Abstract
Introduction
Understanding the impact of past interventions and how it affected transmission dynamics is key to guiding prevention efforts. We estimated the population-level impact of condom, antiretroviral therapy (ART), and prevention of mother-to-child transmission activities on HIV transmission and the contribution of key risk factors on HIV acquisition and transmission.
Methods
An age stratified dynamical model of sexual and vertical HIV transmission among the general population, female sex workers (FSW), and men who have sex with men (MSM) was calibrated to detailed prevalence and intervention data. We estimated the fraction of HIV infections averted by the interventions, and the fraction of incident infections acquired and transmitted by different populations over successive 10-year periods (1976-2015).
Results
Overall, condom use averted 61% (95% Credible Intervals: 56-66%) of all adult infections during 1987-2015 mainly due to increases by FSW (46% of infections averted). In comparison, ART prevented 15% (10-19%) of adult infections during 2010-2015. As a result, FSW initially (1976-1985) contributed 95% (91-97%) of all new infections, declining to 19% (11-27%) during 2005-2015. Older men and clients mixing with non-FSW are currently the highest contributor to transmission. MSM contributed ≤4% of transmission throughout. Young women (15-24 years; excluding FSW) did not transmit more infection than they acquired.
Conclusion
Early increases in condom use, mainly by FSW, have substantially reduced HIV transmission. Clients of FSW and older men have become the main source of transmission whereas young women remain at increased risk. Strengthening prevention and scaling-up of ART, particularly to FSW and CFSW, is important.
Keywords: Compartmental model, Impact evaluation, Ivory Coast, Key populations, Transmission dynamics, West Africa
Introduction
The epidemic in Côte d'Ivoire initially spread quickly1 with HIV prevalence in Abidjan, the country's largest city, reaching 38% of female sex workers (FSW) in 1986 and 3-10% of pregnant women in 19891-3. During those years, AIDS incidence in Abidjan was among the highest observed worldwide4. This prompted the creation of the national anti-AIDS program in 19875,6. Subsidies for condoms were introduced in 1991 and retailed under the Prudence trademark5. Multiple social marketing programs were initiated and Prudence™ condoms became widely available in more than 10,000 selling points nationally. Sales of this brand increased from 2 million in 1991, to 49 million in 1996, and to 120 million in 20036. Given the high prevalence observed among FSW and their clients, core group interventions were also implemented in 1991, mostly in urban areas7.
During the 1990s, the national public health response in Côte d'Ivoire primarily focused on the general population with some interventions targeting FSW and their clients. Despite its politico-military troubles, the country's response became more organized in the 2000s, with the establishment of a dedicated ministry to fight HIV and drafting of the first National Strategic Plan8. The first two national strategic plans (2002-2004 and 2006-2010) recommended focusing activities on a number of key populations (KP). However, in practice, these KP included vast segments of the population, with different degrees of vulnerability to HIV acquisition and transmission8,9. Men who have sex with men (MSM) were included as a KP starting in 2006. The last action plan (2011-2015) re-centered prevention activities to the most vulnerable KP (i.e., FSW, MSM, young people) and prioritized treatment scale-up and prevention of mother-to-child transmission (PMTCT)10.
In the early 2000s, antiretroviral therapy (ART) treatment scale-up was slow and access was limited for the majority of people living with HIV, despite Côte d'Ivoire being part of UNAIDS initiative pilot phase for treatment access11. Under this national initiative only 3,190 individuals received highly active ART between 1998 and 200212. A number of initiatives, including provision of free ART from 2008 onwards, increased the number of people receiving ART to 77,531 by 201013. Similarly, only 636 HIV positive pregnant women benefited from PMTCT in 200214 compared to 11,588 in 201013.
Despite a relatively well-documented HIV epidemic and rapid national response, the impact achieved by existing HIV interventions in Côte d'Ivoire and the long-term contribution of KP such as FSW, MSM, and young women to HIV acquisition and transmission has never been estimated. With a national HIV prevalence of 3.7% in 201215, the lessons learned from an in-depth review and comprehensive analysis of the past national response in Côte d'Ivoire would improve the evidence base for existing and future health policies. Further, recent studies have also shown the importance of understanding which subgroups of susceptible individuals are more likely to acquire new infections (i.e., the source of HIV acquisitions) and which groups of HIV positive individuals are more likely to transmit infections (i.e., the source of HIV transmissions) in the long-term using transmission dynamic models16-18. The source of HIV transmission, also called the transmission Population Attributable Fraction (PAF)19, incorporates not only information on infections from index cases to their partners but also from such secondary infected partners to their partners (i.e., chains of transmission). The transmission PAF metric is more useful than the source of acquisitions to identify epidemic drivers - defined as the group with a risk factor that accounts for most of HIV transmission and for which transmission could be most efficiently prevented if it was blocked. As such, dynamic models of HIV transmission, such has the one used in this study, provide more useful information to inform HIV prevention programs than static model such as the Modes of Transmission metric16,17,20,21.
Therefore, our study aimed 1) to estimate the population-level impact of past condom, ART, and PMTCT interventions on HIV transmission in Côte d'Ivoire and 2) to estimate the long-term contribution of subgroups of individuals with selected risk factors (e.g., age, sex work, sex between men, primary phase of infection) on HIV acquisition and transmission between 1976 and 2015.
Methods
Mathematical model structure
The dynamic compartmental model represents the open population (15-59 years old) of Côte d'Ivoire, growing at a constant rate22, stratified into eight sexual risk categories: low-risk and high-risk women (>1 partner per year), low-risk and high-risk men (>2 partners per year), FSW, their clients (CFSW), MSM who report bisexual practices, and exclusive MSM (Supplement Figure S1). People who inject drugs were omitted because they represent a small minority of the population (∼120 in Abidjan)23. The size of the different risk groups was held constant through time but FSW could leave sex work and integrate the high-risk women group (and vice-versa). For ease of description, we will use the term general population to refer to the heterosexual population, excluding FSW, in the remaining of the text.
The population is further stratified in four age groups: 15-19, 20-24, 25-49, and 50-59 years old. A fraction of susceptible individuals enter the sexually active population at either 15 or 20 years of age. This fraction of 15-19 years old virgins increases with time15,24-26. All individuals aged 20-59 are assumed to be sexually active. Côte d'Ivoire has experienced high rates of immigration, which was accounted for in the model27,28. Age mixing and mixing between low- and high-risk women and men was informed by the 2011-12 Demographic and Health Survey (DHS)15.
Sexually active susceptible individuals can get infected at a per capita force of infection that depends on the annual number of new sexual partners, type of sexual partner, HIV prevalence among these sexual partners, numbers of sexual acts per partner per year, sexual mixing pattern between age and sexual risk groups, type (vaginal/anal) and fraction of sex acts protected by condoms, partner's infectiousness (e.g., varying by disease stage, ART treatment status, and viral suppression status), and the individual's susceptibility (e.g., young women have higher risk of infection29,30). The reduction in HIV susceptibility of male circumcision was not explicitly parameterized but captured in per act transmission probabilities since more than 95% of men in Côte d'Ivoire are circumcised15,26.
Recently HIV infected and untreated individuals are assumed to progress from an initial short highly infectious primary infection through four disease stages of various duration (Figure S2), proxied by CD4 cell counts: >500, 350-500, 200-349, and <200 CD4 cells/μL. Individuals not in the primary phase of infection can get tested at a rate that varies with calendar time and symptomatic status. Once tested, diagnosed HIV positive individuals initiate treatment at a time-dependent rate that is a function of disease stage. Treated individuals become virally suppressed after a short time lag. Individuals on ART no longer progress through the different disease stages and have lower rates of AIDS-related mortality than untreated individuals31,32. Treatment failures and ART discontinuation occur at fixed rates. In such cases, the disease will follow its natural progression unless individuals re-initiate ART. Individuals can exit the population through aging, natural mortality or AIDS-related mortality.
A linked decision tree model was used to estimate vertical transmission of HIV from infected mother to their newborn using information from the dynamic compartmental model on the number of HIV positive females and their ART status (Figure S3). Probability of an HIV positive mother transmitting to her child depends on her CD4 count and PMTCT coverage over time: no intervention (up to 1999), single-dose nevirapine (from 2000-2005), dual prophylaxis (from 2006-2010), and the World Health Organization (WHO) option A/B (from 2010). Peri-partum and postnatal transmission probabilities were abstracted from Rollins33.
Detailed information on sexual mixing, the model's ordinary differential equations, the equations for the force of infection, and those for PMTCT are presented as supplementary materials (Text S1 to S4).
Data sources for model parameterization
The complete list of all model parameters is presented as supplementary materials. Briefly, demographical parameters were abstracted from the World Population Prospect22 (Table S1). Sexual behaviors parameters for the general heterosexual population were informed primarily by the 1994, 1998-99, 2005, and 2011-12 DHS or AIDS Indicator Survey (Table S2). The scientific literature informed biological, PMTCT, and sexual behavior parameters for MSM and FSW (Tables S3-S4). Additional analyses of secondary survey data were also performed to inform MSM and FSW parameters. Data on past intervention trends, such as condom use, treatment, and PMTCT, were abstracted from government reports and the scientific literature (Tables S5-S8). Information on historical trends for these interventions is presented as supplementary material (Text S5). Access to programmatic data was facilitated through on-site visits and face-to-face meetings with program managers.
Model calibration and data sources
Uncertainty in the model's parameter estimates was explicitly taken into account by eliciting prior distributions. Incremental Mixture Importance Sampling was used to better approximate the posterior distributions of interests34. A Latin Hypercube Sampling35 algorithm was used at the initial stage to sample 5 million parameter sets from their prior distributions and to select those that satisfied the pre-specified constraints36,37 for the following model outputs: national HIV prevalence by sex and age groups in the general population15,26,38, HIV prevalence among FSW1,7,39-41, HIV prevalence among CFSW42, HIV prevalence among MSM by age groups43, and ART coverage13,44-49 (Table S9). Some surveys conducted prior to 1991 were adjusted for their imperfect diagnostic assays (Text S6). The model's likelihood was calculated by summing the binomial log-likelihoods of the following outcomes: HIV prevalence by sex and age group (general population), FSW, CFSW, and MSM (Table S9). The importance sampling stage was then performed to obtain the posterior distribution of interests by sampling 1000 parameter sets out of the 41,146 identified (these are summarized using their median, 2.5th, and 97.5th percentiles).
The dynamic system was initialized in 1970 and, in 1975 HIV was seeded in FSW, clients of FSW, and MSM. The model was coded in MATLAB® and solved numerically using a Euler algorithm with a 0.05-year time step.
Estimating intervention impact
The impact of interventions was estimated using averted fractions (AF) derived as the relative difference between the cumulative number of adult incident HIV infections (for PMTCT, impact was assessed among infants) under the observed levels of interventions and a counterfactual scenario with lower coverage. The counterfactual scenarios for ART and PMTCT assumed absence of these interventions over the relevant time period of assessment. However, as condom use may have been promoted for contraception and/or prevention of other sexually transmitted infections, using a counterfactual that assumed no condom use was deemed unrealistic. Instead, three counterfactual scenarios were explored by setting condom use levels constant either to 1) those observed in 1987 when the national response to HIV was first organized, 2) those observed in 1990 before subsidies for condoms were introduced, or 3) 1993 levels, two years after subsidies, but before achieving full coverage. AFs for condom use were computed for the 1987-2015 period for all three counterfactuals. The AF of observed increased condom use among specific risk groups (i.e., general population, FSW, and MSM) were also assessed while maintaining other risk groups at their counterfactual condom use levels. Finally, we investigated the plausibility that the trend in HIV prevalence was in fact the result of the self-reported increases in condom use using the approach described in Williams et al.50 and Pickles et al.51. This was achieved by examining if the model could be calibrated to the observed HIV prevalence data (see Table S9) if we assumed that levels of condom use had not increased beyond their 1987 levels (using the same prior distributions as for the original model calibration).
Estimating sources of acquisition and transmission
We characterized the HIV epidemic by the sources of HIV acquisition and the sources of HIV transmission in the long-term. The first is measured by the distribution of cumulative incident infection (i.e. the fraction of all new HIV infections that are acquired by a specific risk groups over a time period). The second is measured by the transmission PAF of a specific risk factor (i.e. the fraction of all new infections directly transmitted by individuals with certain risk factors as well as the infections transmitted by those who have acquired infections from them: secondary transmission). Both measures were estimated for different risk and age groups for successive 10-years periods over 1976-2015.
Specifically, the PAFt-t0 due to a specific risk factor (incidence with risk factor is noted IRisk) over a given time period (t-t0), is estimated as follows:
INo Risk is derived by setting the probability of HIV transmission from individuals with the selected risk factor to all their partners to zero. This was independently performed for each sex and age group, and selected behavioral (for CFSW, only transmission from clients to women not involved in sex work was set to zero) and biological risk factors (primary infections, undiagnosed infections, untreated diagnosed infections, and treated infections). Because individuals may share more than one risk factors and susceptible individuals can get infected by different partners, it is known that the transmission PAF can sum to more than one52.
Results
Model outputs fitted well HIV prevalence data (Figure 1) and other epidemiological and intervention outcomes (Figures S4-S6).
Figure 1. Observed (points, mean with 95% confidence intervals) and predicted HIV prevalence in A) the general population (15-49 years old, and B) among female sex workers in Côte d'Ivoire.
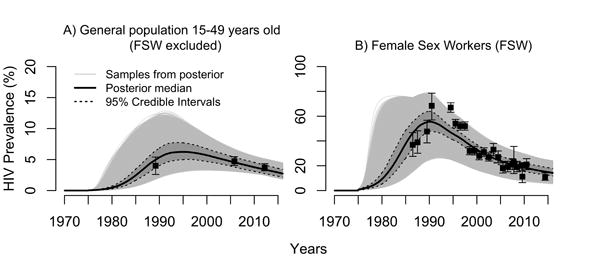
In A) FSW are not assumed to be sampled in the household-based HIV prevalence surveys and were excluded in calculating model-based overall prevalence estimates. Incremental Mixture Importance Sampling (IMIS) resulted in 41,146 unique sets of parameters labelled samples from posterior (grey curves). One thousands of these curves were resampled with probability proportional to their importance weights to derive the median (red) and 95% credible intervals (shaded blue area).
Impact of past interventions
Available data suggests that the rise in condom use in the 1990s was more pronounced among FSW than among the general population and MSM (Figure 2). Model results suggest that condom use averted a median of 61% (95% Credible Interval (CrI): 56-66%), 50% (44-58%), and 23% (20-26%) of all new HIV infections during 1987-2015 that would have occurred if condom use had remained at their 1987, 1991, and 1993 levels, respectively (Figure 2). If condom use had only increased in the general heterosexual population, and remained constant at the 1987 baseline level in other risk groups, only 19% of new infections would have been averted between 1987 and 2015. This contrasts with the 46% of new HIV infections that would have been averted if condoms had been scaled-up only among FSW (while condoms remained at the 1987 baseline levels in other risk groups). Estimates using the 1990 and 1993 counterfactuals yielded slightly more conservative impact estimates (Figure 2). Overall, the AF due to condom use increases among MSM alone were small (≤1%) across counterfactual scenarios. Condoms nevertheless were effective and averted a minimum of 28% of MSM HIV infections (results not shown). The hypothesis that increases in condom use can explain the course of the HIV epidemic in Côte d'Ivoire is highly plausible. In fact, assuming no increase in condom use beyond their 1987 levels was highly inconsistent with the HIV prevalence data: none of the 5 million Latin Hypercube samples fitted our outcomes (see Table S9).
Figure 2. Historical trends in condom use and estimated averted fractions (AF) of HIV infections over 1987-2015 in Cote d'Ivoire, according to different counterfactual scenarios.
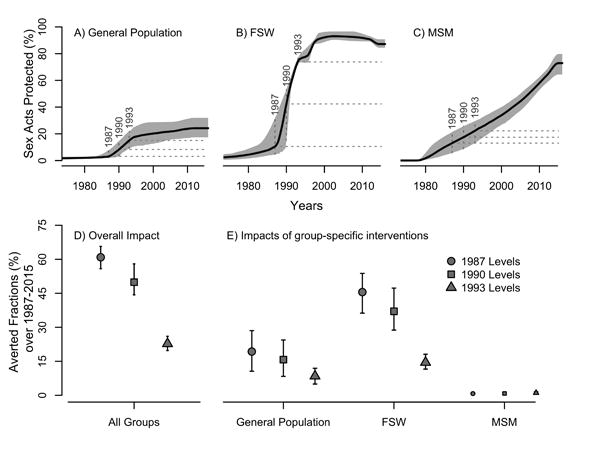
The proportion of sex acts protected by condom estimated from data (Table S5) is shown for A) the general heterosexual population excluding women engaged in sex-work (displayed trends are a weighted average of each age-class specific trends), B) female sex workers (FSW), and C) men who have sex with men (MSM). Dashed horizontal lines represented the three counterfactuals scenarios of condom use trends assumed to derive the AF, corresponding to constant levels from 1987, 1990, and 1993 onward. Panel D) shows AF estimates of increases in condom use by all risk groups for each counterfactual. In panel E) AF due to condom use increases in specific risk group are presented (general heterosexual population not engaging in sex work only, by FSW only, and by MSM only). For these, each AF estimates assumed that condom has not increased in the other risk groups (i.e. condom use remained at their counterfactual levels). Median and 95% credible intervals of the posterior estimates are shown.
Data suggests that coverage of ART and PMTCT activities (Tables S7 and S9) was low prior to 2010 (Figure 3) and remains suboptimal, reflecting in part ART eligibility criteria. These were expanded in 2012 to offer treatment to all HIV patients with a CD4≤350 cells/μL or with WHO stage 4 clinical disease53. The country has yet to adopt the new WHO guidelines for ART, however54. Approximately 8% of all HIV infections were estimated to have been averted by ART overall during 2000-2015 (Figure 3). The largest impact occurred during 2010-2015, where ART prevented an estimated 15% of all HIV infections owing to an estimated increase in ART coverage from 16% (10-19%) in 2010 to 38% (28-42%) of HIV positive individuals in 2015. Similarly, the impact of PMTCT among infants was very modest during 2000-2005 because of suboptimal coverage of HIV positive mothers receiving PMTCT in 2005 (8% coverage; 95%CrI: 6-11%). From 2010-2015, however, PMTCT prevented an estimated 23% of vertically transmitted infections. Yet, an estimated 17% (14-20%) of infants born to HIV positive mothers were still acquiring HIV in 2015.
Figure 3. Predicted coverage and population-level impact of antiretroviral therapy (ART) and of prevention of mother-to-child (PMTCT) activities in Cote d'Ivoire during 2000-2015.
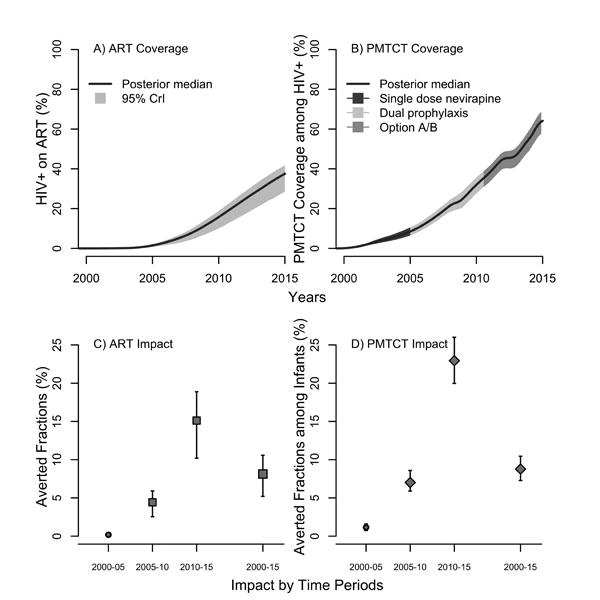
Panels shows A) coverage of ART, B) coverage of PMTCT and C) averted fractions (AF) of all new HIV infections among adults due to scaling-up of ART, and D) averted fractions of new infants infections due to PMTCT. AFs were calculated over the following time periods: 2000-2005, 2005-2010, 2010-2015, and 2000-2015. Medians and 95% credible intervals of the posteriors are presented. The counterfactual scenarios used to estimate the AF assumed the absence of ART and PMTCT interventions.
Source of HIV acquisition
Young women aged 15-24 years old have been disproportionally affected by HIV over the entire 1976-2015 period, representing 26-30% of new acquired infections compared to young men (<21%) (Table 1). The estimated fraction of new infections acquired by FSW, composing only 1.6% (1.2-2.2%) of the modeled female population, declined from 13% in 1976-1985 to 5% in 2005-2015 (Table 2). The fraction of HIV infections acquired by CFSW also decreased over time from 41% to 18%. Only ≤4% of new infections are estimated to have occurred among MSM, which nevertheless represents a high burden of infection for MSM given that they represent 1.2% (0.9-1.6%) of the male population (see Table S1 for the prior distribution regarding the size of this risk group). During 1976-1985, 36% of new infections were acquired by women not engaging in sex work, which increased to 43% over 2005-2015, as a result of HIV spreading from KP to CFSW and further.
Table 1.
A) Estimated distribution of all new HIV infections that were acquired and B) that were transmitted by different age and sex groups in Côte d'Ivoire over successive 10-years periods during 1976-2015.
| Time periods | Age groups | Women* | Men* |
|---|---|---|---|
| A) Who acquired infection | Proportion of acquired infections (95%CrI) | ||
| 1976-1985 | 15-24 years old | 30% (25-37%) | 21% (18-24%) |
| 25-49 years old | 17% (14-20%) | 26% (23-30%) | |
| 50-59 years old | 1% (1-1%) | 4% (3-5%) | |
| 1985-1995 | 15-24 years old | 32% (27-38%) | 16% (14-19%) |
| 25-49 years old | 21% (18-25%) | 24% (20-27%) | |
| 50-59 years old | 1% (1-2%) | 5% (4-6%) | |
| 1995-2005 | 15-24 years old | 28% (22-35%) | 13% (10-15%) |
| 25-49 years old | 25% (21-29%) | 26% (22-30%) | |
| 50-59 years old | 2% (1-2%) | 6% (5-7%) | |
| 2005-2015 | 15-24 years old | 26% (20-32%) | 11% (9-13%) |
| 25-49 years old | 26% (22-30%) | 28% (24-31%) | |
| 50-59 years old | 2% (2-2%) | 7% (5-8%) | |
| B) Who transmitted infections | PAF (95%CrI) | ||
| 1976-1985 | 15-24 years old | 86% (81-90%) | 69% (63-74%) |
| 25-49 years old | 42% (36-60%) | 75% (71-80%) | |
| 50-59 years old | 1% (1-1%) | 10% (8-13%) | |
| 1985-1995 | 15-24 years old | 54% (48-59%) | 32% (27-38%) |
| 25-49 years old | 33% (30-39%) | 56% (52-61%) | |
| 50-59 years old | 1% (1-1%) | 9% (8-11%) | |
| 1995-2005 | 15-24 years old | 33% (29-39%) | 18% (14-24%) |
| 25-49 years old | 37% (31-43%) | 54% (52-59%) | |
| 50-59 years old | 1% (1-2%) | 11% (9-13%) | |
| 2005-2015 | 15-24 years old | 30% (26-36%) | 15% (11-21%) |
| 25-49 years old | 41% (35-47%) | 56% (52-60%) | |
| 50-59 years old | 2% (1-3%) | 13% (11-16%) | |
The ‘women’ category includes female sex workers and the ‘men’ one includes men who have sex with men and clients of female sex workers.
95% CrI=95% Credible intervals; PAF=Population attributable fraction.
PAF for women in the different age groups were calculated by setting the HIV transmission probability between women in a certain age group and their male partners to zero at the beginning of a defined time period. The same procedure was used to calculate PAF for men. Note that, although the proportions of new infections sum to 100% (except for rounding errors), this is not necessarily the case for PAF estimates.
Table 2.
Estimated distribution of new HIV infections by risk (A), and that were transmitted by different behavioral (B) and biological (C) risk factors for HIV infection in Côte d'Ivoire during 1976-2015.
| Time periods | ||||
|---|---|---|---|---|
| 1976-1985 | 1985-1995 | 1995-2005 | 2005-2015 | |
| A) Who acquired infections | ||||
| Women (FSW excluded) | 36% (28-44%) | 48% (41-54%) | 50% (45-57%) | 49% (44-57%) |
| FSW | 13% (10-16%) | 7% (5-10%) | 4% (3-6%) | 5% (3-7%) |
| Men (CFSW and MSM excluded) | 9% (6-12%) | 17% (12-23%) | 24% (18-31%) | 24% (18-31%) |
| CFSW | 41% (35-50%) | 26% (21-32%) | 17% (12-21%) | 18% (13-22%) |
| MSM | 1% (0-2%) | 2% (1-3%) | 3% (2-5%) | 4% (2-6%) |
| Young women (15-24 years old; no FSW)* | 22% (17-28%) | 28% (22-33%) | 26% (20-31%) | 24% (20-29%) |
| B) Who transmitted infections (by behavioral risk factors; PAF 95%CrI)† | ||||
| High-risk women (FSW excluded) | 10% (6-18%) | 13% (8-22%) | 18% (11-28%) | 18% (11-29%) |
| Sex work | 95% (91-97%) | 60% (50-70%) | 21% (13-28%) | 19% (11-27%) |
| High-risk men (MSM and CFSW excluded) | 2% (1-5%) | 6% (3-14%) | 13% (7-26%) | 15% (8-29%) |
| CFSW to non-FSW | 51% (47-70%) | 61% (53-68%) | 50% (42-59%) | 44% (35-54%) |
| MSM | 1% (0-2%) | 2% (1-3%) | 4% (2-5%) | 4% (2-7%) |
| Young women (15-24 years old; no FSW) | 19% (12-30%) | 23% (17-30%) | 25% (20-32%) | 24% (19-30%) |
| C) Who transmitted infections (by biological risk factors; PAF 95%CrI)† | ||||
| Primary infection phase | 86% (77-93%) | 44% (32-58%) | 25% (17-36%) | 25% (17-36%) |
| Undiagnosed infections | 100% (100-100%) | 100% (100-100%) | 99% (97-99%) | 87% (80-91%) |
| Untreated diagnosed infections§ | 0% (0-0%) | 0% (0-0%) | 4% (2-6%) | 24% (16-34%) |
| Treated infections§ | 0% (0-0%) | 0% (0-0%) | 0% (0-0%) | 6% (3-9%) |
CFSW=clients of female sex workers; FSW=female sex workers; PAF=population attributable fractions; MSM=men who have sex with men.
The following risk factors/behaviors are represented: high-risk women (>1 partner year-1), FSW, high-risk men (>2 partners year-1), CFSW transmitting to non-FSW partners, MSM, and individuals in the primary HIV infection phase.
For the proportions of acquired infections to sum to 100% for each time periods (except for rounding errors), “Young women (15-24 years old, no FSW)” should not be double-counted as they are already included in the “Women (FSW excluded)” categories.
Median population attributable fractions with 95% Credible Intervals.
PAF for untreated diagnosed infections and treated infections prior to 1995 are equal to zero because testing rates, and recruitment into treatment, were assumed to be zero.
Source of HIV transmission
At the beginning of the epidemic (1976-1985), sex work accounted for most new HIV transmission (PAF=95%; 95%CrI: 91-97%; Table 2). In comparison, sexual mixing between CFSW and their non-FSW partners alone contributed 51% of new transmissions; consistently more than high-risk men or high-risk women (Table2). The contribution of FSW and CFSW declined over time as HIV prevalence increased in other risk groups and condom use in commercial sex acts rose. Early on, 15-24 years old women and 25-49 years old men, which also includes young FSW and CFSW, respectively transmitted 86% and 69% of new infections declining to 30% and 15% in 2005-2015 (Table 1). If we exclude FSW, young women consistently acquired more infections than they transmitted, except over 2005-2015 where they acquired the same proportion of infections as they transmitted (Table 2). As HIV prevalence increased over time, the relative contribution of the different age groups to HIV transmission shifted to older ones, particularly for older men who transmitted three times more than young men in the latest period. Despite the high HIV prevalence observed among MSM (18% in 2015; 95% CrI: 12-22%), model estimates suggest that the contribution of MSM to overall HIV transmission has remained marginal over the course of the epidemic (<4%).
Comparatively to the behavioral risk factors, the contribution to transmission of individuals in the primary phase of infection decreased from 86% in 1976-1985 to 25% during 2005-2015 (Table 2), reflecting that proportionally more people are in a later and less infectious HIV stage in mature HIV epidemics. Over 2005-2015, 87% of new HIV infections were attributed to undiagnosed infections, 24% to untreated but diagnosed infections, and 6% to treated infections.
Discussion
With renewed worldwide impetus to sustainably curb the HIV epidemic55, understanding changes in transmission dynamics is an important step towards informing future health policies. Our results show how the contribution of different risk groups to HIV acquisition and transmission has evolved over time in Côte d'Ivoire, mirroring the maturation of the HIV epidemic and past interventions. As for other epidemics driven by unprotected sex work56,57, commercial sex contributed markedly to HIV transmission early in the HIV epidemic. This contribution diminished with time, however, partly due to rising condom use by FSW, reflecting the importance of sustaining FSW interventions50,58,59.
Importantly, bridging of CFSW with their non-FSW partners steadily transmitted around 44% of new HIV; more than the contribution of acute infections (>25%) and similar to older adult males. Thus, interventions targeting CFSW - promoting condom use with their partners not involved in sex work and/or increasing ART coverage in this group - could be important components of the future HIV response60. Focusing activities on this risk group, however, has its own challenges61. Studies of venue-based interventions have nevertheless shown that clients can, at least partly, be reached 42,62-64 and structural interventions to increase safety of sex work environment have the potential to limit HIV acquisition and transmission by CFSW65. The contribution of high-risk men and women to the overall epidemics, which was initially modest has increased slightly over time, never exceeding 18%. This reflects that members of these groups have relatively high number of partners and lower condom use than FSW. Interventions targeting high-risk men and women could also help reduce their contribution to transmission; perhaps less cost-effectively than for FSW who represent a much smaller fraction of the population and who are easier to identify and reach.
Importantly young women have been, and remain, a vulnerable population as they consistently acquired more infections than other age groups and men. However, their contribution to transmission, especially early on, was mainly mediated through sex work. After excluding young FSW, the contribution of young women to transmission never exceeded their contribution to HIV acquisition at any epidemic stages, highlighting their vulnerability and lesser role as epidemic driver. This contribution also needs to be interpreted in light that young women represent a sizeable fraction of the female population (∼38%) compared to FSW or CFSW.
In line with available empirical estimates, our model projected an HIV prevalence of 18% among MSM in 2015, much higher than that of the general population, and slightly above that of FSW during 2005-2015. Nevertheless, MSM contributed little over time to new HIV infections acquired and transmitted. This reflects in part the limited data available suggesting a smaller size of the MSM population, lower number of partners, and more limited sexual mixing with the general population, as compared to FSW. As sex between men remains a sensitive topic in Côte d'Ivoire66, obtaining accurate estimates of the size of this group is a challenge. Yet, given the high prevalence and modeled incidence, both MSM and FSW remain disproportionally vulnerable to HIV despite uncertainties in their respective population size estimates67.
In general, our estimates of the proportion of infections acquired by the different key populations over 2005-2010 are quite different to the 2010 Modes of Transmission (MOT) assessment for Côte d'Ivoire68. For example, we estimated that FSW and MSM respectively acquired between 3-7% and 2-6% of all new HIV infections over 2005-2010. MOT estimates for 2010 of the proportion of HIV infection acquired was much lower for FSW (1-2%) and higher for MSM (6-19%)68. These differences could be explained by the fact that MOT model used slightly different estimates for the size of those key populations, only examined HIV acquisition over one year, did not include the changing infectivity of HIV by disease stage or the impact of ART, did not account for differential sexual mixing by age, and has not been calibrated to epidemiological data. For these reasons, concerns have been raised regarding the reliability of MOT outputs for sub-Saharan Africa21.
Our model suggests that condom interventions have been very effective in averting infections so far. This corroborates findings that behavior changes, through increased condom use, can curb transmission in diverse settings of sub-Saharan Africa69,70. In Côte d'Ivoire, this impact is predominantly due to the documented increases in condom use by FSW7, which may have prevented up to 46% of all infections. This is comparable to results of condom interventions for FSW implemented in Benin50 and Burkina Faso58. Condom use among MSM had a more limited impact on overall transmission because of limited sexual mixing of MSM with the general population. However, increasing condom use by MSM, currently estimated at 73% of sex acts protected, could avert more infections in this group. Other interventions, not currently implemented in Côte d'Ivoire, such as pre-exposure prophylaxis could be considered if current demonstration projects found this to be cost-effective71,72. More generally, our results highlight the importance of high risk groups and targeted HIV prevention for high-groups. Indeed, such targeted preventions have been shown to be highly cost-effective51,73.
As for the more recent ART and PMTCT interventions, they have yet to achieve their full population-level effectiveness because of suboptimal coverage, especially during the early 2000s. Over the last five years (2010-2015), however, ART is estimated to have prevented 15% of all infections in adults. A slightly higher relative impact was observed over the same period for PMTCT interventions, which averted 23% of vertically transmitted infections. Elimination of MTCT would nevertheless still require improving interventions' access to all HIV positive pregnant women.
Limitations of this study are chiefly due to data issues. First, behavioral and epidemiological data on MSM populations are scarce in Côte d'Ivoire such that assumptions for the whole country needed to be extrapolated from MSM surveys conducted in few urban areas. Similarly, studies concerning FSW, albeit more numerous, were mostly conducted in Abidjan. The majority of parameters extracted from triangulations of the various FSW were, however, consistent. Second, and not unique to our study, self-reported data on sexual behaviors could be biased. Finally, the PMTCT sub-model was not calibrated due to the absence of reliable national data on vertical HIV transmission. However, results from this decision tree on the number of HIV positive infants were cross-validated and consistent with UNAIDS estimates.
Our study has many strengths as it is the first transmission modeling exercise that characterized in details the HIV epidemic in Côte d'Ivoire, identifying long term drivers of HIV acquisition and transmission over serial time periods16. This exercise is based on a comprehensive review of all available epidemiological and programmatic data, which was complemented by on-site interviews with program managers74. Second, our impact measures explicitly incorporate the dynamical nature of HIV and are estimated from reliable simulations of the epidemic against well-defined counterfactual scenarios. Third, the Bayesian framework employed enables us to explicitly integrate parameters uncertainty in our inferences.
This study modeled the HIV epidemic in Côte d'Ivoire at the national level. Using a similar approach, important additional insights could be gained by examining how geographical heterogeneities in demography, sexual behaviors, and intervention coverage, influence transmission dynamics and effectiveness of interventions. Further, model-based epidemic appraisal that distinguish between infections acquired and transmitted over the short and longer term at different stages of the epidemic can be used to develop a framework informing the choice of risk groups to target and interventions to adopt.
This paper suggests that the national response in Côte d'Ivoire, especially early condom interventions targeting FSW, was effective at preventing an important number of HIV infections. This is particularly important since the greater allocation of financial resources for ART over the last few years resulted in funding declines for condoms and prevention activities for KP74. Our results support the strengthening of KP condom activities for the national response to be sustainable. Notwithstanding challenges in reaching these men, enhanced prevention among clients, often a neglected risk group, could further reduce transmission. Concomitantly, further efforts are required to develop interventions packages to increase access and uptake of ART, as well as to increase PMTCT coverage. The greatest contributors to new infections in the treatment cascade were undiagnosed infections, highlighting the importance of increasing testing and linkage to care75. Detailed implementation research on how best to scale-up this intervention would be valuable. Continuous evaluation of these interventions is recommended if they are to achieve their maximal population-level effectiveness.
Supplementary Material
Acknowledgments
MMG's work was supported by Bisby Fellowship Prize and a HIV/AIDS Health Services/Population Health Fellowship from the Canadian Institutes of Health Research. We thank the UNAIDS Regional Office for West and Central Africa and the HPTN Modelling Centre (HPTN), which is funded by the US National Institutes of Health (NIH UM1 AI068617) through HPTN, for partial funding of this work.
We also thank Josephine Aho, Avi Hakim, and Sheree Schwartz for providing additional information on surveys of FSW and MSM conducted in Côte d'Ivoire.
Sources of support: Canadian Institutes of Health Research, UNAIDS Regional Office for West and Central Africa, HPTN Modelling Centre.
Footnotes
Competing interest: The authors have declared that no competing interests exist.
Authors' contributions: MCB and MA designed the study. JFV, MCB, and MMG developed the mathematical model. JFV and MMG implemented the coding. MMG and SD performed the comprehensive literature review and gathered programmatic and epidemiological data to inform model parameters. SD conducted the on-site interviews with program managers. MMG calibrated the model and performed the analyses. MMG and MCB interpreted the results. MMG wrote the paper while DD, JFG, KA, MA, MCB, and SD critically reviewed it. All authors have read and approved the final version of the paper.
Additional File: Supplementary Appendix 1.
References
- 1.Koffi K, Gershy-Damet GM, Peeters M, Soro B, Rey JL, Delaporte E. Rapid spread of HIV infections in Abidjan, Ivory Coast, 1987-1990. Eur J Clin Microbiol Infect Dis. 1992;11(3):271–273. doi: 10.1007/BF02098100. [DOI] [PubMed] [Google Scholar]
- 2.Odehouri K, De Cock KM, Krebs JW, et al. HIV-1 and HIV-2 infection associated with AIDS in Abidjan, Côte d'Ivoire. AIDS. 1989;3(8):509–512. doi: 10.1097/00002030-198908000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Denis F, Barin F, Gershy-Damet G, et al. Prevalence of human T-lymphotropic retroviruses type III (HIV) and type IV in Ivory Coast. Lancet. 1987;1(8530):408–411. doi: 10.1016/s0140-6736(87)90118-8. [DOI] [PubMed] [Google Scholar]
- 4.De Cock KM, Porter A, Odehouri K, et al. Rapid emergence of AIDS in Abidjan, Ivory Coast. Lancet. 1989;2(8660):408–411. doi: 10.1016/s0140-6736(89)90590-4. [DOI] [PubMed] [Google Scholar]
- 5.Amat-Roze J. Sexually Transmitted Diseases and HIV/AIDS in Côte d'Ivoire. In: Setel P, Lewis M, Lyons M, editors. Histories of Sexually Transmitted Diseases and HIV/AIDS in Sub-Saharan Africa. Westport, CT: Greenwood Publishing Group; 1999. pp. 43–64. [Google Scholar]
- 6.MLS. Communication pour le changement de comportement dans le domaine du VIH/SIDA en Côte d'Ivoire: Analyse des stratégies et de la réponse de 1985 à 2004. Abidjan, Côte d'Ivoire: Ministère de la lutte contre le SIDA, Projet Retro-CI, Centre Africain de Recherche et d'Intervention en Dévleoppement, Johns Hoplkins Bloomberg School of Public Health / Center for Communication Programs; 2005. [Google Scholar]
- 7.Ghys PD, Diallo MO, Ettiègne-Traoré V, et al. Increase in condom use and decline in HIV and sexually transmitted diseases among female sex workers in Abidjan, Côte d'Ivoire, 1991-1998. AIDS. 2002;16(2):251–258. doi: 10.1097/00002030-200201250-00015. [DOI] [PubMed] [Google Scholar]
- 8.MLS. Plan stratégique national de lutte contre le VIH/SIDA 2002-2004. Abidjan, Côte d'Ivoire: Ministère Délégué auprès du Premier Ministre, chargé de la lutte contre le SIDA; 2002. [Google Scholar]
- 9.CNLS. Plan stratégique national de lutte contre le VIH/SIDA 2006-2010. Abidjan, Côte d'Ivoire: Secrétariat technique du Conseil National de Lutte contre le Sida; 2006. [Google Scholar]
- 10.CNLS. Plan stratégique national de lutte contre l'infection a VIH, le SIDA et les IST 2011-2015. Abidjan, Côte d'Ivoire: Conseil National de Lutte contre le SIDA; 2011. [Google Scholar]
- 11.Msellati P, Vidal L, Moatti J. L'accès aux traitements du VIH/Sida en Côte d'Ivoire - Évaluation de l'initiative Onusida/Ministère Ivoirien de la Santé Publique: aspects économiques, sociaux et comportementaux. Paris, France: Agence nationale de recherches sur le sida; 2001. [Google Scholar]
- 12.MSLS. Suivi de la déclaration d'engagement sur le VIH/SIDA (UNGASS) Période Janvier - Décembre 2002. Abidjan, Côte d'Ivoire: Ministère de la Santé et de la Lutte contre le Sida; 2003. [Google Scholar]
- 13.DIPE. Rapport annuel des indicateurs VIH du secteur Santé en Côte d'Ivoire 2010. Abidjan, Côte d'Ivoire: Direction de l'Information, de la Planification et de l'Évaluation Ministère de la Santé et de l'Hygiène Publique; 2011. [Google Scholar]
- 14.MLS. Stratégie nationale de communication pour le changement de comportement face au VIH/SIDA 2005-2008. Abidjan, Côte d'Ivoire: Ministère de la Lutte contre le SIDA; 2005. [Google Scholar]
- 15.INS, ICF International. Enquête Démographique et de Santé et à Indicateurs Multiples de Côte d'Ivoire 2011-2012. Calverton, MD: Institut National de la Statistique et ICF International; 2012. [Google Scholar]
- 16.Mishra S, Pickles M, Blanchard JF, Moses S, Boily MC. Distinguishing sources of HIV transmission from the distribution of newly acquired HIV infections: why is it important for HIV prevention planning? Sex Transm Infect. 2014;90(1):19–25. doi: 10.1136/sextrans-2013-051250. [DOI] [PubMed] [Google Scholar]
- 17.Shubber Z, Mishra S, Vesga JF, Boily MC. The HIV Modes of Transmission model: a systematic review of its findings and adherence to guidelines. J Int AIDS Soc. 2014;17:18928. doi: 10.7448/IAS.17.1.18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishra S, Sgaier SK, Thompson LH, et al. HIV epidemic appraisals for assisting in the design of effective prevention programmes: shifting the paradigm back to basics. PLoS One. 2012;7(3):e32324. doi: 10.1371/journal.pone.0032324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishra S, Steen R, Gerbase A, Lo YR, Boily MC. Impact of high-risk sex and focused interventions in heterosexual HIV epidemics: a systematic review of mathematical models. PLoS One. 2012;7(11):e50691. doi: 10.1371/journal.pone.0050691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mishra S, Boily MC, Schwartz S, et al. Data and methods to characterize the role of sex work and to inform sex work programs in generalized HIV epidemics: evidence to challenge assumptions. Ann Epidemiol. 2016;26(8):557–569. doi: 10.1016/j.annepidem.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prudden HJ, Watts CH, Vickerman P, et al. Can the UNAIDS modes of transmission model be improved? A comparison of the original and revised model projections using data from a setting in west Africa. AIDS. 2013;27(16):2623–2635. doi: 10.1097/01.aids.0000432476.22616.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.UNDP. World Population Prospects:The 2015 Revision - Key Findings and Advance Tables. New York, NY: Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat; 2015. [Google Scholar]
- 23.Bouscaillou J, Evanno J, Prouté M, et al. Santé des personnes usagères de drogue à Abidjan en Côte d'Ivoire - Prévalence et pratiques à risque d'infection par le VIH, les hépatites virales, et autres infections. Médecins du Monde et le Fonds Mondial - Alliance Côte d'Ivoire. 2014 [Google Scholar]
- 24.N'Cho S, Kouassi L, Koffi A, et al. Enquête Démographique et de Santé, Côte d'Ivoire 1994. Calverton, MD: Institut National de la Statistique et Macro International Inc; 1995. [Google Scholar]
- 25.INS, ORC Macro. Enquête Démographique et de Santé, Côte d'Ivoire 1998-1999. Calverton, MD: Institute National de la Statistique et ORC Macro; 2001. [Google Scholar]
- 26.INS, MLS, ORC Macro. Enquête sur les Indicateurs du Sida, Côte d'Ivoire 2005. Calverton, MD: Institut National de la Statistique (INS), Ministère de la Lutte contre le Sida [Côte d'Ivoire] et ORC Macro; 2006. [Google Scholar]
- 27.RGPH. Recensement général de la population et de l'habitat 2014 - Principaux résultats préliminaires. Abidjan, Côte d'Ivoire: Secrétariat Technique Permanent du Comité Technique du RGPH; 2014. [Google Scholar]
- 28.Zanou B. Rapport d'analyse du recensement général de la population et de l'habitation - 1998 Thème 2: Migrations. Abidjan, Côte d'Ivoire: Institut national de la statistique; 2001. [Google Scholar]
- 29.Mackelprang RD, Baeten JM, Donnell D, et al. Quantifying ongoing HIV-1 exposure in HIV-1-serodiscordant couples to identify individuals with potential host resistance to HIV-1. J Infect Dis. 2012;206(8):1299–1308. doi: 10.1093/infdis/jis480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naicker N, Kharsany AB, Werner L, et al. Risk Factors for HIV Acquisition in High Risk Women in a Generalised Epidemic Setting. AIDS Behav. 2015;19(7):1305–1316. doi: 10.1007/s10461-015-1002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 32.Ray M, Logan R, Sterne JA, et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS. 2010;24(1):123–137. doi: 10.1097/QAD.0b013e3283324283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rollins N, Mahy M, Becquet R, Kuhn L, Creek T, Mofenson L. Estimates of peripartum and postnatal mother-to-child transmission probabilities of HIV for use in Spectrum and other population-based models. Sex Transm Infect. 2012;88(Suppl 2):i44–51. doi: 10.1136/sextrans-2012-050709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raftery AE, Bao L. Estimating and projecting trends in HIV/AIDS generalized epidemics using Incremental Mixture Importance Sampling. Biometrics. 2010;66(4):1162–1173. doi: 10.1111/j.1541-0420.2010.01399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blower S, Dowlatabadi H. Sensitivity and uncertainty analysis of complex models of disease transmission: an HIV model, as an example. International Statistical Review. 1994;62(2):229–243. [Google Scholar]
- 36.Alkema L, Raftery AE, Brown T. Bayesian melding for estimating uncertainty in national HIV prevalence estimates. Sex Transm Infect. 2008;84(Suppl 1):i11–i16. doi: 10.1136/sti.2008.029991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poole D, Raftery AE. Inference for Deterministic Simulation Models: The Bayesian Melding Approach. Journal of the American Statistical Association. 2000;95(452):1244–1255. [Google Scholar]
- 38.Benoit SN, Gershy-Damet GM, Coulibaly A, et al. Seroprevalence of HIV infection in the general population of the Côte d'Ivoire, West Africa. J Acquir Immune Defic Syndr. 1990;3(12):1193–1196. [PubMed] [Google Scholar]
- 39.Vuylsteke B, Semdé G, Sika L, et al. HIV and STI prevalence among female sex workers in Côte d'Ivoire: why targeted prevention programs should be continued and strengthened. PLoS One. 2012;7(3):e32627. doi: 10.1371/journal.pone.0032627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bamba A, Grover E, Ezouatchi R, et al. Étude biologique et comportementale des IST/VIH/SIDA chez les professionnelles du sexe du district d'Abidjan et examen des interventions en direction des populations clefs en Côte d'Ivoire. Ministère de la Santé et de la Lutte contre le SIDA, ENDA Santé, Johns Hopkins University; 2014. [Google Scholar]
- 41.Schwartz S, Papworth E, Thiam-Niangoin M, et al. An urgent need for integration of family planning services into HIV care: the high burden of unplanned pregnancy, termination of pregnancy, and limited contraception use among female sex workers in Côte d'Ivoire. J Acquir Immune Defic Syndr. 2015;68(Suppl 2):S91–98. doi: 10.1097/QAI.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 42.Vuylsteke BL, Ghys PD, Traoré M, et al. HIV prevalence and risk behavior among clients of female sex workers in Abidjan, Côte d'Ivoire. AIDS. 2003;17(11):1691–1694. doi: 10.1097/00002030-200307250-00014. [DOI] [PubMed] [Google Scholar]
- 43.Hakim AJ, Aho J, Semde G, et al. The Epidemiology of HIV and Prevention Needs of Men Who Have Sex with Men in Abidjan, Cote d'Ivoire. PLoS One. 2015;10(4):e0125218. doi: 10.1371/journal.pone.0125218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DIPE. Rapport annuel VIH/Sida du secteur santé en Côte d'Ivoire 2007-2008. Abidjan, Côte d'Ivoire: Direction de l'Information, de la Planification et de l'Évaluation. Ministère de la Santé et de l'Hygiène Publique; 2009. [Google Scholar]
- 45.DIPE. Rapport annuel des indicateurs VIH du secteur santé en Côte d'Ivoire 2009. Abidjan, Côte d'Ivoire: Direction de l'Information, de la Planification et de l'Évaluation. Ministère de la Santé et de l'Hygiène Publique; 2010. [Google Scholar]
- 46.DIPE. Rapport annuel des indicateurs VIH du secteur santé en Côte d'Ivoire 2011. Abidjan, Côte d'Ivoire: Direction de l'Information, de la Planification, et de l'Évaluation. Ministère de la Santé et de la Lutte contre le Sida; 2012. [Google Scholar]
- 47.DIPE. Rapport annuel des indicateurs VIH du secteur santé en Côte d'Ivoire 2012. Abidjan, Côte d'Ivoire: Direction de l'Information, de la Planification et de l'Évaluation. Ministère de la Santé et de la Lutte contre le SIDA; 2013. [Google Scholar]
- 48.DIPE. Rapport annuel des indicateurs VIH du secteur santé en Côte d'Ivoire 2013. Abidjan, Côte d'Ivoire: Direction de l'Information, de la Planification et de l'Évaluation; 2014. [Google Scholar]
- 49.DIPE. Rapport annuel des indicateurs VIH du secteur santé en Côte d'Ivoire 2014 - Non consolidé. Abidjan, Côte d'Ivoire: Direction de l'Information, de la Planification et de l'Évaluation. Ministère de la santé et de la lutte contre le sida; 2015. [Google Scholar]
- 50.Williams JR, Alary M, Lowndes CM, et al. Positive impact of increases in condom use among female sex workers and clients in a medium HIV prevalence epidemic: modelling results from Project SIDA1/2/3 in Cotonou, Benin. PLoS One. 2014;9(7):e102643. doi: 10.1371/journal.pone.0102643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pickles M, Boily MC, Vickerman P, et al. Assessment of the population-level effectiveness of the Avahan HIV-prevention programme in South India: a preplanned, causal-pathway-based modelling analysis. Lancet Globlal Health. 2013;1(5):e289–299. doi: 10.1016/S2214-109X(13)70083-4. [DOI] [PubMed] [Google Scholar]
- 52.Rowe AK, Powell KE, Flanders WD. Why population attributable fractions can sum to more than one. Am J Prev Med. 2004;26(3):243–249. doi: 10.1016/j.amepre.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 53.MSLS. Recueuil des protocoles thérapeutiques nationaux de pathologies. Abidjan, Côte d'Ivoire: Ministère de la Santé et de la Lutte contre le SIDA; 2013. [Google Scholar]
- 54.WHO. Global update on the health sector response to HIV, 2014. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 55.Piot P, Abdool Karim SS, Hecht R, et al. Defeating AIDS--advancing global health. Lancet. 2015;386(9989):171–218. doi: 10.1016/S0140-6736(15)60658-4. [DOI] [PubMed] [Google Scholar]
- 56.Djomand G, Quaye S, Sullivan PS. HIV epidemic among key populations in west Africa. Curr Opin HIV AIDS. 2014;9(5):506–513. doi: 10.1097/COH.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Papworth E, Ceesay N, An L, et al. Epidemiology of HIV among female sex workers, their clients, men who have sex with men and people who inject drugs in West and Central Africa. J Int AIDS Soc. 2013;16(Suppl 3):18751. doi: 10.7448/IAS.16.4.18751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Low A, Nagot N, Konate I, et al. Potential impact of existing interventions and of antiretroviral use in female sex workers on transmission of HIV in Burkina Faso: a modeling study. J Acquir Immune Defic Syndr. 2015;68(Suppl 2):S180–188. doi: 10.1097/QAI.0000000000000441. [DOI] [PubMed] [Google Scholar]
- 59.Adjorlolo-Johnson G, De Cock KM, Ekpini E, et al. Prospective comparison of mother-to-child transmission of HIV-1 and HIV-2 in Abidjan, Ivory Coast. JAMA. 1994;272(6):462–466. [PubMed] [Google Scholar]
- 60.Lowndes CM, Alary M, Labbé AC, et al. Interventions among male clients of female sex workers in Benin, West Africa: an essential component of targeted HIV preventive interventions. Sex Transm Infect. 2007;83(7):577–581. doi: 10.1136/sti.2007.027441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Faugier J, Cranfield S. Reaching male clients of female prostitutes: the challenge for HIV prevention. AIDS Care. 1995;7(Suppl 1):S21–32. doi: 10.1080/09540129550126795. [DOI] [PubMed] [Google Scholar]
- 62.Espirito Santo ME, Etheredge GD. How to reach clients of female sex workers: a survey by surprise in brothels in Dakar, Senegal. Bull World Health Organ. 2002;80(9):709–713. [PMC free article] [PubMed] [Google Scholar]
- 63.Leonard L, Ndiaye I, Kapadia A, et al. HIV prevention among male clients of female sex workers in Kaolack, Senegal: results of a peer education program. AIDS Educ Prev. 2000;12(1):21–37. [PubMed] [Google Scholar]
- 64.Pitpitan EV, Strathdee SA, Semple SJ, Chavarin CV, Magis-Rodriguez C, Patterson TL. Buffering Syndemic Effects in a Sexual Risk-Reduction Intervention for Male Clients of Female Sex Workers: Results From a Randomized Controlled Trial. Am J Public Health. 2015;105(9):1866–1871. doi: 10.2105/AJPH.2014.302366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shannon K, Strathdee SA, Goldenberg SM, et al. Global epidemiology of HIV among female sex workers: influence of structural determinants. Lancet. 2015;385(9962):55–71. doi: 10.1016/S0140-6736(14)60931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aho J, Hakim A, Vuylsteke B, et al. Exploring risk behaviors and vulnerability for HIV among men who have sex with men in Abidjan, Cote d'Ivoire: poor knowledge, homophobia and sexual violence. PLoS One. 2014;9(6):e99591. doi: 10.1371/journal.pone.0099591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tanser F, de Oliveira T, Maheu-Giroux M, Bärnighausen T. Concentrated HIV subepidemics in generalized epidemic settings. Curr Opin HIV AIDS. 2014;9(2):115–125. doi: 10.1097/COH.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.UNAIDS. New HIV infections by mode of transmission in West Africa: a multi-country analysis. Dakar, Sénégal: UNAIDS - Regional Support Team for West and Central Africa; 2010. [Google Scholar]
- 69.Johnson LF, Hallett TB, Rehle TM, Dorrington RE. The effect of changes in condom usage and antiretroviral treatment coverage on human immunodeficiency virus incidence in South Africa: a model-based analysis. J R Soc Interface. 2012;9(72):1544–1554. doi: 10.1098/rsif.2011.0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hallett TB, Aberle-Grasse J, Bello G, et al. Declines in HIV prevalence can be associated with changing sexual behaviour in Uganda, urban Kenya, Zimbabwe, and urban Haiti. Sex Transm Infect. 2006;82(Suppl 1):i1–8. doi: 10.1136/sti.2005.016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Verguet S, Stalcup M, Walsh JA. Where to deploy pre-exposure prophylaxis (PrEP) in sub-Saharan Africa? Sex Transm Infect. 2013;89(8):628–634. doi: 10.1136/sextrans-2012-050891. [DOI] [PubMed] [Google Scholar]
- 72.Venter WD, Cowan F, Black V, Rebe K, Bekker LG. Pre-exposure prophylaxis in Southern Africa: feasible or not? J Int AIDS Soc. 2015;18(4 Suppl 3):19979. doi: 10.7448/IAS.18.4.19979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vassall A, Pickles M, Chandrashekar S, et al. Cost-effectiveness of HIV prevention for high-risk groups at scale: an economic evaluation of the Avahan programme in south India. Lancet Glob Health. 2014;2(9):e531–540. doi: 10.1016/S2214-109X(14)70277-3. [DOI] [PubMed] [Google Scholar]
- 74.Diabaté S, Maheu-Giroux M, Vesga J, Boily M, Alary M. Rapport présenté à ONUSIDA - Région d'Afrique de l'ouest et du centre. Plan d'accélération de la réponse nationale au VIH en Côte d'Ivoire. 2015 [Google Scholar]
- 75.Maheu-Giroux M, Tanser F, Boily MC, Pillay D, Joseph SA, Barnighausen T. Determinants of time from HIV infection to linkage-to-care in rural KwaZulu-Natal, South Africa. AIDS. 2017 doi: 10.1097/QAD.0000000000001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


