Abstract
Aims
To identify variables that can predict upgrade for magnetic resonance imaging (MRI)-detected atypical ductal hyperplasia (ADH).
Methods and results
We reviewed 1655 MRI-guided core biopsies between 2005 and 2013, yielding 100 (6%) cases with ADH. The pathological features of ADH and MRI findings were recorded. An upgrade was considered when the subsequent surgical excision yielded invasive carcinoma (IC) or ductal carcinoma in situ (DCIS). The rate of ADH between institutions was 3.3–7.1%, with an average of 6%. A total of 15 (15%) cases had upgrade, 12 DCIS and three IC. When all cases were included, only increased number of involved cores was statistically significant (P= 0.02). When cases with concurrent lobular neoplasia (LN) were excluded (n= 14), increased number of ADH foci and increased number of involved cores were statistically significant (P= 0.002, P= 0.009). We analysed the data separately from a single institution (n= 61). Increased number of foci, increased number of total cores and involved cores and larger ADH size predicted upgrade with statistical significance.
Conclusions
The incidence of ADH in MRI-guided core biopsy is rare. The rate of upgrade is comparable to mammographically detected ADH, warranting surgical excision. Similar to mammographically detected lesions, the volume of the ADH predicts the upgrade
Keywords: atypical ductal hyperplasia, breast, magnetic resonance imaging, upgrade
Introduction
Breast magnetic resonance imaging (MRI) is being used increasingly for screening women at high risk for developing breast cancer and for identifying sites of additional disease in women with known breast cancer, as well as for other specific clinical indications.1 The pathophysiological basis of MRI detection of malignancies is that it demonstrates rapid uptake and washout kinetics after administration of intravenous contrast when compared to normal breast tissue. However, benign and high-risk lesions may also demonstrate a similar pattern of enhancement. While the benefit of MRI is its higher sensitivity compared to the other breast imaging modalities, such as mammography and ultrasound, its specificity is similar to that of mammography, with published positive predictive values in the range of 35–64%.2,3 Given that benign and high-risk lesions are found not uncommonly at MRI-guided biopsy, their management remains of considerable importance.
Most patients who have atypical ductal hyperplasia (ADH) in a core biopsy undergo surgical excision due to the relative high risk of underestimating the residual disease, such as ductal carcinoma in situ (DCIS) or invasive carcinoma. However, a considerable number of these patients do not have residual disease, and are therefore being overtreated. Therefore, predicting upgrade of ADH diagnosed on core biopsy to a more advanced lesion (DCIS or invasive carcinoma) has attracted more attention in recent years. Studies have shown that certain pathological, clinical or mammographic features can predict upgrade. These include increased number of foci, increased number of involved cores, older patient age, mammographic mass lesion and residual mammographic microcalcifications, among other features.4–11 All these studies investigated stereotactic or ultrasound-guided core biopsies. MRI-guided core biopsies of the breast have increased in recent years, which have increased the number of cases with a diagnosis of ADH. Many studies have focused on the incidence of atypia including ADH in MRI-guided core biopsies,12–26 but unlike mammographically detected ADH, little is known about the factors predicting upgrade of MRI-detected ADH. This is due mainly to the small number of cases at any given single institution. The aim of the current study was to identify clinical, histological and/or radiological variables that can predict upgrade of ADH detected by MRI-guided biopsy to invasive carcinoma or DCIS. In order to conduct this, we reviewed a relatively large number of paired cases (ADH in a MRI-guided core biopsy and subsequent surgical excision) pooled from multiple academic centres.
Material and methods
Cases
At Roswell Park Cancer Institute (RPCI), the radiology records were searched for breast MRI-guided biopsy, yielding 358 cases. The pathology reports were reviewed. Twenty-seven cases had a diagnosis of ADH. Upon slide review by the breast pathologist (T.K.), five cases were excluded due to downgrading to UDH (n = 3) or the presence of concurrent papilloma (n = 2). Cases were retrieved from the University of Pittsburgh Medical Center (UPMC) through a computer-based search in CoPath for the words ‘breast MRI core biopsy’, yielding 862 cases, 61 of which had a diagnosis of ADH. Upon slide review by the breast pathologist (Z.L.), all 61 (7.1%) cases met our criteria and were included in the study. Cases were retrieved from the Washington University (WU) through a computer-based search in CoPath for the words ‘MRI’ in all fields and ‘corebiopsy’ and ‘breast’ in a final diagnosis field yielding 335 cases, 14 of which had ADH. Upon slide review by the breast pathologist (S.S.), 11 (3.3%) cases met our criteria. Two cases were excluded because of concurrent papilloma and one case downgraded to UDH. For Montefiore Medical Center (MMC) cases, the Picture Archiving and Communication System (PACS) was searched yielding 100 cases. The pathology reports were reviewed for a diagnosis of ADH among MRI-guided biopsies, yielding eight cases. Upon slide review by the breast pathologist (R.K.), six (6%) cases met our criteria. Two cases were excluded because of concurrent papilloma.
Demographic data including age, race, hormone intake, previous or concurrent history of cancer and menopausal status were collected for each patient.
This study was approved by the internal review board (IRB) in all four participating institutions [RPCI (EDR#189310, 2011), WU (IRB#201203144,2012), UPMC (PR013080150, 2013) and MMC (Ref#001154, IRB# 2013-2701, 2013)]. The study falls under existing data review category. Therefore, no patients’ consents were required.
Histological Interpretation
All cases from 2005 to 2013 were reviewed by a breast pathologist (T.K., Z.L., S.S., R.K.) to verify the correct diagnosis of ADH. There have been many proposed criteria to differentiate ADH from low-grade DCIS.27–30 In our study, a case was considered ADH if it met the following criteria: (i) regardless of size and degree of involvement, nuclear grade has to be low (monotonous, uniform rounded cells with fine chromatin); and (ii) if size is greater than 2 mm, the terminal duct lobular unit has to be only partially involved.
Histological features of ADH were recorded. The focus was considered completely involved when a monotonous cell proliferation completely filled all spaces. Partial involvement was considered when flat epithelial atypia or usual ductal hyperplasia intermixed with a monomorphic neoplastic proliferation that had a non-flat growth pattern (micropapillary, solid or cribriform). The number of large ducts and/or terminal duct-lobular units affected by ADH was counted. Foci were considered separate when they were present in two different cores or when separated by specialized lobular stroma. We also recorded the number of biopsy cores, the number of cores involved with ADH and the presence or absence of concurrent lobular neoplasia (LN, atypical lobular hyperplasia or lobular carcinoma in situ) and/or flat epithelial atypia.
The excisional specimens which were performed within a maximum of 3 months were reviewed without knowledge of the core biopsy findings. The presence of DCIS, invasive carcinoma, LN, fibrocystic changes, papilloma, radial scar, UDH, fat necrosis and a combination between these changes (other than DCIS and invasive carcinoma) was recorded. To ensure that the targeted lesion was removed, we evaluated the presence of the previous biopsy site. These results were then compared with the extent of variables in the core biopsies.
When the patient presented with concurrent ipsilateral carcinoma, verification from the pathology report and histological review was required to ensure that the lesion was not contiguous to the main tumour. In order to verify this, two biopsy sites had to be recognized, corresponding to each of the two lesions (the main tumour and the studied biopsy with ADH) and had to be separated by noninvolved breast tissue. All cases with concurrent ipsilateral breast cancer were found to be eligible for this study.
Radiological Interpretation
The candidate lesion was evaluated on the contrast-enhanced MRI performed immediately prior to MRI-guided biopsy. The following lesion characteristics were recorded: whether the finding represented a mass or non-mass enhancement and its longest diameter. The images were reviewed by the radiologist in RPCI (P.K.) and MMC (B.R.). The radiology variables were abstracted from the radiology reports in WU and UPMC. Second-look ultrasound was performed prior to MRI-guided biopsy per institutional protocol if the imaging finding was a mass, but not in cases of non-mass enhancement. For all institutions, the radiology biopsy report was reviewed to determine the number of cores obtained and the gauge of the biopsy needle. The report was also reviewed to determine the reason for MRI (such as for staging, high-risk screening or a clinical finding such as nipple discharge).
In the univariate statistical analysis, the outcome upgrade was correlated with predictors using Fisher's exact test for categorical variables and the Wilcoxon non-parametric test for continuous variables, at a nominal significance level of 0.05. Multiple analyses were performed, one including all cases from all institutions, one including pure ADH (excluding LN) and one including only UPMC cases. Multivariate analyses were not performed, due to the fact that only one variable was statistically significant. The statistical analysis was performed using R version 3.0.1 (http://www.r-project.org).
Results
A total of 1655 MRI-guided core biopsy cases were identified [RPCI (n = 358, 21.6%), UPMC (n = 862, 52.1%), MMC (n = 100, 6%) and WU (n = 335, 20.3%)]. The total number of cases with ADH was 100 (6%), ranging from 3.3% at WU to 7.1% at UPMC. The total number of cases with upgrade was 15 (15%). The rate of upgrade ranged from 9.1% for WU to 33% for MMC cases. There was no statistically significant difference in the rate of ADH diagnosis or the rate of upgrade among the contributing institutions (Table 1). The final diagnosis on the excisional biopsy for the cases with upgrade was DCIS (n = 12) or invasive carcinoma of no special type (n = 3). The histological changes observed in the rest of the cases (n = 85) were as follows (including 11 cases with two histological changes each): ADH (n = 51), LN (n = 18), fibrocystic changes (n = 15), papilloma (n = 4), radial scar (n = 3), UDH (n = 4) and fat necrosis (n = 1). Eleven of these cases had a combination of two of these changes, ADH/LN (n = 8), ALH/papilloma (n = 1), ADH/papilloma (n = 1) and ADH/radial scar (n = 1).
Table 1. Case distribution among different institutions.
| Institution | Total | ADH, N (%) | Upgrade, N (%) |
|---|---|---|---|
| RPCI | 358 | 22 (6.1) | 4 (18.2) |
| UPMC | 862 | 61 (7.1) | 8 (13.1) |
| WU | 335 | 11 (3.3) | 1 (9.1) |
| MMC | 100 | 6 (6) | 2 (33) |
| Total | 1655 | 100 (6) | 15 (15) |
RPCI, Roswell Park Cancer Institute; UPMC, University of Pittsburgh Medical Center; WU, Washington University; MMC, Montefiore Medical Center.
When all cases were included in the analysis (with or without LN), only the number of involved cores was statistically significant (Figure 1A). The median number of involved cores for cases with upgrade was 2 (range 1–8), while the median number was 1 for cases with no upgrade (range 1–5) (P = 0.02) (Table 2). Although the median size of the largest histological focus was slightly larger in cases with upgrade (2.1 mm versus 2.0 mm) (Figure 1B), this variable was not statistically significant. The reason for the biopsy for the vast majority of cases was either staging (51%) or high risk (38%). The rate of upgrade for the former was 15.7% versus 13.2% for the latter, with no statistically significant difference. Mass lesions seen on MRI (Figure 2A,B) had a higher risk of upgrade (20.6% of 34 cases) than non-mass enhancement (Figure 2C,D) (12.1% in 66 cases), but this difference was not statistically significant. We found that all patients with invasive carcinoma (n = 3) who presented with a mass on MRI had either concurrent or past history of invasive carcinoma. Although core biopsies with the smaller needle gauge detected more cases with upgrade [seven of 34 (20.6%)] than the larger needle gauge [eight of 66 (12.1%)], this difference was not statistically significant. The other variables were not statistically significant.
Figure 1.
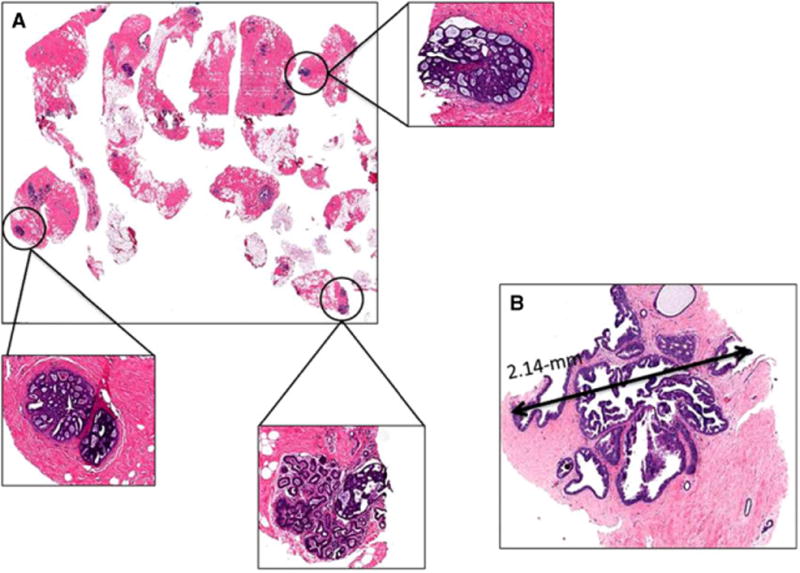
Histological features predicting upgrade. A, multiple cores involved with atypical ductal hyperplasia (ADH), figure with scanning magnification shows three foci of ADH (in circles) with corresponding figures with higher magnification (in boxes); B, ADH focus measuring >2 mm (2.14 mm), the excision shows low nuclear grade ductal carcinoma in situ.
Table 2. ADH (with or without LN) clinicopathological and radiological variables correlation with upgrade.
| Variables | Upgrade | P-value | ||
|---|---|---|---|---|
| Yes (n = 15) | No (n = 85) | |||
| Clinical | ||||
| Age* | 57 (31,75) | 61 (40, 72) | 57 (31, 75) | 0.37 |
| Race | African American (n = 8) | 2 (25.0) | 6 (75.0) | 0.58 |
| Caucasian (n = 89) | 13 (14.6) | 76 (85.4) | ||
| Menopause status | Post (n = 66) | 11 (16.7) | 55 (83.3) | 0.79 |
| Pre (n = 34) | 4 (11.8) | 30 (88.2) | ||
| Hormonal intake | No (n = 41) | 7 (17.1) | 34 (82.9) | 0.55 |
| Yes (n = 51) | 6 (11.8) | 45 (88.2) | ||
| Breast carcinoma history | ||||
| Concurrent ipsilateral | No (n = 68) | 11 (16.2) | 57 (83.8) | 0.8 |
| Yes (n = 32) | 4 (12.5) | 28 (87.5) | ||
| Concurrent contralateral | No (n = 75) | 10 (13.3) | 65 (86.7) | 0.52 |
| Yes (n = 25) | 5 (20.0) | 20 (80.0) | ||
| Past ipsilateral | No (n = 97) | 13 (13.4) | 84 (86.6) | 0.058 |
| Yes (n = 3) | 2 (66.7) | 1 (33.3) | ||
| Past contralateral | No (n = 88) | 14 (15.9) | 74 (84.1) | 0.69 |
| Yes (n = 12) | 1 (8.3) | 11 (91.7) | ||
| Pathology | ||||
| Concurrent FEA | No (n = 90) | 14 (15.6) | 76 (84.4) | 1 |
| Yes (n = 10) | 1 (10.0) | 9 (90.0) | ||
| Concurrent LN | No (n = 86) | 13 (15.1) | 73 (84.9) | 1 |
| Yes (n = 14) | 2 (14.3) | 12 (85.7) | ||
| No. ADH foci* | 1 (1, 11) | 2 (1, 11) | 1 (1, 5) | 0.21 |
| No. cores (total)* | 8 (3, 17) | 8 (5, 12) | 8 (3, 17) | 0.25 |
| No. cores (involved)* | 1 (1, 8) | 2 (1, 8) | 1 (1, 5) | 0.02 |
| Largest ADH size* | 2 (0.5, 8) | 2.1 (0.5, 4.5) | 2 (0.5, 8) | 0.24 |
| Radiology | ||||
| Gauge | 11–14 (n = 34) | 7 (20.6) | 27 (79.4) | 0.38 |
| 9 (n = 66) | 8 (12.1) | 58 (87.9) | ||
| 8–13 (72) | 10 (13.9) | 62 (86.1) | 0.76 | |
| 14 (28) | 5 (35.7) | 23 (64.3) | ||
| MRI reason | Clinical finding (n = 8) | 1 (12.5) | 7 (87.5) | 0.94 |
| Equivocal mammography (n = 3) | 1 (33.3) | 2 (66.7) | ||
| High risk (n = 38) | 5 (13.2) | 33 (86.8) | ||
| Staging (n = 51) | 8 (15.7) | 43 (84.3) | ||
| Mass versus NME | Mass (n = 34) | 7 (20.6) | 27 (79.4) | 0.38 |
| NME (n = 66) | 8 (12.1) | 58 (87.9) | ||
ADH, Atypical ductal hyperplasia; LN, Lobular neoplasia[atypical lobular hyperplasia (ALH) or lobular carcinoma in situ (LCIS)]; FEA, Flat epithelial atypia; NME, Non-mass enhancement.
Continuous variables with median and range; all other variables n (%).
Figure 2.
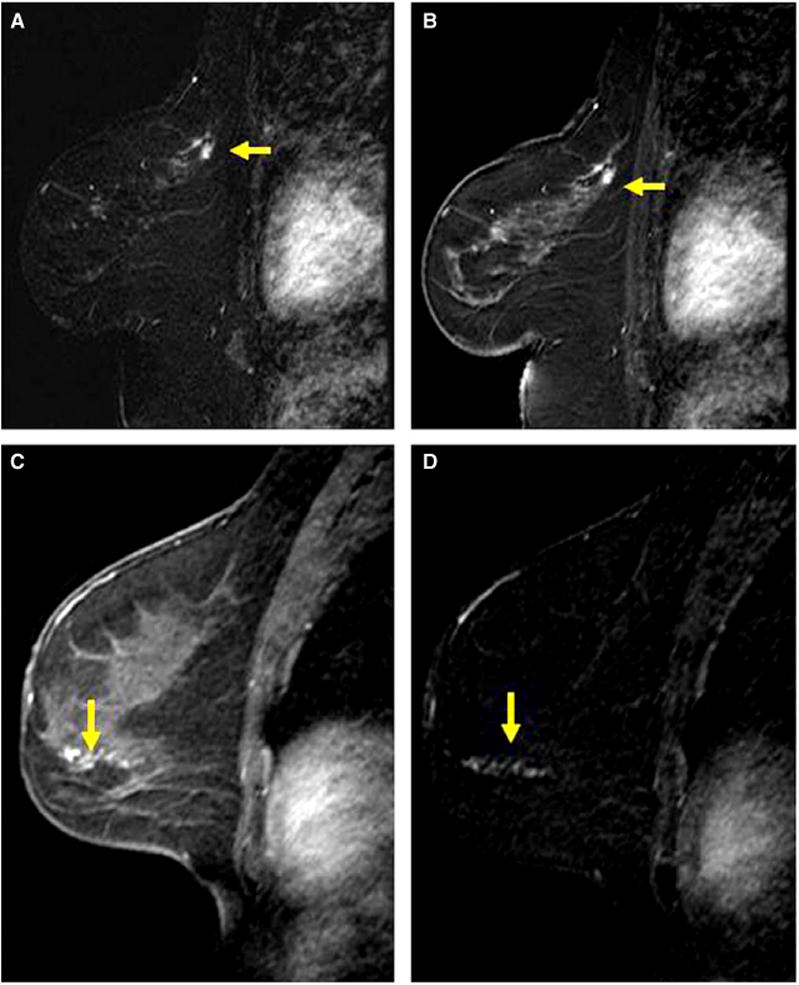
Examples of mass and non-mass enhancement. A, B, Sagittal post-contrast magnetic resonance (MR) image (A) and corresponding subtraction image (B) of the left breast in a 59-year-old woman demonstrates a 0.8-cm irregular mass in the posterior superior breast (yellow arrows). Magnetic resonance imaging (MRI)-guided biopsy yielded atypical ductal hyperplasia (ADH) and excision yielded ADH and sclerosingadenosis. C, D, sagittal post-contrast MR image (C) and corresponding subtracted image (D) of the left breast in a 40-year-old woman demonstrate 4 cm of non-mass enhancement in a linear distribution (arrows). MRI-guided core biopsy yielded ADH and excision yielded low to intermediate grade ductal carcinoma in situ.
When cases with concurrent LN were excluded (n = 14), the increased number of ADH foci and increased number of involved cores were statistically significant. The median and range for the first was 2 (1–11) versus 1 (1–5) (P = 0.002) and for the second was 2 (1–8) versus 1 (1–5) (P = 0.009).
We performed a similar analysis for single institutions (RPCI and UPMC). For RPCI cases (n = 22), none of the variables was significant. For UPMC cases (n = 61), increased number of foci, decreased number of total cores, increased number of involved cores and larger ADH size were all statistically significant (Table 3). All other clinical and radiological variables were not statistically significant.
Table 3. ADH pathological upgrade from UPMC.
| Variables | Upgrade | P-value | ||
|---|---|---|---|---|
| Yes (n = 8) | No (n = 53) | |||
| No. ADH foci* | 1 (1, 3) | 2 (1, 3) | 1 (1, 3) | 0.015 |
| No. cores (total)* | 8 (5, 16) | 6.5 (5, 8) | 8 (5, 16) | 0.026 |
| No. cores (involved)* | 1 (1, 2) | 2 (1, 2) | 1 (1, 2) | 0.003 |
| Largest ADH size* | 2.5 (0.7, 5) | 3.5 (1.5, 4.5) | 2.5 (0.7, 5) | 0.021 |
ADH, Atypical ductal hyperplasia; UPMC: University of Pittsburgh Medical Center.
Continuous variables with median and range.
Discussion
In this study, we report the largest series of ADH identified on MRI-guided biopsy in a single study (n = 100) from the largest series of MRI-guided biopsies (n = 1655). We also report the largest single-institution series of 61 cases (from UPMC).
There have been multiple studies investigating the rate of upgrade for MRI-detected high-risk lesions,12–26 as summarized elegantly by Heller et al.12 There were 15 published studies that documented the total number of lesions, total number of high-risk lesions, frequency of ADH cases, number of excised ADH lesions and the rate of upgrade in the subsequent excision with the type of upgrade (DCIS or invasive carcinoma). The combined total number of MRI-guided biopsies in these studies was 3117, with 142 (4.6%) yielding ADH. The range of the frequency of ADH in all MRI-guided biopsies was 1–14% among the individual studies. The total number of cases that underwent excisional biopsy was 136, 51 (3 7.5%) of which had an upgrade with a range of 25–100%. The type of upgrade was DCIS (n = 3 7, 72.5%) and invasive carcinoma (n = 14, 27.5%). The largest study, from a single institution by Heller et al., studied 35 (3.06% of total MRI cases) ADH cases, 12 (34.3%) of which had anupgrade.31
We found a 6% frequency of ADH among all MRI-guided biopsies, which is concordant with the overall rate in the literature. Our upgrade rate of 15% is lower than the overall rate in the literature. This may be due to our higher number of cases and a more standardized definition of ADH.
The rate of upgrade for MRI-detected ADH is comparable to that seen in mammography-detected ADH.3–11 However, the patient populations undergoing mammography and MRI differ. Mammography is performed for all women aged more than 40 years in the United States. MRI is performed for different reasons. Many surgeons elect to perform MRI to identify the disease extent in certain situations; for instance, dense breasts where the disease extent is not well visualized mammographically, oestrogen receptor (ER)-negative breast cancer, invasive lobular cancers and mammographically occult breast cancers presenting as palpable lumps. In addition, large trials have shown that pre-operative MRI identifies additional occult breast cancers in up to 27% of patients and detects cancer in the contralateral breast in 3.1% of patients,32,33 which is reason alone for some surgeons to use pre-operative MRI in most of their cases. Therefore, patients with MRI lesions are at baseline higher risk for breast cancer than patients with mammographic lesions. Mammographically detected ADH is usually in the form of microcalcifications, while mass lesions are less common (11.3%).11 ADH detected by MRI may be a mass lesion or non-mass enhancement (34% masses and 66% non-mass enhancement in this study). Interestingly, in the current report we found that all invasive carcinoma cases that had been interpreted as masses on MRI had either concurrent or past history of invasive carcinoma. This observation could suggest that a subgroup of patients could be at higher risk for upgrade in the form of invasive carcinoma. More cases are needed to investigate this observation.
It has been proposed that enhancement detected by MRI is due to neo-angiogenesis in the lesion, with an increase in vessel number and size as well as increased vascular permeability.34 This mechanism could explain pathologies that produce neo-angiogenesis such as invasive cancer. It is known that intraductal neoplasias such as DCIS and ADH are confined to the basement membrane, and do not have direct contact with the stroma. Therefore, no neo-angiogenesis could be expected to form in these lesions. However, Jansen et al. explained the mechanism underlying the enhancement in DCIS by proposing that gadolinium diffuses out of capillaries into the extraductal space, reaches leaky duct basement membranes and collects and distributes in the largely unobstructed duct lumen.35 Regardless of the exact underlying mechanism that drives the enhancement in ADH lesions, it is evidently quite different from the formation of microcalcifications that would be detected mammographically. Of note, patients undergoing breast MRI are almost always required to have had a recent mammogram, and any finding undergoing MRI-guided biopsy is not likely to have a mammographic equivalent.
Large studies of mammographically detected ADH (with greater than 100 cases) show that 3-14% of large-gauge (11-gauge or larger) vacuum-assisted biopsies yield ADH with an upgrade rate of 21–27%.36 Factors associated with upgrade to malignancy after ADH diagnosed by stereotactic vacuum-assisted biopsy include the number of ADH foci and the number of involved cores and, to a lesser extent, ADH size, mass lesion, older age and micropapillary ADH histological type.4–11 We have recently developed a nomogram that can be used to predict upgrade after stereotactic vacuum-assisted biopsy. We found an increased number of involved cores, larger ADH size, solid histological type, mass lesion, age, postmenopausal status, history of hormonal intake and history of breast cancer useful in producing the nomogram.11 In the current study we investigated whether any of these variables can predict upgrade for ADH detected by breast MRI.
We found that an increased number of involved cores is significant in predicting upgrade in all cases from all institutions combined with or without LN, which is also a risk factor for upgrade of mammographically detected ADH. When we excluded caseswith concurrent LN (n = 14), we found the increased number of foci and increased number of involved cores to be statistically significant. When we analysed cases from the largest contributing single institution, we found in addition that decreased number of cores (total core samples) to be statistically significant in predicting upgrade. This variable has never been reported in mammography-detected ADH as being a factor for upgrade.4–11 The majority (12 of 15, 80%) of upgraded cases in the current study were DCIS. DCIS is defined as neoplastic ductal epithelial proliferation involving at least two spaces and measuring at least 2 mm.28 Therefore, they are more likely to be under-represented than invasive carcinoma, where the biopsy procedure captures a small proportion of the lesion, rendering a diagnosis of ADH rather than DCIS. In mammography-guided biopsy the target is mass or microcalcifications, while in MRI-guided biopsy the target is an area of enhancement. Therefore, we believe that DCIS is possibly under-represented in MRI-guided biopsy compared to mammography-guided biopsy. This may be because mammography-guided biopsies are more likely to sample the lesion of concern, as a radiograph of the biopsy specimen can confirm the presence of the target within the specimen; in contrast, enhancement cannot be confirmed within the specimen after an MRI-guided biopsy and therefore it cannot usually be confirmed that the target was removed. Larger ADH size was also found to be statistically significant in predicting upgrade, consistent with prior mammography studies.5,8,11 The only common variable that remained significant among different analyses (with versus without LN or in a single institution) was the increased number of involved cores. One of the reasons for this variability might be due to the intraobserver variability, which is known to be high in evaluating ADH.37,38 However, the reviewing pathologists were specialized in breast pathology and examples of the described variables were exchanged to decrease this variability. The other possible reason is differences in the institution setting. While RPCI is a tertiary cancer centre, the other three institutions were general hospitals.
In mammographically detected ADH, mass lesions had a higher risk of upgrade than microcalcifications.36,39 In MRI-detected lesions, we also found that mass enhancement had a higher risk of upgrade than non-mass enhancement. However, the difference was not statistically significant. Interestingly, we found that all patients who had upgrade in the form of invasive carcinoma had masses on MRI. Mass lesions typically undergo second-look ultrasound, and if visualized will be biopsied using ultrasound guidance rather than MRI guidance. Therefore, mass lesions that are included in this study may have different characteristics to masses that would be sonographically visible. It would be useful for future studies to investigate the radiological characteristics of the enhancement and correlate with the risk of upgrade, including margin, shape and type of internal enhancement for mass lesions and the type of enhancement in the non-mass, including focal, linear or segmental. These variables were not possible to study due to the relatively small sample size.
We conclude that the incidence of ADH in MRI-guided core biopsy is rare. The rate of upgrade is comparable to mammographically detected ADH, warranting surgical excision. Similar to mammo-graphically detected lesions, the volume of the ADH predicts the upgrade. This can be used to guide therapy (excision versus observation) in some clinical situations. The MRI findings (mass versus non-mass enhancement) do not predict upgrade.
References
- 1.Kuhl C. Current status of breast MR imaging. Part 2. Radiology. 2007;244:672–691. doi: 10.1148/radiol.2443051661. [DOI] [PubMed] [Google Scholar]
- 2.Kuhl C. The current status of breast MR imaging. Part 1. Radiology. 2007;244:356–378. doi: 10.1148/radiol.2442051620. [DOI] [PubMed] [Google Scholar]
- 3.Gutierrez RL, DeMartini WB, Silbergeld JJ, et al. High cancer yield and positive predictive values: outcomes at a center routinely using preoperative breast MRI for staging. Am J Radiol. 2011;196:293–299. doi: 10.2214/AJR.10.4804. [DOI] [PubMed] [Google Scholar]
- 4.Ely KA, Carter BA, Jensen RA, Simpson JF, Page DL. Core biopsy of the breast with atypical ductal hyperplasia. A probabilistic approach to reporting. Am J Surg Pathol. 2001;25:1017–1021. doi: 10.1097/00000478-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Wagoner MJ, Laronga C, Acs G. Extent and histologic pattern of atypical ductal hyperplasia present on core biopsy specimens of the breast can predict ductal carcinoma in situ. Anat Pathol. 2009;131:112–121. doi: 10.1309/AJCPGHEJ2R8UYFGP. [DOI] [PubMed] [Google Scholar]
- 6.Chae BJ, Lee A, Song BJ, Jung SS. Predictive factors for breast cancer in patients diagnosed atypical ductal hyperplasia at core biopsy. World J Surg Oncol. 2009;7:77–81. doi: 10.1186/1477-7819-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allison KH, Eby PR, Kohr J, Demartini WB, Lehman CD. Atypical ductal hyperplasia on vacuum-assisted breast biopsy: suspicion for ductal carcinoma in situ can stratify patients at high risk for upgrade. Hum Pathol. 2010;42:41–50. doi: 10.1016/j.humpath.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Ko E, Han W, Lee JW, et al. Scoring system for predicting malignancy in patients diagnosed with atypical ductal hyperplasia at ultrasound-guided core biopsy. Breast Cancer Res Treat. 2008;112:189–195. doi: 10.1007/s10549-007-9824-0. [DOI] [PubMed] [Google Scholar]
- 9.Sneige N, Lim SC, Whitman GJ, et al. Atypical ductal hyperplasia diagnosis by directional vacuum-assisted stereotactic biopsy of breast microcalcifications. Considerations for surgical excision. Am J Clin Pathol. 2003;119:248–253. doi: 10.1309/0GYV-4F2L-LJAV-4GFN. [DOI] [PubMed] [Google Scholar]
- 10.Menes TS, Rosenberg R, Balch S, et al. Upgrade of high-risk breast lesions detected on mammography in the breast cancer Surveillance Consortium. Am J Surg. 2014;207:24–31. doi: 10.1016/j.amjsurg.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khoury T, Chen X, Wang D, et al. Nomogram to predict the likelihood of upgrade of atypical ductal hyperplasia diagnosed on a core needle biopsy in mammographically detected lesions. Histopatholology. 2015;67:106–120. doi: 10.1111/his.12635. [DOI] [PubMed] [Google Scholar]
- 12.Heller SL, Hernandez O, Moy L. Radiologic-pathologic correlation at breast MR imaging: what is the appropriate management for high-risk lesions? Magn Reson Imaging Clin N Am. 2013;21:583–599. doi: 10.1016/j.mric.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Liberman L, Morris EA, Dershaw DD, et al. Fast MRI guided vacuum-assisted breast biopsy: initial experience. Am J Roentgenol. 2003;181:1283–1293. doi: 10.2214/ajr.181.5.1811283. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Lehman CD, Dee KE. MRI-guided breast biopsy: clinical experience with 14-gauge stainless steel core biopsy needle. Am J Roentgenol. 2004;182:1075–1080. doi: 10.2214/ajr.182.4.1821075. [DOI] [PubMed] [Google Scholar]
- 15.Liberman L, Bracero N, Morris E, et al. MRI-guided 9-gauge vacuum-assisted breast biopsy: initial clinical experience. Am J Roentgenol. 2005;185:183–193. doi: 10.2214/ajr.185.1.01850183. [DOI] [PubMed] [Google Scholar]
- 16.Lehman CD, Deperi ER, Peacock S, et al. Clinical experience with MRI-guided vacuum-assisted breast biopsy. Am J Roentgenol. 2005;184:1782–1787. doi: 10.2214/ajr.184.6.01841782. [DOI] [PubMed] [Google Scholar]
- 17.Ghate SV, Rosen EL, Soo MS, et al. MRI-guided vacuum-assisted breast biopsy with a handheld portable biopsy system. Am J Roentgenol. 2006;186:1733–1736. doi: 10.2214/AJR.05.0551. [DOI] [PubMed] [Google Scholar]
- 18.Perlet C, Heywang-Kobrunner SH, Heinig A, et al. Magnetic resonance-guided, vacuum-assisted breast biopsy: results from a European multicenter study of 538 lesions. Cancer. 2006;106:982–990. doi: 10.1002/cncr.21720. [DOI] [PubMed] [Google Scholar]
- 19.Orel SG, Rosen M, Mies C, et al. MR imagingguided 9-gauge vacuum-assisted core-needle breast biopsy: initial experience. Radiology. 2006;238:54–61. doi: 10.1148/radiol.2381050050. [DOI] [PubMed] [Google Scholar]
- 20.Liberman L, Holland AE, Marjan D, et al. Underestimation of atypical ductal hyperplasia at MRI guided 9-gauge vacuum-assisted breast biopsy. Am J Roentgenol. 2007;188:684–690. doi: 10.2214/AJR.06.0809. [DOI] [PubMed] [Google Scholar]
- 21.Mahoney MC. Initial clinical experience with a new MRI vacuum-assisted breast biopsy device. J Magn Reson Imaging. 2008;28:900–905. doi: 10.1002/jmri.21549. [DOI] [PubMed] [Google Scholar]
- 22.Malhaire C, El Khoury C, Thibault F, et al. Vacuum-assisted biopsies under MR guidance: results of 72 procedures. Eur Radiol. 2010;20:1554–1562. doi: 10.1007/s00330-009-1707-9. [DOI] [PubMed] [Google Scholar]
- 23.Perretta T, Pistolese CA, Bolacchi F, et al. MR imaging-guided 10-gauge vacuum-assisted breast biopsy: histological characterisation. Radiol Med. 2008;113:830–840. doi: 10.1007/s11547-008-0289-y. [DOI] [PubMed] [Google Scholar]
- 24.Strigel RM, Eby PR, Demartini WB, et al. Frequency, upgrade rates, and characteristics of high-risk lesions initially identified with breast MRI. Am J Roentgenol. 2010;195:792–798. doi: 10.2214/AJR.09.4081. [DOI] [PubMed] [Google Scholar]
- 25.Tozaki M, Yamashiro N, Sakamoto M, et al. Magnetic resonance-guided vacuum-assisted breast biopsy: results in 100 Japanese women. Jpn J Radiol. 2010;28:527–533. doi: 10.1007/s11604-010-0464-7. [DOI] [PubMed] [Google Scholar]
- 26.Crystal P, Sadaf A, Bukhanov K, et al. High-risk lesions diagnosed at MRI-guided vacuum-assisted breast biopsy: can underestimation be predicted? Eur Radiol. 2011;21:582–589. doi: 10.1007/s00330-010-1949-6. [DOI] [PubMed] [Google Scholar]
- 27.Page DL, Dupont WD, Rogers LW, Rados MS. Atypical hyperplastic lesions of the female breast: a long-term follow-up study. Cancer. 1985;55:2698–2708. doi: 10.1002/1097-0142(19850601)55:11<2698::aid-cncr2820551127>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 28.Tavassoli FA, Norris HJ. A comparison of the results of longterm follow-up for atypical intraductal hyperplasia and intraductal hyperplasia of the breast. Cancer. 1990;65:518–529. doi: 10.1002/1097-0142(19900201)65:3<518::aid-cncr2820650324>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 29.Purcell CA, Norris HJ. Intraductal proliferations of the breast: a review of histologic criteria for atypical intraductal hyperplasia and ductal carcinoma in situ, including apocrine and papillary lesions. Ann Diagn Pathol. 1998;2:135–145. doi: 10.1016/s1092-9134(98)80051-8. [DOI] [PubMed] [Google Scholar]
- 30.Ellis IO. Intraductal proliferative lesions of the breast: morphology, associated risk and molecular biology. Mod Pathol. 2010;23:51–57. doi: 10.1038/modpathol.2010.56. [DOI] [PubMed] [Google Scholar]
- 31.Heller SL, Elias K, Gupta A, Greenwood HI, Mercado CL, Moy L. Outcome of high-risk lesions at MRI-guided 9-gauge vacuum-assisted breast biopsy. Am J Roentgenol. 2014;202(23):7–245. doi: 10.2214/AJR.13.10600. [DOI] [PubMed] [Google Scholar]
- 32.Liberman L, Morris EA, Dershaw DD, Abramson AF, Tan LK. MR imaging of the ipsilateral breast in women with percutaneously proven breast cancer. Am J Roentgenol. 2003;180:901–991. doi: 10.2214/ajr.180.4.1800901. [DOI] [PubMed] [Google Scholar]
- 33.Lehman CD, Gatsonis C, Kuhl CK, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med. 2007;356:1295–1303. doi: 10.1056/NEJMoa065447. [DOI] [PubMed] [Google Scholar]
- 34.Teifke A, Behr O, Schmidt M, et al. Dynamic MR imaging of breast lesions: correlation with microvessel distribution pattern and histologic characteristics of prognosis. Radiology. 2006;239:351–360. doi: 10.1148/radiol.2392050205. [DOI] [PubMed] [Google Scholar]
- 35.Jensen SA, Paunesku T, Fan X, et al. Ductal carcinoma in situ: X-ray fluorescence microscopy and dynamic contrast-enhanced MR imaging reveals gadolinium uptake within neoplastic mammary ducts in a murine model. Radiology. 2009;253:399–406. doi: 10.1148/radiol.2533082026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deshaies I, Provencher L, Jacob S, et al. Factors associated with upgrading to malignancy at surgery of atypical ductal hyperplasia diagnosed on core biopsy. Breast. 2011;20:50–55. doi: 10.1016/j.breast.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Rosai J. Borderline epithelial lesions of the breast. Am J Surg Pathol. 1991;15:209–221. doi: 10.1097/00000478-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Schnitt SJ, Connolly JL, Tavassoli FA. Interobserver reproducibility in the diagnosis of ductal proliferative breast lesions using standardized criteria. Am J Surg Pathol. 1992;16:1133–1143. doi: 10.1097/00000478-199212000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Jackman RJ, Bridwell RL, Ikeda DM. Atypical ductal hyperplasia: can some lesions be defined as probably benign after stereotactic 11-gauge vacuum assisted biopsy, eliminating the recommendation for surgical excision? Radiology. 2002;224:548–554. doi: 10.1148/radiol.2242011528. [DOI] [PubMed] [Google Scholar]


