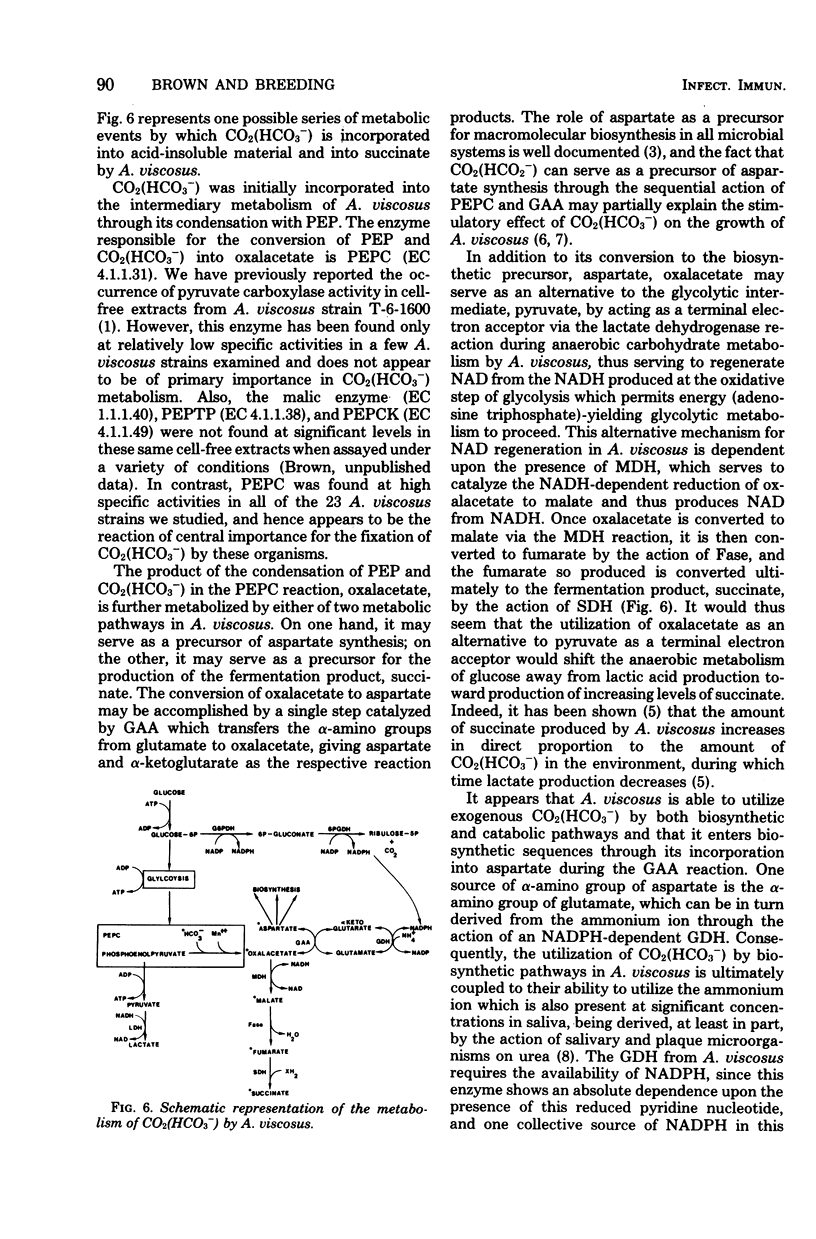
Abstract
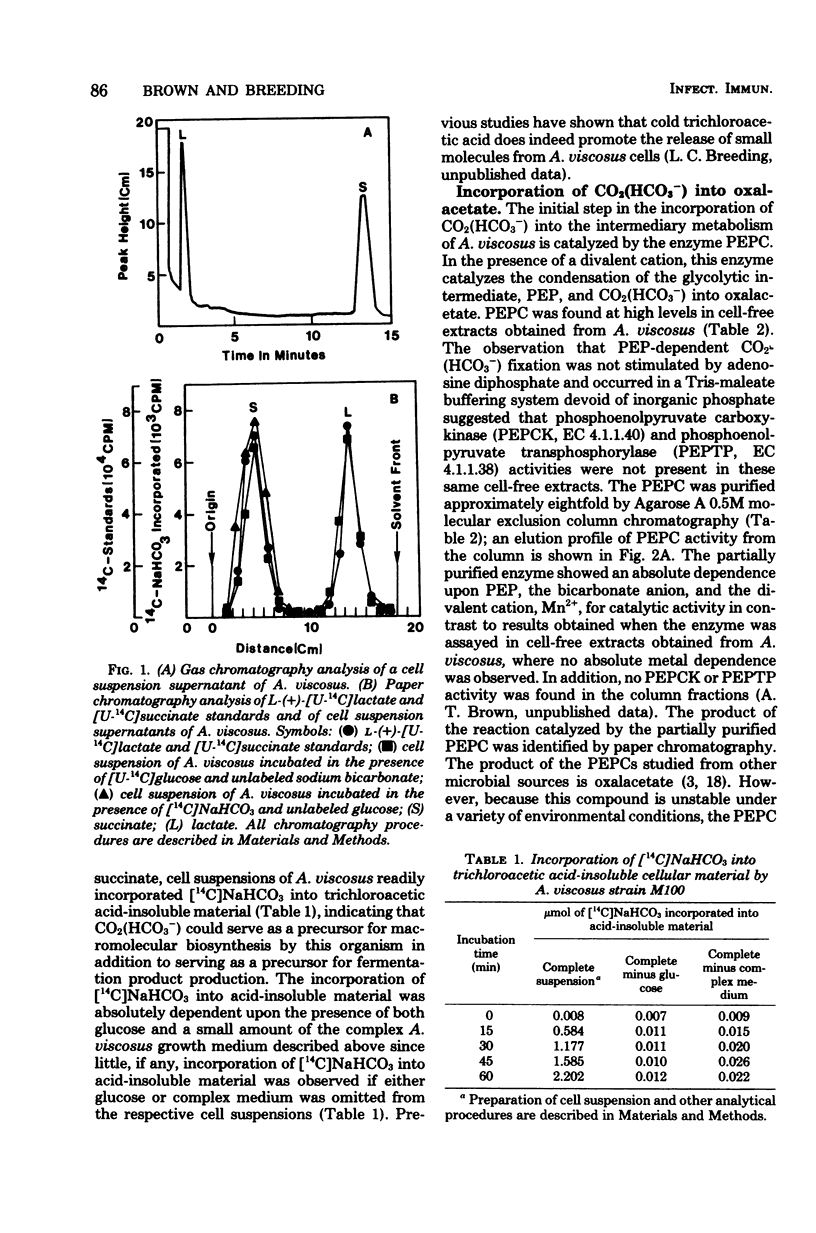
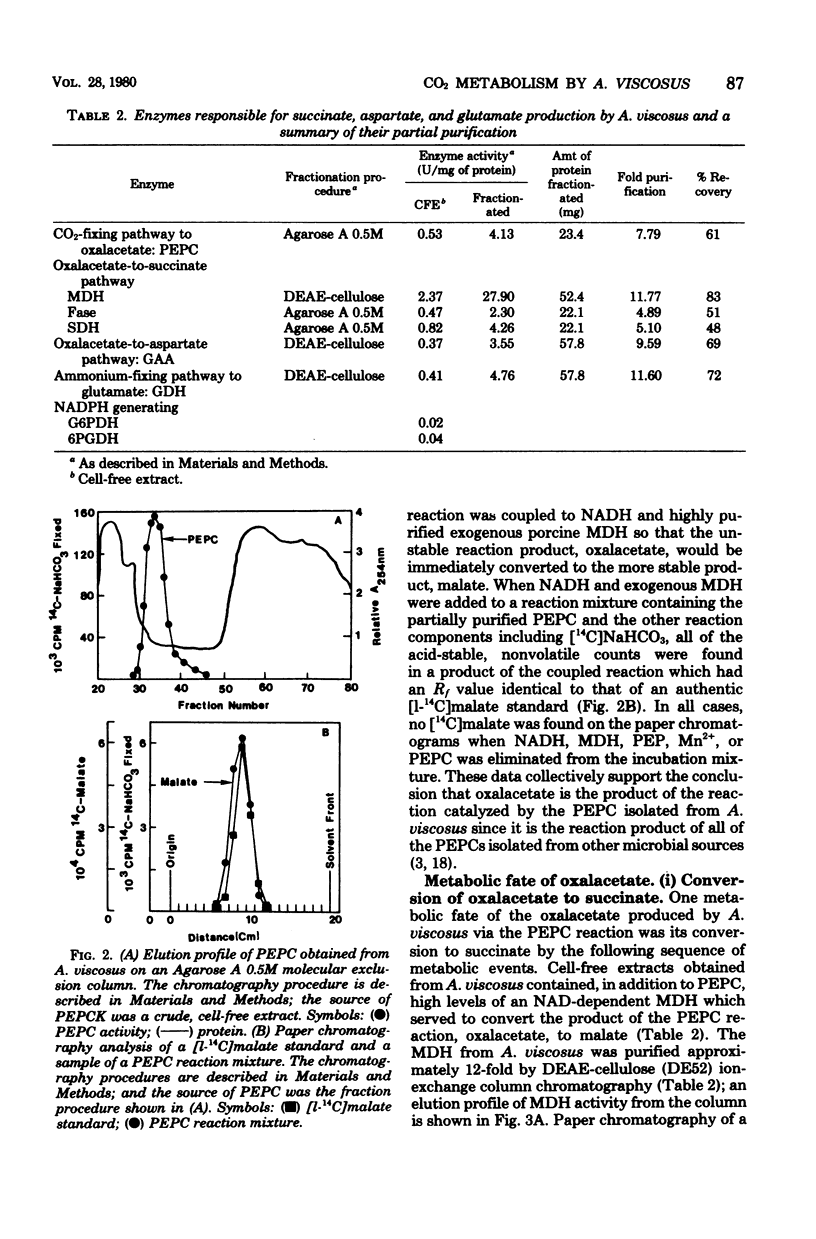
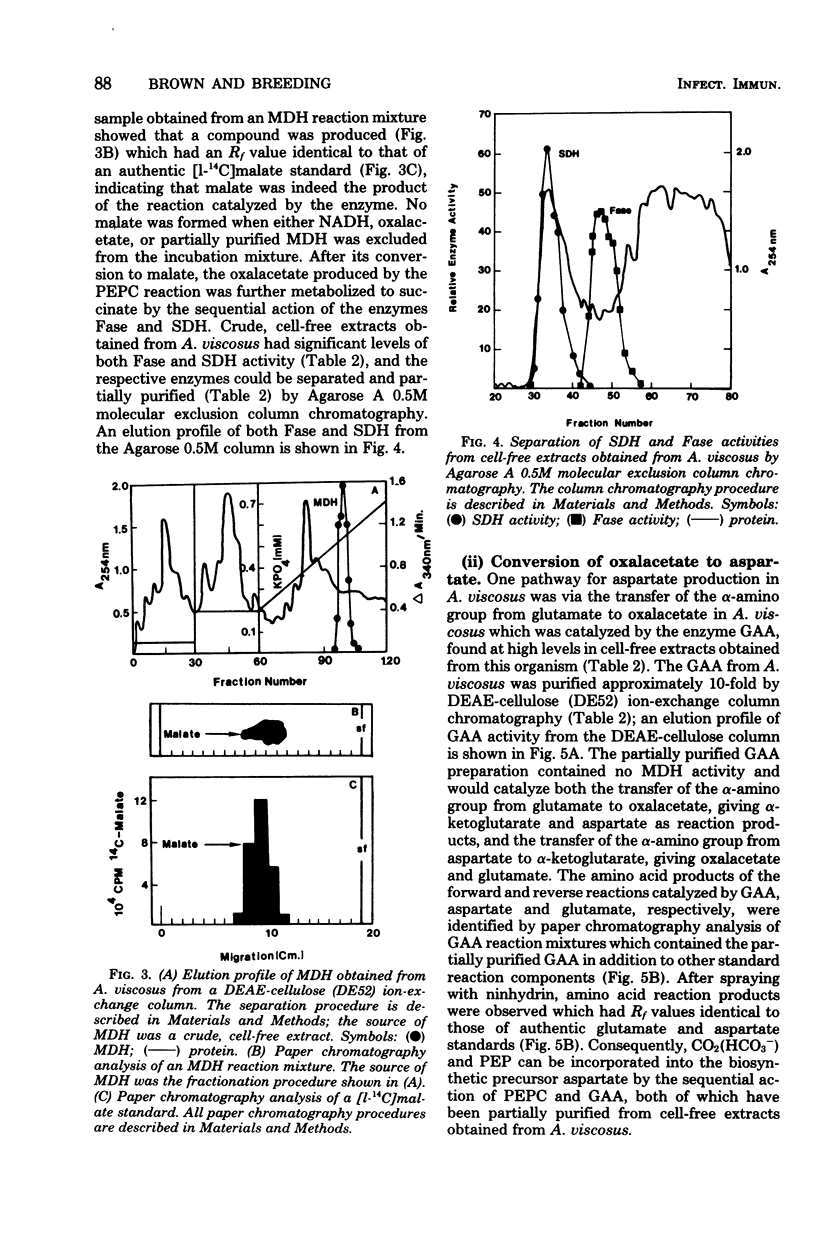
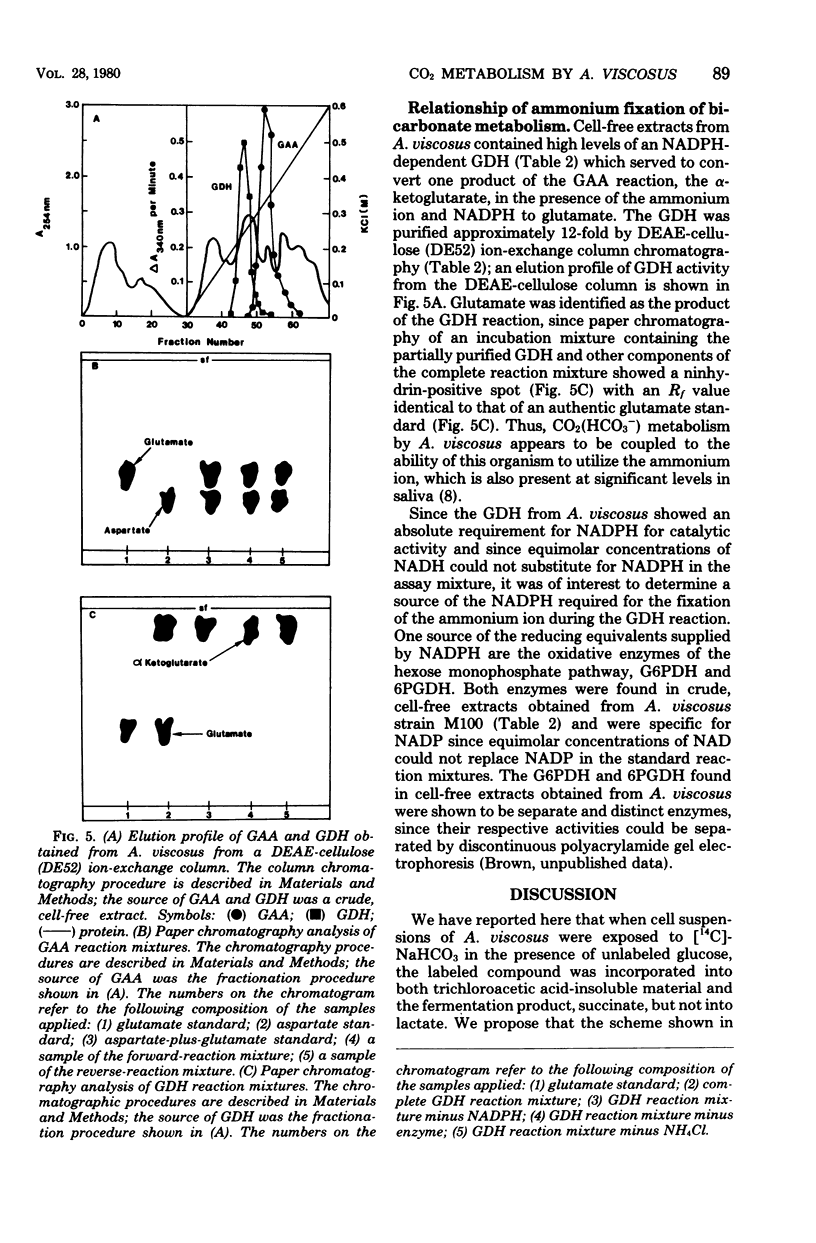
14C-labeled bicarbonate was incorporated into trichloroacetic acid-insoluble material by cell suspensions of A. viscosus strain M100 and also into the four-carbon fermentation product, succinate, but not into the three-carbon fermentation product, lactate. The initial step in the conversion of 14C-labeled bicarbonate into both trichloroacetic acid-insoluble material and succinate was catalyzed by the enzyme phosphoenolypyruvate carboxylase, which served to convert the glycolytic intermediate, phosphoenolpyruvate, and bicarbonate to the four-carbon compound, oxalacetate. The metabolic fate of oxalacetate was its conversion to either trichloroacetic acid-insoluble material or succinate. One pathway by which oxalacetate may be metabolized into acid-insoluble material is via its conversion to the biosynthetic precursor aspartate by the action of glutamate aspartate aminotransferase. One source of the alpha-amino group of aspartate was the ammonium ion, which could be incorporated into glutamate, the substrate of the glutamate aspartate aminotransferase reaction, by the action of a reduced nicotinamide adenine dinucleotide phosphate-dependent glutamate dehydrogenase whose reducing equivalents could be derived from the nicotinamide adenine dinucleotide phosphate-dependent oxidative reactions of the hexose monophosphate pathway catalyzed by glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase. Alternatively, oxalacetate was converted to the fermentation product, succinate, through the sequential action of malate dehydrogenase, fumarase, and succinic dehydrogenase. The resolution and partial purification of phosphoenolpyruvate carboxylase, glutamate aspartate aminotransferase, glutamate dehydrogenase, malate dehydrogenase, fumarase, and succinic dehydrogenase are also reported.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. T., Christian C. P., Eifert R. L. Purification, characterization, and regulation of a nicotinamide adenine dinucleotide-dependent lactate dehydrogenase from Actinomyces viscosus. J Bacteriol. 1975 Jun;122(3):1126–1135. doi: 10.1128/jb.122.3.1126-1135.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. T., Wittenberger C. L. Mechanism for regulating the distribution of glucose carbon between the Embden-Meyerhof and hexose-monophosphate pathways in Streptococcus faecalis. J Bacteriol. 1971 May;106(2):456–467. doi: 10.1128/jb.106.2.456-467.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank R. M., Guillo B., Llory H. Caries dentaires chez le rat gnotobiote inoculé avec Actinomyces viscosus et Actinomyces naeslundii. Arch Oral Biol. 1972 Sep;17(9):1249–1253. doi: 10.1016/0003-9969(72)90157-4. [DOI] [PubMed] [Google Scholar]
- Howell A., Jr A filamentous microorganism isolated from periodontal plaque in hamsters. 1. Isolation, morphology and general cultural characteristics. Sabouraudia. 1963 Oct;3(1):81–92. doi: 10.1080/00362176485190131. [DOI] [PubMed] [Google Scholar]
- Howell A., Jr, Jordan H. V. A filamentous microorganism isolated from periodontal plaque in hamsters. II. Physiological and biochemical characteristics. Sabouraudia. 1963 Oct;3(1):93–105. doi: 10.1080/00362176485190141. [DOI] [PubMed] [Google Scholar]
- JORDAN H. V., KEYES P. H. AEROBIC, GRAM-POSITIVE, FILAMENTOUS BACTERIA AS ETIOLOGIC AGENTS OF EXPERIMENTAL PERIODONTAL DISEASE IN HAMSTERS. Arch Oral Biol. 1964 Jul-Aug;9:401–414. doi: 10.1016/0003-9969(64)90025-1. [DOI] [PubMed] [Google Scholar]
- Jordan H. V., Fitzgerald R. J., Stanley H. R. Plaque formation and periodontal pathology in gnotobiotic rats infected with an oral actinomycete. Am J Pathol. 1965 Dec;47(6):1157–1167. [PMC free article] [PubMed] [Google Scholar]
- Jordan H. V., Hammond B. F. Filamentous bacteria isolated from human root surface caries. Arch Oral Biol. 1972 Sep;17(9):1333–1342. doi: 10.1016/0003-9969(72)90166-5. [DOI] [PubMed] [Google Scholar]
- Jordan H. V., Keyes P. H., Bellack S. Periodontal lesions in hamsters and gnotobiotic rats infected with actinomyces of human origin. J Periodontal Res. 1972;7(1):21–28. doi: 10.1111/j.1600-0765.1972.tb00627.x. [DOI] [PubMed] [Google Scholar]
- KEYES P. H., JORDAN H. V. PERIODONTAL LESIONS IN THE SYRIAN HAMSTER. III. FINDINGS RELATED TO AN INFECTIOUS AND TRANSMISSIBLE COMPONENT. Arch Oral Biol. 1964 Jul-Aug;9:377–400. doi: 10.1016/0003-9969(64)90024-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Llory H., Guillo B., Frank R. M. A cariogenic Actinomyces viscosus--a bacteriological and gnotobiotic study. Helv Odontol Acta. 1971 Oct;15(2):134–138. [PubMed] [Google Scholar]
- Repaske R., Clayton M. A. Control of Escherichia coli growth by CO2. J Bacteriol. 1978 Sep;135(3):1162–1164. doi: 10.1128/jb.135.3.1162-1164.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valley G., Rettger L. F. THE INFLUENCE OF CARBON DIOXIDE ON BACTERIA. J Bacteriol. 1927 Aug;14(2):101–137. doi: 10.1128/jb.14.2.101-137.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]



