability to cannulate with standard hydrophilic wire and catheter. This procedure has been previously described (7), as has use of the Colapinto needle. If these methods were unsuccessful then percutaneous transhepatic puncture of the TIPS could be considered, but within the setting of thrombolysis, percutaneous access may be less preferable due to the increased bleeding risk.
In our patient, there was thrombus extending to the portal vein, which led us to choose thrombolysis rather than primary venoplasty and stent re-lining. Progression of thrombus to involve portal and superior mesenteric veins after TIPS occlusion has been previously documented (8). Extensive thrombosis risks mesenteric venous ischemia, underlining the need for timely intervention.
In the context of de novo acute mesenteric venous thrombosis, various treatment options have been described, with TIPS creation for access to the portovenous system a preferred approach in the setting of cirrhosis; this would facilitate use of ultrasound-assisted thrombolysis, though we are unaware of any published descriptions of this approach.
A disadvantage of ultrasound-assisted thrombolysis is the additional equipment required for treatment. In contrast to the relatively cheap catheters required for standard catheter-directed thrombolysis, the EKOS system requires an expensive single use catheter and purchase of a reusable base generator unit. However, the advantage of decreased thrombolysis times leading to theoretically reduced bleeding complications may offset this outlay. It should also be noted that an alternative method of TIPS revision using mechanical thrombectomy also requires relatively expensive equipment.
In conclusion, we describe the application of ultrasound-assisted thrombolysis to TIPS thrombosis, a technique that can allow shorter treatment times and therefore decreased exposure to the risks associated with thrombolytic agents. Where conventional angioplasty and stent-graft re-lining is unsuccessful this technique may be a useful approach to revision of the occluded TIPS.
Main points.
Transjugular intrahepatic portosystemic shunt (TIPS) dysfunction can manifest as shunt thrombosis, with early presentation often related to technical factors.
Ultrasound-assisted thrombolysis has been documented to shorten duration of catheter-directed thrombolysis in the setting of pulmonary emboli or peripheral arterial thromboembolism.
In the setting of TIPS occlusion with portal vein thrombosis we demonstrate ultrasound-assisted thrombolysis to be a useful adjunct to traditional techniques.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Bureau C, Pagan JC, Layrargues GP, et al. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long-term results of a randomized multicentre study. Liver Int. 2007;27:742–747. doi: 10.1111/j.1478-3231.2007.01522.x. https://doi.org/10.1111/j.1478-3231.2007.01522.x. [DOI] [PubMed] [Google Scholar]
- 2.Cura M, Cura A, Suri R, et al. Causes of TIPS dysfunction. AJR Am J Roentgenol. 2008;191:1751–1757. doi: 10.2214/AJR.07.3534. https://doi.org/10.2214/AJR.07.3534. [DOI] [PubMed] [Google Scholar]
- 3.Owens CA. Ultrasound-Enhanced Thrombolysis: EKOS EndoWave Infusion Catheter System. Semin Intervent Radiol. 2008;25:37–41. doi: 10.1055/s-2008-1052304. https://doi.org/10.1055/s-2008-1052304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin PH, Annambhotla S, Bechara CF, et al. Comparison of percutaneous ultrasound-accelerated thrombolysis versus catheter-directed thrombolysis in patients with acute massive pulmonary embolism. Vascular. 2009;17( Suppl 3):S137–147. doi: 10.2310/6670.2009.00063. https://doi.org/10.2310/6670.2009.00063. [DOI] [PubMed] [Google Scholar]
- 5.Schrijver AM, van Leersum M, Fioole B, et al. Dutch Randomized Trial Comparing Standard Catheter-Directed Thrombolysis and Ultrasound-Accelerated Thrombolysis for Arterial Thromboembolic Infrainguinal Disease (DUET) J Endovasc Ther. 2015;22:87–95. doi: 10.1177/1526602814566578. https://doi.org/10.1177/1526602814566578. [DOI] [PubMed] [Google Scholar]
- 6.Dumantepe M, Tarhan A, Yurdakul I, Özler A. US-accelerated catheter-directed thrombolysis for the treatment of deep venous thrombosis. Diagn Interv Radiol. 2013;19:251–258. doi: 10.5152/dir.2012.004. [DOI] [PubMed] [Google Scholar]
- 7.Ferral H, Banks B, Wholey M, et al. Techniques for transjugular intrahepatic portosystemic shunt revision. AJR Am J Roentgenol. 1998;171:1041–1047. doi: 10.2214/ajr.171.4.9762993. https://doi.org/10.2214/ajr.171.4.9762993. [DOI] [PubMed] [Google Scholar]
- 8.Beheshti MV, Jones MP. Shunt occlusion and acute portal, splenic, and mesenteric venous thrombosis complicating placement of a transjugular intrahepatic portosystemic shunt. J Vasc Interv Radiol. 1996;7:277–281. doi: 10.1016/s1051-0443(96)70780-7. https://doi.org/10.1016/S1051-0443(96)70780-7. [DOI] [PubMed] [Google Scholar]


