Abstract
Shape memory polymer foams have been previously investigated for their safety and efficacy in treating a porcine aneurysm model. Their biocompatibility, rapid thrombus formation, and ability for endovascular catheter-based delivery to a variety of vascular beds makes these foams ideal candidates for use in numerous embolic applications, particularly within the peripheral vasculature. This study sought to investigate the material properties, safety, and efficacy of a shape memory polymer peripheral embolization device in vitro. The material characteristics of the device were analyzed to show tunability of the glass transition temperature (Tg) and the expansion rate of the polymer to ensure adequate time to deliver the device through a catheter prior to excessive foam expansion. Mechanical analysis and flow migration studies were performed to ensure minimal risk of vessel perforation and undesired thromboembolism upon device deployment. The efficacy of the device was verified by performing blood flow studies that established affinity for thrombus formation and blood penetration throughout the foam and by delivery of the device in an ultrasound phantom that demonstrated flow stagnation and diversion of flow to collateral pathways.
Keywords: Embolization, shape memory polymer, varicose veins, pelvic congestion syndrome, varicocele
1. Introduction
In peripheral venous disorders, abnormal blood flow through specific vessels can result in high rates of morbidity including pain, hemorrhage, dysfunction, and a decrease in quality of life for thousands of patients every year. These venous conditions include chronic venous insufficiency, pelvic congestion syndrome, varicoceles, and varicosities associated with portal vein hypertension. In each of these conditions, venous valves are weakened, become incompetent, and allow regurgitation of blood in peripheral vessels, causing a sudden rise in venous pressure and the subsequent formation of varicose veins (Alguire and Mathes, 1997; Beebe-Dimmer et al., 2005; Laborda et al., 2013; Masson and Brannigan, 2014). To treat many of these disorders, physicians rely on peripheral occlusion devices to block or divert blood flow from the susceptible region of incompetent veins, thus forcing blood flow through healthy vessels. This diversion significantly reduces the pain and other morbidities, as well as the risk of hemorrhage associated with varicosities. Fibered platinum coils, such as the Nester® Embolization Coils (Cook Medical, Inc., Bloomington, IN), are one common type of embolization device used to permanently occlude peripheral vessels. However, several coils are often required to achieve complete occlusion, and recanalization, or the recurrence of blood flow through a previously occluded vessel, that requires retreatment can occur in up to 20% of patients (Enriquez et al., 2013). Additionally, complete thrombotic occlusion of the target vessel may not occur for up to 19 minutes after treatment with fibered coils, leading to increased procedural costs and radiation exposure (Dudeck et al., 2011).
Hemorrhage as a result of traumatic vessel or organ injury can also be managed using peripheral occlusion devices. In cases involving vascular trauma, every minute required to achieve stable vessel occlusion may be critical to the patient’s survival. As such, the ideal peripheral occlusion device should minimize time to achieve complete occlusion, require only one device to achieve stable vessel occlusion, minimize the potential for recanalization, and be delivered minimally invasively. To address the prolonged time to occlusion and the need to implant multiple devices, some physicians utilize embolic plugs, such as the AMPLATZER™ Vascular Plugs (AVP, St. Jude Medical, St. Paul, MN). These plugs provide a single device solution to achieve complete vessel occlusion. Embolic plugs typically consist of a fine nitinol mesh with or without a polytetrafluoroethylene (PTFE) fabric incorporated into the structure (Emmert et al., 2013). However, vascular plugs may require up to 20 minutes for stable thrombus formation and they can typically only be used in vessels with limited tortuosity due to the stiffness of the devices (Wang, 2010). The drawbacks of current coils and vascular plug devices highlight the need to continue improving on peripheral embolization technology.
Shape memory polymer (SMP) foams have been thoroughly investigated as advantageous embolic devices for stabilizing porcine sidewall aneurysms and vascular anomalies (Hwang et al., 2013; Ortega et al., 2013; Rodriguez et al., 2012; Rodriguez et al., 2014b). Previously, the biocompatibility of SMP foams has been demonstrated in porcine models (Rodriguez et al., 2014a; Rodriguez et al., 2014b). In these studies, significant connective tissue infiltration was seen throughout the implant, which caused complete, stable occlusion of the treated aneurysms. Connective tissue deposition and scar formation is a critical step in preventing recanalization (Bavinzski et al., 1999; Molyneux et al., 1995). The proposed SMP foams aim to minimize time to mature healing by providing a scaffold morphology that readily supports a healing response involving the initial clotting of blood within the scaffold. Then, over time, replacement of the clot with mature connective tissue will occur. With reduced time to mature healing, the risk of recanalization is significantly reduced. This, in turn, decreases the need for follow-up imaging with a corresponding decrease in the overall cost of treatment. SMP foams also have the unique ability to be stored in a compressed geometry and subsequently expand to fill large volumes upon contact with circulating blood (Singhal et al., 2012). The shape memory capacity of these foams results in an ideal material for minimally invasive devices which provide limited friction during catheter delivery. However, they are still capable of expanding up to ten times their crimped diameter to fill large volumes and create rapid occlusion of vessels with a single device. The affinity for rapid clot formation is primarily due to the high surface area and porous morphology of the foam that creates numerous recirculation and stagnation zones that activate rapid thrombosis (Friedrich and Reininger, 1995; Ortega et al., 2013).
The following study consists of a series of in vitro tests aimed at verifying the safety and efficacy of a first-generation shape memory polymer peripheral embolization device (PED) to be used for arterial and venous occlusion in peripheral vessels accessible with a 4–6Fr guide catheter. Material characterization was conducted on various SMP foam formulations to ensure the proposed formulation would remain crimped at room temperature but allow expansion when exposed to a 37°C aqueous environment. The shape recovery of the PED was also analyzed to ensure the foam expanded slowly enough to allow delivery to the treatment site via endovascular catheter. Mechanical analysis was conducted on the foam and coil anchor components of the device (figure 1) to ensure the radial force of the device is unlikely to cause vessel perforation or rupture. Then a temperature and pressure-controlled flow system was used to test the device’s susceptibility to migration and undesired thromboembolism. The same flow system was used to perfuse bovine blood through SMP foam devices to investigate the distribution of fibrin and erythrocytes throughout the device over time and ensure that blood is able to penetrate throughout the foam volume. This is critical for ensuring there are no regions devoid of access to circulating blood which may delay healing in the treatment vessel and create a predisposition for recanalization after treatment. Finally, the device was delivered under ultrasound guidance to a vascular phantom by a vascular surgeon and interventional radiologist to determine the overall performance and echogenicity of the device, as researchers have investigated for other occlusive devices (Caivano et al., 2012; Porciello et al., 2014).
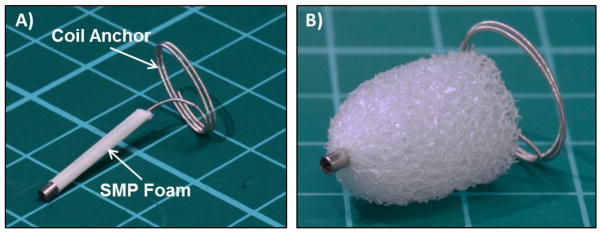
Figure 1.
Image of the crimped (A) and expanded (B) embodiment of the SMP peripheral occlusion device investigated within this study. The device consists of a distal platinum alloy coil anchor and a proximal length of SMP foam that is crimped for delivery through a guide catheter and subsequently undergoes up to 100X volume expansion to fill large volumes upon deployment.
2. Materials and Methods
2.1 Foam Fabrication
Each foam composition was fabricated using the three-step protocol described previously (Hasan et al., 2014). In short, isocyanate (NCO) prepolymers were synthesized with appropriate molar ratios of N,N,N’,N’-Tetrakis(2-hydroxypropyl)ethylenediamine (HPED, 99%; Sigma-Aldrich Inc., St. Louis, MO), triethanolamine (TEA, 98%; Sigma-Aldrich Inc.), and hexamethylene diisocyanate (HDI, TCI America Inc., Portland, OR). The prepolymers were reacted for 2 days with a temperature ramp from room temperature to 50°C at a rate of 20°C/hr, held isothermally at 50°C for 16 hours, and passively allowed to cool back to room temperature. A hydroxyl (OH) mixture was blended with the remaining molar equivalents of HPED and TEA. This mixture also contained deionized (DI) water (> 17 M Ω cm purity; Millipore water purifier system; Millipore Inc.), and catalysts (T-131 and BL-22, Air Products and Chemicals, Inc., Allentown, PA). During the foaming step, the NCO prepolymer and the OH mixture were combined in a foaming cup along with surfactants (DC 198 and DC 5943, Air Products and Chemicals, Inc., Allentown, PA) and the physical blowing agent, Enovate 245fa (Honeywell International, Inc., Morristown, NJ). This solution was mixed in a FlackTek Speedmixer (FlackTek, Inc., Landrum, SC) and poured into a bucket to form a foam. The foam was cured at 60°C for 5 minutes before passively cooling to room temperature for further processing. Various foam formulations and pore sizes were fabricated to create foams with differing crosslink densities, glass transition temperatures (Tg), rate of moisture plasticization, and subsequent foam expansion rates. Foam formulations are denoted as H20-H60, where the numerical value appearing after “H” corresponds to the ratio of HPED to TEA equivalents in the polymer premix. Both foam formulation and pore size were used to control the expansion rate of the foams and the resultant working time to enable catheter delivery.
2.2 Foam Processing
After fabrication, foams were cut into blocks 2cm thick, 7cm long, and 6cm wide. These blocks were then reticulated using the same method described previously (Rodriguez et al., 2014b). In short, the foams were penetrated by a floating pin array while subjected to low amplitude, high frequency perturbations, which allowed the creation of pinholes in the foam pore membranes. These pinholes create interconnected pores throughout the foam which allow blood flow and eventual connective tissue deposition to penetrate throughout the entire device. After reticulation, the foams were cut with disposable biopsy punches (Sklar Surgical Instruments, West Chester, PA, USA) for three different device sizes- 6, 8, and 12mm. These device sizes were used to enable delivery through 4, 5, and 6Fr catheters, respectively, and the ability to treat vessels with diameters between approximately 2–11mm. After the foams were cut into their final geometry, they were cleaned to remove any plasticizers and unreacted monomers from the foams. Each cleaning cycle lasted 15 minutes and was performed under sonication in a 40°C water bath. The first two cleaning cycles consisted of submerging the foams in 99% isopropyl alcohol (VWR, Radnor, PA). Then the foams were rinsed with reverse osmosis (RO) water before being cleaned in four cycles of Contrad 70 liquid detergent (Decon Labs, King of Prussia, PA). Each foam was then rinsed with RO water until no Contrad 70 residue was evident. Finally, the foams were cleaned for two cycles in RO water. After cleaning, the damp foams were frozen in a −20°C freezer for 12 hours before freeze-drying in a FreeZone Freeze Dryer (Labconco, Kansas City, MO) for 24 hours.
2.3 Material Analysis
Differential Scanning Calorimetry (DSC) was used to assess the Tg of each foam formulation (H20–H60) through the use of a Q200 DSC (TA Instruments, New Castle, DE, USA), to ensure the crimped devices would remain compressed at typical storage and shipping temperatures. The Tg was also analyzed to verify the tunability of the polymer chemistry, as a higher Tg corresponds to slower shape recovery. Four samples of each foam composition were analyzed using DSC, which provides a simple means of addressing any excessive expansion of the PED while still inside the delivery catheter. This premature expansion can occur as a result of foam contact with blood or saline that plasticizes the foam and depresses the Tg sufficiently to initiate foam expansion at body temperature. Every sample was weighed to ensure a mass of 3–10mg, cooled −40°C, and heated at a rate of 10°C/min to 120°C. This cycle was repeated twice; the last heating cycle was used for quantification of the Tg for each foam formulation. The Tg was calculated using the half-height method in the TA Instruments software.
2.4 Expansion Studies
Expansion studies were conducted to ensure the foams do not undergo premature expansion within the delivery catheter, preventing the device from deploying properly at the target site. Expansion studies were conducted using a water bath heated to 37°C. Foams with pore sizes of approximately 0.5, 1.0, and 1.5mm were crimped over a 0.010” nitinol wire using the SC250 Stent Crimper (Machine Solutions Inc., Flagstaff, AZ, USA). Samples were imaged before and after crimping using a Leica MZ16 Digital Video Microscope (Leica Microsystems, Wetzlar, Hesse, DEU) with a Jenoptik CF Scan Camera (Jenoptik AG, Jena, Thuringia, DEU) and loaded into a fixture which held the nitinol wire taut. The entire fixture was submerged in the heated water bath and imaged at 0.5, 1.0, 1.5, and 2.0 minutes, as well as every minute thereafter until 10 minutes had elapsed. Each picture was then converted to a binary image and the 2-D projected surface area of the foam was calculated in each image using MATLAB (MathWorks, Inc., Natick, MA, USA). The projected surface area at each time point was then divided by the length of the sample to obtain an average diameter of the foam at each time point. This analysis aids in determining which foam formulations and pore sizes will provide sufficient working time for physicians to deliver the devices before excessive foam expansion prevents advancing the device through the catheter.
2.5 Device Fabrication
Due to the low radial force of the SMP foams, a coil anchor is incorporated into the PED to enable implantation in both arteries and veins with minimal risk of device migration. To fabricate the coil anchors used for in vitro device verification tests, 0.018” diameter 90/10% platinum/iridium coils with an inner diameter of 0.010” were threaded over 0.005”, 0.006”, and 0.008” diameter superelastic nitinol wire for the 6, 8, and 12mm PED devices, respectively. The coils were then wrapped around a stainless steel mandrel that had been machined to each device diameter and shape-set in a 550°C furnace for 15 minutes. After 15 minutes, the mandrels were immediately quenched in room temperature water to set the final shape of the coil. The coils were removed from the mandrel and a straight section of the coil was manually threaded through the center of the foam before crimping.
2.6 Device Mechanical Analysis
One critical design consideration for a peripheral occlusion device, to prevent vessel perforation or rupture, is to ensure the device does not exert radial forces in excess of the radial strength of the treatment region. To analyze variations in radial strength based on foam formulation and pore size, a J-Crimp Radial Compression Station (Blockwise Engineering LLC, Tempe, AZ, USA) was used with an Instron Model 5966 Dual Column Test System (Illinois Tool Works Inc., Norwood, MA, USA) to perform radial force measurements during foam expansion. For this test, foams were cut into cylinders 4, 6, 8, and 10mm in diameter and 2cm in length using biopsy punches. Samples were then crimped to approximately 1mm in diameter using a SC250 Stent Crimper (Machine Solutions Inc., Flagstaff, AZ, USA). The lumen of the J-Crimp compression station was adjusted such that the expanded diameters of the foam devices were 50% oversized to the lumen, the maximum percentage of oversizing indicated within the Instructions for Use (IFU) for other embolic plug devices like the AVP. Then, each sample was placed inside the J-Crimp compression station while heated to 100°C. Each sample was allowed to freely expand for 20 minutes within the compression station while the radial force of the device was monitored on the Instron.
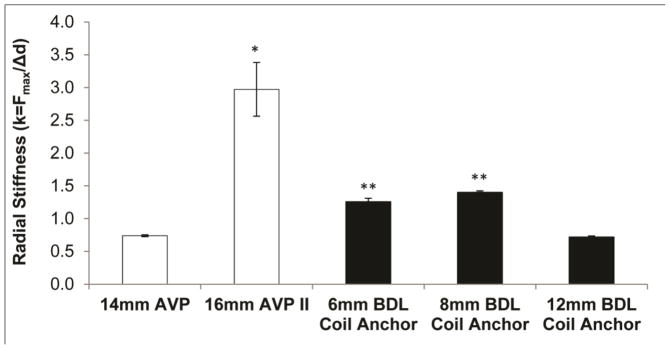
The radial stiffness of the PED anchor coil was also analyzed and compared to a 14mm AVP and 16mm AVP II. The same J-Crimp Radial Compression Station was used for this analysis. To begin each test, the lumen of the J-Crimp was set to the expanded diameter of the device of interest. Then the diameter of the J-Crimp was decreased at a rate of 10mm/min until the expanded device diameter was 50% oversized to the J-Crimp diameter, again aligned with common sizing parameters detailed in the IFU for other embolic devices. The total radial force throughout device compression was continually recorded to determine the maximum force experienced during compression. The maximum force was then divided by the change in diameter to obtain the radial stiffness (k) of each device.
In order to estimate the total surface area of devices in contact with the endothelium of the vessels, a 16mm AVP II and 8mm PED anchor coil were deployed into flexible polyvinyl chloride (PVC) tubing with an inner diameter of 10 and 5mm, respectively, and imaged using the same Leica microscope previously implemented for expansion studies. The images were analyzed using ImageJ software to estimate the total surface area of the flexible tubing that was deflected by the radial force of each respective device- providing an estimation of the total surface area of the devices contacting the vessel lumen.
2.7 Device Migration and Unintended Thromboembolism
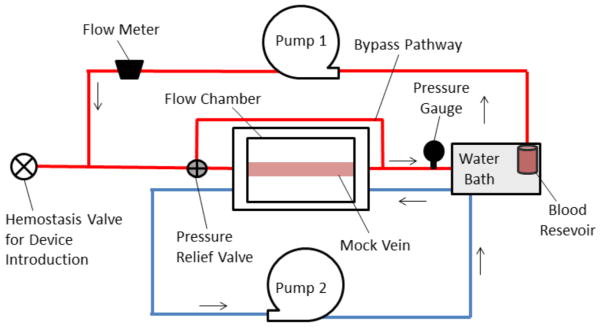
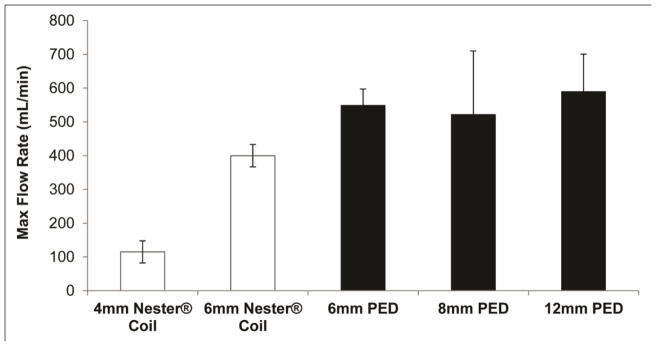
A critical test that must be performed on a new peripheral occlusion device is one that verifies the device will not become an embolus or dislodge from the treatment region and migrate to a non-target area resulting in thromboembolism. To monitor device stability, samples were delivered and analyzed in the flow loop shown in figure 2 without the bypass pathway to force flow through a single vessel and represent the worst case scenario with no collateral vessels. Devices were delivered to the test section when the flow system was equilibrated at 37±2°C. The test sections consisted of flexible PVC tubing (McMaster-Carr, Douglasville, GA, USA) 5, 7, and 10mm in diameter for the 6, 8, and 12mm PED, respectively. Each tubing size was chosen such that each device diameter would be approximately 20% oversized to the target vessel- the hypothesized lowest degree of device oversizing that would be indicated for the PED device. Due to limitations in commercial flexible PVC tubing sizes, the 8mm device was only oversized by 14% to the test section, providing a more rigorous stability test than the other device sizes. When the foams were fully expanded in the test section, Pump 1 was set to circulate a 36.7% (vol) glycerol-water mixture at room temperature through the lumen of the mock vein. This glycerol solution was used to ensure the inner diameter of the mock vein was lubricious and the circulating fluid matched the kinematic viscosity of blood (Nguyen et al., 2004). The flow rate was then gradually ramped up in increments of approximately 20mL/min while maintaining the pressure within the flow system at 207±52mmHg. At each flow rate interval, the devices were imaged for 2 minutes with a stationary Canon PowerShot SX230 HS Digital Camera (Canon U.S.A., Inc., Melville, NY, USA) positioned above the test section to monitor migration. Displacement of more than 1mm in 2 minutes was deemed a device failure. The flow interval prior to the interval at which failure occurred was noted as the maximum flow rate for each device. This procedure was used for both PED and Cook coils.
Figure 2.
Schematic of flow system used for in vitro device stability and blood perfusion studies. Pump 1 is a peristaltic pump which circulates fluid through the test section, and Pump 2 is also a peristaltic pump which circulates heated water into the flow chamber surrounding the mock vein to maintain the test section in a 37°C aqueous environment.
2.8 Blood Perfusion
In order to monitor the degree of blood infiltration throughout the PED and investigate the potential for zones devoid of blood contact within the foam volume, bovine blood was incorporated into the flow system shown in figure 2. Histology was subsequently performed on foam devices to verify blood infiltration throughout the foam. The histological analysis was also used to investigate the affinity for fibrin deposition within the foam, which serves as a precursor to stable scar formation and the prevention of recanalization. To accomplish this, bovine blood was obtained as part of a tissue share program with the Rosenthal Meat Science and Technology Center at Texas A&M University in College Station, TX. All blood used in this study was obtained from animals euthanized for purposes unrelated to this research. Blood was collected immediately following sacrifice of the animals and citrated in a 5 gallon bucket containing approximately 2.1 liters of 3.2% sodium citrate to prevent clotting, as recommended by Adcock et al. (Adcock et al., 1997). The 3.2% sodium citrate solution was prepared by mixing 2,103mL of phosphate buffered saline (PBS) with a pH of 7.4 (Sigma-Aldrich Inc.) with 67.3g of sodium citrate (Santa Cruz Biotechnology Inc., Dallas, TX, USA).
Before perfusing blood through foam devices, Activated Clotting Time (ACT) tests were conducted using a Hemochron® 401 (International Technidyne Corporation, Edison, NJ, USA) and kaolin-activated test vials (Accriva Diagnostics, Piscataway, NJ, USA). ACT tests were conducted to determine the proper amount of 0.1M calcium chloride (CaCl2, Flinn Scientific Inc., Batavia, IL, USA) to add to the blood to restore the clotting capabilities of the blood. The target ACT value for the bovine blood was between 120 and 180 seconds to mimic normal, healthy ACT of cattle (Riley and Lassen, 1979). Various amounts of CaCl2 were added to 2mL of citrated blood in the kaolin-activated test vials until the ACT fell into the desired range. Ultimately, 105μL of CaCl2 for every 1mL of citrated blood resulted in an average ACT of 173 seconds. This ratio of CaCl2 to blood was used throughout the blood flow studies. All blood flow studies were conducted within 24 hours after blood collection, and less than six hours elapsed between the first and last flow study.
To begin the blood flow studies, 37°C PBS was perfused through the flow system for 10 minutes to prime the tubing and fittings. The pressure relief valve proximal to the test section was set to approximately 450mmHg to ensure all flow was forced through the test section until the fittings of the flow system were at risk of failure. All tubing used in the flow loop was 1/4”ID x 3/8”OD S-50-HL Tygon®, an ISO 10993 certified, non-pyrogenic, non-hemolytic, non-toxic tubing commonly used in biologic applications (Kim et al., 1974; Neumann et al., 2003; Zweens and Schiphof, 1976). While the flow system was primed, 500mL of citrated blood was warmed within a container inside a water bath until it reached 37°C. When the flow system and blood had equilibrated at 37°C, 105μL of CaCl2 was added for every 1mL of blood in the warmed container. The blood and CaCl2 was lightly stirred for 5 seconds before the inlet of peristaltic pump 1 was inserted into the blood container to begin perfusion through the flow system. Foams were perfused with blood for 30, 90, 150, 210, and 270 seconds at a flow rate of 40mL/min. Perfused blood was then captured in a waste container after exiting the test section so that it would not recirculate through the test system. At each time point, the tubing within the flow chamber was cut proximal and distal to the foam and quickly rinsed with PBS to remove any non-adherent cells. Each sample was then fixed in formalin for 7 days.
2.9 Histological Analysis
After fixation, each sample was transected perpendicular to the long axis at three points to obtain cross-sections through the sample from proximal, middle, and distal regions relative to the direction of flow. Each section was dehydrated in increasing concentrations of ethanol and cleared with Pro-Par Clearant (Anatech Ltd., Battle Creek, MI, USA) using the Citadel 1000 Tissue Processor (Shandon Inc., Pittsburgh, PA, USA). Samples were then infiltrated with and embedded in paraffin wax using the Citadel 1000 tissue processor and Tissue-Tek TEC III Tissue Embedder (Miles Laboratories Inc., Naperville, IL, USA). Paraffin blocks were sectioned at 5μm thicknesses on a Microm HM355S Rotary Microtome (Thermo Fisher Scientific Inc., Waltham, MA, USA), placed on the M7654-1 SP Tissue Flotation Bath (Cardinal Health, Dublin, OH, USA) set to 45°C, floated onto glass slides, and dried in a Lipshaw Model 218 Slide Dryer (Shandon Inc., Pittsburgh, PA, USA). Once slides were dry, they were deparaffinized with xylenes, rehydrated, and stained with Hematoxylin and Eosin (H&E) and Phosphotungstic Acid Hematoxylin (PTAH). Slides were then imaged with a virtual microscope using a 40x objective (Olympus Corporation, Tokyo, JPN).
To analyze the percent of fibrin coverage on each histology slide, Adobe Photoshop was used to isolate the fibrin, stained bluish purple by the phosphotungstic acid, from the erythrocytes and foam struts, stained pinkish red by the hematoxylin. When each stain was isolated, the slides were converted to binary images and analyzed for the surface area of pixels corresponding to each stain using ImageJ. H&E was used as a counterstain to aid in verifying the presence of fibrin and leukocytes.
2.10 Ultrasound Investigation
Two physicians with over 15 years of experience in their respective fields performing various peripheral embolization procedures, delivered the PED into the flow system shown in figure 2, where a homemade ultrasound phantom was inserted in place of the mock vein. The ultrasound phantom was created by bringing 3 cups of water to a boil, mixing in nine 0.25-ounce packets of gelatin and 4 tablespoons of sugar free Metamucil®, and pouring the mixture into a greased circular mold containing a ” PVC tube that would create a lumen through the phantom upon removal. The mold was placed in a refrigerator for 12 hours before the phantom was removed from the mold and placed into the flow system. While circulating the same 36.7% (vol) glycerol-water solution used for device migration studies through the vascular phantom, an M-Turbo® SonoSite ultrasound machine was used with a HFL38x planar probe (FUJIFILM SonoSite, Inc., Bothell, WA, USA) to image a device delivery procedure and analyze the efficacy and echogenicity of the PED.
3. Results
3.1 Material Analysis
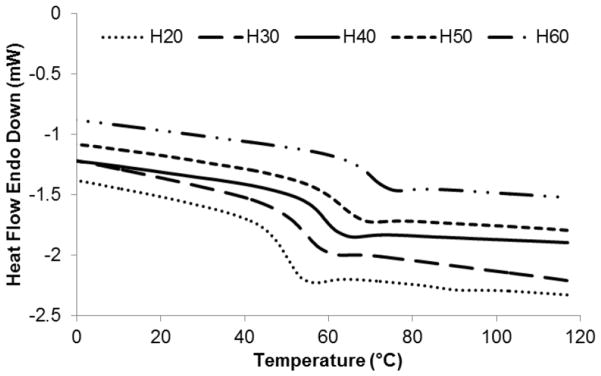
DSC was used to assess the ability to control the activation temperature of the proposed devices, which corresponds to the Tg of the materials under investigation. It is critical that the actuation temperature of these devices is greater than the temperature at which they are stored to prevent premature expansion of the foams. The Tg for all foam formulations ranged between 49 and 70°C. Figure 3 shows representative thermograms for each foam composition used in this study, where H20–H60 correspond to foam compositions with 20–60% molar equivalents of HPED. The thermograms demonstrate a single transition with no indication of a secondary transition, as well as a nearly linear relationship between increasing Tg as the ratio of HPED to TEA also increases. The average Tg found for each foam formulation and the corresponding standard deviations are shown in table 1.
Figure 3.
DSC thermograms showing increased glass transition temperatures with increasing ratios of HPED to TEA.
Table 1.
Average glass transition temperature and standard deviation for each foam formulation based on analysis of four samples of each foam formulation.
| Foam Composition | Average Tg (°C) | Standard Deviation |
|---|---|---|
| H20 | 49.85 | 0.15 |
| H30 | 53.33 | 0.58 |
| H40 | 58.83 | 0.28 |
| H50 | 62.49 | 0.26 |
| H60 | 69.44 | 0.39 |
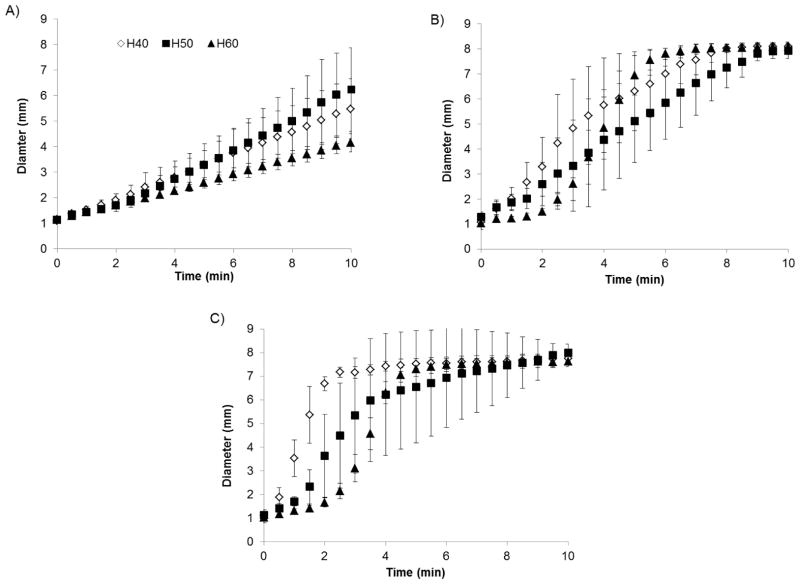
Based on pilot expansion and delivery studies, devices fabricated using H20 and H30 formulations expanded too rapidly to allow delivery of devices via catheter. For this reason, only devices fabricated from H40, H50, and H60 foams were investigated further as potential foam formulations to incorporate into the peripheral occlusion device. Figure 4 shows the results from the expansion studies conducted in 37°C water. As expected, there is a general trend of decreasing expansion rate, within the first three minutes of submersion in 37°C water, as the crosslink density of the foam increases (higher HPED content). The first three minutes of exposure to aqueous environments is critical as the PED is designed to be delivered within three minutes after first contacting blood or saline. Pore size also had a dramatic effect on expansion rate, as shown in figure 4, where expansion rate decreased as the pore size decreased due to increased foam density delaying water diffusion into the foam matrix. However, regardless of pore size and foam composition, all samples experienced 100% shape recovery in less than 20 minutes.
Figure 4.
Average expanded diameter of crimped 8mm foam cylinders with 0.5mm pores (A), 1.0mm pores (B), and 1.5mm pores (C) at 30 second intervals after immersion in 37°C water (mean ± one standard deviation, n = 5). Foams demonstrated controllable expansion rates based on varying foam composition and pore size. Again for reference, H40, H50, and H60 refer to foam compositions with 40%, 50%, and 60%, respectively, molar equivalents of HPED.
3.2 Device Mechanical Analysis
Radial force tests demonstrated that the radial force of foam devices consistently increased as the device diameter increases. The results for the radial force tests are summarized below in Table 2. These tests show the radial force of SMP foams with varied foam chemistries (H40, H50, H60). A pore size of 0.5 ± 0.1mm was chosen for analysis of all chemistries after testing the radial force of foam samples with 0.5, 1, and 1.5mm pore sizes, which revealed that foams with the smallest pore size exert the greatest radial force due to increased foam density. Constrained recovery tests demonstrated that the maximum force exerted on the vessel walls by foam expansion when the foam is 50% oversized to the target vessel is significantly lower than the 107N of force required to rupture autologous veins commonly used in bypass procedures, if we assume a uniform cylindrical surface area of the foam (Konig et al., 2009).
Table 2.
Maximum radial force (mean ± one standard deviation, n = 5) exerted by H40 (A), H50 (B), and H60 (C) foams during actuation when the foam expanded diameter is 50% oversized to the target vessel. Graph shows a positive relationship between device diameter and the maximum radial force of the foam.
| Foam Composition | Device Diameter (mm) | Vessel Diameter (mm) | Radial Force (N) |
|---|---|---|---|
|
| |||
| H40 | 4 | 2.7 | 0.08 ± 0.03 |
| 6 | 4.0 | 0.16 ± 0.03 | |
| 8 | 5.3 | 0.24 ± 0.05 | |
| 10 | 6.7 | 0.3 ± 0.05 | |
|
| |||
| H50 | 4 | 2.7 | 0.13 ± 0.07 |
| 6 | 4.0 | 0.17 ± 0.04 | |
| 8 | 5.3 | 0.21 ± 0.04 | |
| 10 | 6.7 | 0.25 ± 0.03 | |
|
| |||
| H60 | 4 | 2.7 | 0.2 ± 0.04 |
| 6 | 4.0 | 0.29 ± 0.07 | |
| 8 | 5.3 | 0.38 ± 0.05 | |
| 10 | 6.7 | 0.36 ± 0.09 | |
The radial stiffness of each different sized anchor was compared to the stiffness of two vascular plugs currently on the market in figure 5. During radial force testing to determine device stiffness values, the 8mm devices exerted an average maximum radial force of 4.0N, while the AVP II exerted an average maximum radial force of 15.8N when oversized by 50% to the target vessel. Microscopic imaging of the 8mm PED anchor coil revealed that approximately 30% of the coil surface area is in contact with the vessel endothelium, which corresponds to 0.43cm2 of surface area. Given the estimated surface area of coils in contact with the vessel lumen, the PED anchor would exert a pressure of approximately 700mmHg on the vessel endothelium- less than half the pressure required to cause rupture in an autologous vein graft (Konig et al., 2009). When a 16mm AVP II was deployed within a flexible PVC tube with an inner diameter of 10mm, it was estimated that approximately 0.85cm2 of device surface area was in contact with the inner diameter of the tubing, resulting in a radial pressure of approximately 1,400mmHg. Given the proven safety and efficacy of the AVP II device that led to its FDA approval, and the markedly reduced radial force and pressure exerted by the PED anchor, it is unlikely that the PED coil anchor would cause vessel rupture or perforation in vivo. Prior to verification tests, it was hypothesized that the coil anchor would account for the vast majority of the radial force exerted by the PED. Radial stiffness testing revealed that this was indeed the case, as demonstrated by a maximum radial force of less than 0.5N for any foams tested.
Figure 5.
Maximum radial stiffness (mean ± one standard deviation, n = 5) in N/mm of a 14mm AVP and 16mm AVP II (St. Jude Medical, Inc.) compared to platinum alloy coils fabricated within the Biomedical Device Laboratory (BDL) at Texas A&M. The maximum radial force was measured while each device was radially compressed until the lumen diameter corresponded to a vessel size for which each device is 50% oversized. The maximum radial force was then divided by the change in diameter to produce a device stiffness constant that enables estimations of the total radial force exerted by each device when implanted in any sized vessel.*p<0.05 vs. all other devices, and **p<0.05 vs. 14mm AVP for a two-tail paired Student’s t-test.
3.3 Device Migration and Unintended Thromboembolism
To ensure the PED has a limited risk of migrating downstream and causing unintended thrombosis, studies were conducted in which the maximum flow rate was determined for each PED size for comparison to one of the market-leading embolic coils, Cook Medical’s NesterR Embolic Coil. Figure 6 summarizes the results from this analysis, which showed the PED can withstand equivalent or higher flow rates than Nester® coils. This analysis was also performed with only one Nester® coil within the mock vein, whereas at least three coils are typically implanted to achieve complete vessel occlusion in the clinic (Kipshidze et al., 2005). If three coils were implanted into the test section, the pressure drop across the device mass would drastically increase, and the maximum flow rate for these coils would likely decrease further.
Figure 6.
Comparison of the maximum flow rate (mean ± one standard deviation, n = 3 for Nester® Coils and n = 4 for PED devices) commercial embolic coils and the PED technology under investigation can withstand without migrating downstream and causing undesired thromboembolism.
3.4 Blood Perfusion Studies and Histology
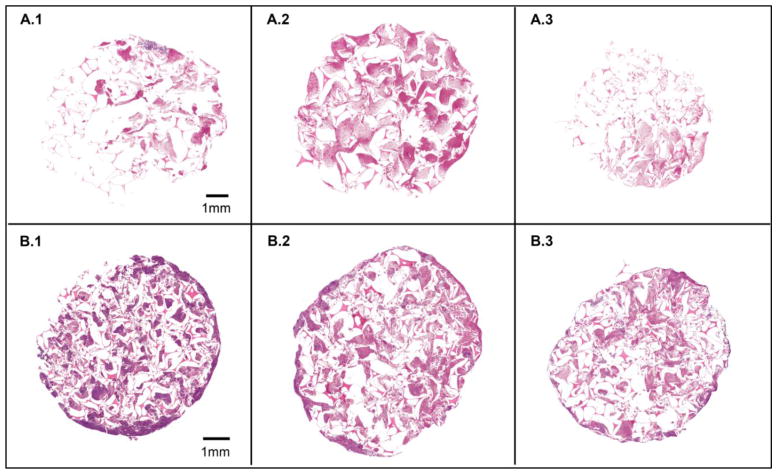
During in vitro blood flow studies, complete occlusion of the foam device was observed at 270 seconds. Complete occlusion was evidenced by flow diverting through the pressure relief valve. At this point, the pressure within the vein model created by thrombus formation exceeded the pressure relief valve setting and flow was diverted through the bypass pathway. In order to show the most dramatic change in cellular deposition throughout the foam device, figure 7 shows a histologic comparison between samples perfused with blood for 30 and 270 seconds.
Figure 7.
Histology results showing foam samples perfused with blood for 30sec in row A, and samples perfused for 270sec in row B. Samples 1, 2, and 3 correspond to proximal, middle, and distal locations within the device, respectively. All samples were analyzed using PTAH to stain erythrocytes pinkish red and fibrin and leukocytes purple.
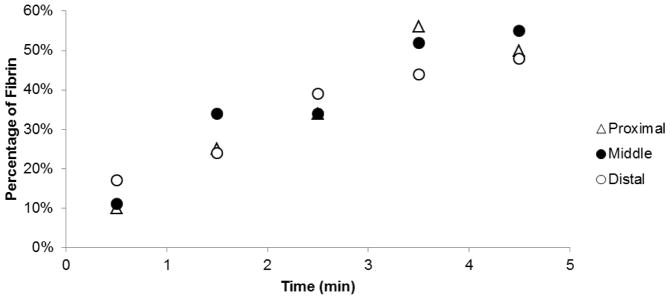
Each image sequence shows blood is penetrating throughout the entire volume of the foam device, demonstrating the effectiveness of reticulation in creating interconnected pathways along the length of the device. After 30 seconds of blood perfusion, each cross section consists of primarily erythrocytes enmeshed in loose, interspersed fibrin. At 270sec, approximately 50% of the proximal section of foam consisted of dense fibrin (figure 8), which likely contributed to the complete vessel occlusion which occurred at this time point.
Figure 8.
Average percentage of fibrin at proximal, middle, and distal locations within the SMP foam device perfused with bovine blood for varying durations of time. Percentage of fibrin quantified using colorimetric analysis.
3.5 Ultrasound Investigation
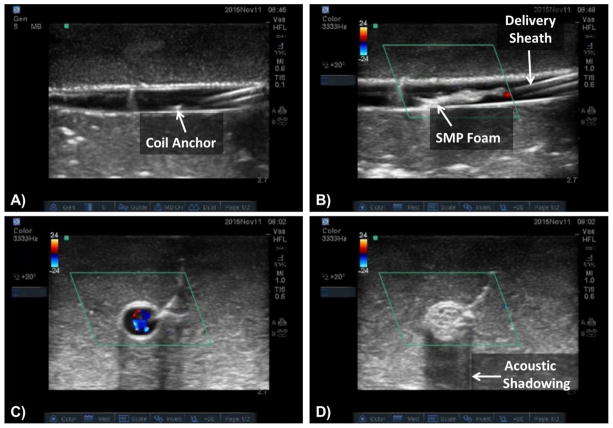
When delivering the PED under ultrasound, both the SMP and platinum coil anchor provided sufficient echogenicity to allow visualization, as shown in figure 9. Image A shows the platinum coil anchor exiting the catheter. Image B shows the foam’s natural echogenicity allowing easy device visualization. In image C, a cross section of the vessel is shown with flow before foam expansion, as indicated by the blue and red colorations typical of flow when using Doppler ultrasound (Zamboni et al., 2009). Flow stagnation and the reduction of flow beyond detectable limits are shown in image D, as indicated by the lack of color mapping within the image after foam expansion. This image also shows significant acoustic shadowing indicative of an acoustically dense material, providing further evidence that the PED is likely to cause rapid occlusion upon expansion in vivo. This shadowing is the same phenomenon used by physicians to identify dense, calcified lesions within arteries with intravascular ultrasound (IVUS) (Gao et al., 2014).
Figure 9.
Doppler ultrasound images showing the catheter tip and coil anchor deployment (A), foam expansion and the parallel hyperechoic lines indicating the placement of the delivery sheath (B), a cross-sectional view of the mock vessel with flow (C), and a cross-sectional view of the vessel showing flow stagnation and significant acoustic shadowing after foam expansion (D).
4. Discussion
Through the use of DSC, precise control of the actuation temperature of SMP foams by altering the ratio of HPED to TEA was verified, which has been demonstrated previously by Wilson et al. and Singhal et al. (Singhal et al., 2012; Wilson et al., 2007). The increase in Tg as the amount of HPED increases is a result of the increased crosslink density associated with additional HPED and the steric hindrance provided by the molecular structure of HPED which limits chain mobility. The values obtained in this study for the Tg of each formulation were slightly higher than reported by Singhal et al. (Singhal et al., 2012). This is likely due to the extensive foam cleaning protocol incorporated into this study which removes any unreacted surfactants and catalysts that may have plasticized the previous samples. The ability to control the Tg of SMP foam devices is highly useful for controlling the actuation rate of the device when exposed to circulating blood. This provides a simple means of altering the expansion kinetics of the foam to satisfy the unique specifications required by clinicians for different device indications.
Since the activation of SMPs is entropy-driven and body temperature is lower than the Tg of each foam formulation, the polymers must experience plasticization in the blood or saline injection inside the delivery catheter in order to depress the Tg sufficiently to initiate expansion. Although the transition temperature of these foams are significantly greater than 37°C, the Tg of the foams is depressed to approximately 10°C when exposed to 100% humidity, as shown previously (Yu et al., 2011). This transition temperature depression is what allows the foams to expand in the 37°C aqueous environment within the body. However, one potential complication of this actuation method is premature expansion of the foam within the delivery catheter and the inability to successfully deliver the implant. The expansion studies demonstrated the ability to tune the working time of the proposed device, defined as the point at which the expanded diameter of the foam is four times the inner diameter of the delivery catheter. By altering the ratio of HPED to TEA during foam fabrication and the foam pore size, devices can be fabricated with working times varying from one to five minutes, which is within the working time confines of the first hydrogel-containing coils used in clinical embolization procedures (Kallmes and Fujiwara, 2002).
Konig et al. found the average burst pressure of human saphenous veins to be approximately 1,575mmHg (Konig et al., 2009). Based on this burst pressure and a PED 8mm in diameter and 2cm long device, the radial force of the foams must not exceed 107N to prevent vessel rupture in the venous system. This maximum radial force assumes a uniform distribution of radial force exerted along the length and circumference of the device. Based on this information, radial force tests demonstrated that the SMP foams exert a radial force on the vessel wall that is drastically smaller than would be required for vessel rupture. This is also considering that the foams are oversized by 50% to the inner diameter of the vessel, which is the common sizing practice when selecting an appropriately sized vascular plug (Abdel-Aal et al., 2014; Ozyurtlu et al., 2015). This test demonstrated that the risk of rupturing the target vessel with this device as a result of foam expansion is extremely low, regardless of which foam formulation is used. The more likely device component to cause vessel perforation or rupture is the coil anchor. Although the radial force of the coil exceeds that of foam, it exerts nearly an order of magnitude less force than commercially available vascular plugs used for peripheral occlusion, as well as less than 50% of the pressure required to rupture saphenous vein grafts and approximately 50% less pressure than FDA-approved embolic plugs currently on the market. The mechanical forces exerted by the PED implicate that the risk of vessel rupture as a result of the coil anchor is low.
Device migration studies demonstrated that the PED device is at least as stable as Cook Nester® Coils. Although migration studies were performed on AVP and AVP II devices, the data is not shown in figure 6 as a maximum flow rate could not be determined due to limitations in the maximum flow rate attainable with the peristaltic pump. Although the PED device provided equal or superior resistance to undesired thromboembolism, device stiffness testing demonstrated that the PED is significantly less stiff than current embolic plugs used on the market, such as the AVP II. The reduction in stiffness and use of a coil anchor for stability rather than an expandable nitinol mesh, allows the PED to be delivered to small, tortuous vessels that may not be accessible to other embolic plugs due to the risk of catheter deflection and excessive force required to advance the device.
Although the authors hypothesized the SMP foams would provide sufficient echogenicity to enable delivery using ultrasound guidance, this study represents the first instance of verifying this hypothesis. The ability to deliver these devices using ultrasound guidance also creates additional possibilities to design devices consisting entirely of SMP foam with no metallic components that can still be delivered and visualized using endovascular techniques. Although dependent on the depth of the treatment vessel within the body, ultrasound imaging provides a means of delivering these devices without subjecting the patient to any radiation or potential side effects of the contrast injections required during fluoroscopy.
In vitro blood perfusion studies and the subsequent histological analysis of SMP foam devices revealed that blood penetrated throughout the device. No foam sections appeared to be devoid of thrombus deposition, and a dense fibrin mesh was clearly visible at proximal, middle, and distal locations of the device at the moment in which complete vessel occlusion occurred. Complete occlusion was witnessed after 270 seconds of blood perfusion through the device. The pressure setting used in this experiment (450mmHg) served as a rigorous test of in vitro clotting of the device as flow would likely be diverted, thereby creating clinical occlusion, from the treatment vessel at much lower pressures in vivo. Considering the absence of tissue factor VII and any influence from the extrinsic clotting cascade on thrombus formation, complete occlusion in less than five minutes is a significant achievement; especially considering certain FDA-approved peripheral embolization devices may require more than 5 minutes to achieve vessel occlusion (Abdel Aal et al., 2009; Ferro et al., 2010). However, this clotting time may have been affected by clotting activation during blood collection, as well as activation of the clotting cascade from contact with the tubing and storage container in the flow system. In certain clinical settings, the time to occlusion would also likely be increased due to heparin administration.
Although in vitro studies using animal blood cannot be directly correlated to clinical clotting times in humans, previous in vivo studies using the same SMP foam formulation as the devices investigated here demonstrated complete vessel occlusion in less than 90 seconds in a porcine model (Rodriguez et al., 2014b). This finding was similar to the clotting times reported in porcine studies investigating the clotting efficacy of the AVP II (Mordasini et al., 2010); indicating the SMP foam device under investigation would likely result in analogous occlusion times to the AVP II device.
5. Conclusions
This research verified the mechanical properties of the shape memory polymer PED device are safe and unlikely to cause vessel perforation or rupture. At the same time, these studies demonstrated that the likelihood of device migration and undesired thromboembolism to be minimal. The PED accomplished this while also demonstrating a significant reduction in overall device stiffness compared to commercially available vascular plugs, which allows the PED to be delivered to tortuous vessels that may not be accessible using conventional embolic devices. The results of this work also verified the efficacy of the PED in causing complete vessel occlusion and encouraging rapid thrombus formation, as well as ease of visualization of the PED using ultrasound. However, the safety and efficacy of this device must also be verified through in vivo studies and extensive biocompatibility testing before it can be recommended for clinical use.
Acknowledgments
This work was supported in part by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number R43EB022016. The content within this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Financial Conflicts of Interest
Shape Memory Therapeutics, Inc. (SMT) holds the commercial license for clinical vascular embolization application of the technology described here. The authors wish to disclose that Dr. Duncan Maitland is a founder, board member, and shareholder of SMT, and Dr. Fred Clubb and Dr. Alan Glowczwski are shareholders in SMT. In addition, Mr. Todd Landsman and Dr. Sayyeda Marziya Hasan are employed by and hold stock options at SMT.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Aal AK, Elsabbagh A, Soliman H, Hamed M, Underwood E, Saddekni S. Percutaneous Embolization of a Postnephrectomy Arteriovenous Fistula With Intervening Pseudoaneurysm Using the Amplatzer Vascular Plug 2. Vasc Endovasc Surg. 2014;48:516–521. doi: 10.1177/1538574414561230. [DOI] [PubMed] [Google Scholar]
- Abdel Aal AK, Hamed MF, Biosca RF, Saddekni S, Raghuram K. Occlusion time for Amplatzer vascular plug in the management of pulmonary arteriovenous malformations. AJR American journal of roentgenology. 2009;192:793–799. doi: 10.2214/AJR.08.1534. [DOI] [PubMed] [Google Scholar]
- Adcock DM, Kressin DC, Marlar RA. Effect of 3.2% vs 3.8% sodium citrate concentration on routine coagulation testing. American journal of clinical pathology. 1997;107:105–110. doi: 10.1093/ajcp/107.1.105. [DOI] [PubMed] [Google Scholar]
- Alguire PC, Mathes BM. Chronic venous insufficiency and venous ulceration. Journal of general internal medicine. 1997;12:374–383. doi: 10.1046/j.1525-1497.1997.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavinzski G, Talazoglu V, Killer M, Richling B, Gruber A, Gross CE, Plenk H., Jr Gross and microscopic histopathological findings in aneurysms of the human brain treated with Guglielmi detachable coils. Journal of neurosurgery. 1999;91:284–293. doi: 10.3171/jns.1999.91.2.0284. [DOI] [PubMed] [Google Scholar]
- Beebe-Dimmer JL, Pfeifer JR, Engle JS, Schottenfeld D. The epidemiology of chronic venous insufficiency and varicose veins. Annals of epidemiology. 2005;15:175–184. doi: 10.1016/j.annepidem.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Caivano D, Birettoni F, Fruganti A, Rishniw M, Knafelz P, Moise NS, Porciello F. Transthoracic echocardiographically-guided interventional cardiac procedures in the dog. J Vet Cardiol. 2012;14:431–444. doi: 10.1016/j.jvc.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Dudeck O, Bulla K, Wieners G, Ruehl R, Ulrich G, Amthauer H, Ricke J, Pech M. Embolization of the Gastroduodenal Artery Before Selective Internal Radiotherapy: A Prospectively Randomized Trial Comparing Standard Pushable Coils with Fibered Interlock Detachable Coils. Cardiovascular and interventional radiology. 2011;34:74–80. doi: 10.1007/s00270-010-9845-7. [DOI] [PubMed] [Google Scholar]
- Emmert MY, Venbrux A, Rudakov L, Cesarovic N, Radvany MG, Gailloud P, Falk V, Plass A. The endovascular occlusion system for safe and immediate peripheral vessel occlusion during vascular interventions. Interactive cardiovascular and thoracic surgery. 2013;17:882–885. doi: 10.1093/icvts/ivt318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriquez J, Javadi S, Murthy R, Ensor J, Jr, Mahvash A, Abdelsalam ME, Madoff DC, Wallace MJ, Avritscher R. Gastroduodenal artery recanalization after transcatheter fibered coil embolization for prevention of hepaticoenteric flow: incidence and predisposing technical factors in 142 patients. Acta radiologica. 2013;54:790–794. doi: 10.1177/0284185113481696. [DOI] [PubMed] [Google Scholar]
- Ferro C, Rossi UG, Bovio G, Petrocelli F, Seitun S. The Amplatzer vascular plug 4: preliminary experience. Cardiovascular and interventional radiology. 2010;33:844–848. doi: 10.1007/s00270-009-9749-6. [DOI] [PubMed] [Google Scholar]
- Friedrich P, Reininger AJ. Occlusive Thrombus Formation on Indwelling Catheters - in-Vitro Investigation and Computational Analysis. Thromb Haemostasis. 1995;73:66–72. [PubMed] [Google Scholar]
- Gao Z, Guo W, Liu X, Huang W, Zhang H, Tan N, Hau WK, Zhang YT, Liu H. Automated detection framework of the calcified plaque with acoustic shadowing in IVUS images. PloS one. 2014;9:e109997. doi: 10.1371/journal.pone.0109997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan SM, Raymond JE, Wilson TS, Keller BK, Maitland DJ. Effects of Isophorone Diisocyanate on the Thermal and Mechanical Properties of Shape-Memory Polyurethane Foams. Macromol Chem Phys. 2014;215:2420–2429. doi: 10.1002/macp.201400407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang W, Singhal P, Miller MW, Maitland DJ. In Vitro Study of Transcatheter Delivery of a Shape Memory Polymer Foam Embolic Device for Treating Cerebral Aneurysms. J Med Devices. 2013:7. [Google Scholar]
- Kallmes DF, Fujiwara NH. New expandable hydrogel-platinum coil hybrid device for aneurysm embolization. AJNR American journal of neuroradiology. 2002;23:1580–1588. [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Lee RG, Oster H, Coleman D, Andrade JD, Lentz DJ, Olsen D. Platelet adhesion to polymer surfaces. Transactions - American Society for Artificial Internal Organs. 1974;20 B:449–455. [PubMed] [Google Scholar]
- Kipshidze N, Sadzaglishvili K, Panarella M, Rivera EA, Virmani R, Leon MB. Evaluation of a novel endoluminal vascular occlusion device in a porcine model: early and late follow-up. Journal of endovascular therapy : an official journal of the International Society of Endovascular Specialists. 2005;12:486–494. doi: 10.1583/05-1543.1. [DOI] [PubMed] [Google Scholar]
- Konig G, McAllister TN, Dusserre N, Garrido SA, Iyican C, Marini A, Fiorillo A, Avila H, Wystrychowski W, Zagalski K, Maruszewski M, Jones AL, Cierpka L, de la Fuente LM, L'Heureux N. Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials. 2009;30:1542–1550. doi: 10.1016/j.biomaterials.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laborda A, Medrano J, de Blas I, Urtiaga I, Carnevale FC, de Gregorio MA. Endovascular treatment of pelvic congestion syndrome: visual analog scale (VAS) long-term follow-up clinical evaluation in 202 patients. Cardiovascular and interventional radiology. 2013;36:1006–1014. doi: 10.1007/s00270-013-0586-2. [DOI] [PubMed] [Google Scholar]
- Masson P, Brannigan RE. The varicocele. The Urologic clinics of North America. 2014;41:129–144. doi: 10.1016/j.ucl.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Molyneux AJ, Ellison DW, Morris J, Byrne JV. Histological findings in giant aneurysms treated with Guglielmi detachable coils. Report of two cases with autopsy correlation. Journal of neurosurgery. 1995;83:129–132. doi: 10.3171/jns.1995.83.1.0129. [DOI] [PubMed] [Google Scholar]
- Mordasini P, Gralla J, Brekenfeld C, Schroth G, Hoppe H. Preliminary Experimental Evaluation of the Immediate Angiographic Occlusion Time with Use of the AMPLATZER Vascular Plug II for Carotid Artery Occlusion. J Vasc Interv Radiol. 2010;21:1873–1877. doi: 10.1016/j.jvir.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Neumann T, Nicholson BS, Sanders JE. Tissue engineering of perfused microvessels. Microvascular research. 2003;66:59–67. doi: 10.1016/s0026-2862(03)00040-2. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Biadillah Y, Mongrain R, Brunette J, Tardif JC, Bertrand OF. A method for matching the refractive index and kinematic viscosity of a blood analog for flow visualization in hydraulic cardiovascular models. Journal of biomechanical engineering. 2004;126:529–535. doi: 10.1115/1.1785812. [DOI] [PubMed] [Google Scholar]
- Ortega JM, Hartman J, Rodriguez JN, Maitland DJ. Virtual Treatment of Basilar Aneurysms Using Shape Memory Polymer Foam. Annals of biomedical engineering. 2013;41:725–743. doi: 10.1007/s10439-012-0719-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozyurtlu F, Acet H, Ozpelit ME, Pekel N. Optimal treatment of unligated side branch of internal mammary artery: Coil, amplatzer vascular plug or graft stent? A case report and literature review. Turk Kardiyoloji Dernegi arsivi : Turk Kardiyoloji Derneginin yayin organidir. 2015;43:376–380. doi: 10.5543/tkda.2015.43826. [DOI] [PubMed] [Google Scholar]
- Porciello F, Caivano D, Giorgi ME, Knafelz P, Rishniw M, Moise NS, Bufalari A, Fruganti A, Birettoni F. Transesophageal Echocardiography as the Sole Guidance for Occlusion of Patent Ductus Arteriosus using a Canine Ductal Occluder in Dogs. J Vet Intern Med. 2014;28:1504–1512. doi: 10.1111/jvim.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JH, Lassen ED. Activated coagulation times of normal cows. Veterinary clinical pathology / American Society for Veterinary Clinical Pathology. 1979;8:31–33. doi: 10.1111/j.1939-165x.1979.tb00879.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez JN, Yu YJ, Miller MW, Wilson TS, Hartman J, Clubb FJ, Gentry B, Maitland DJ. Opacification of shape memory polymer foam designed for treatment of intracranial aneurysms. Annals of biomedical engineering. 2012;40:883–897. doi: 10.1007/s10439-011-0468-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JN, Clubb FJ, Wilson TS, Miller MW, Fossum TW, Hartman J, Tuzun E, Singhal P, Maitland DJ. In vivo response to an implanted shape memory polyurethane foam in a porcine aneurysm model. Journal of biomedical materials research Part A. 2014a;102:1231–1242. doi: 10.1002/jbm.a.34782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JN, Miller MW, Boyle A, Horn J, Yang CK, Wilson TS, Ortega JM, Small W, Nash L, Skoog H, Maitland DJ. Reticulation of low density shape memory polymer foam with an in vivo demonstration of vascular occlusion. Journal of the mechanical behavior of biomedical materials. 2014b;40:102–114. doi: 10.1016/j.jmbbm.2014.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal P, Rodriguez JN, Small W, Eagleston S, Van de Water J, Maitland DJ, Wilson TS. Ultra Low Density and Highly Crosslinked Biocompatible Shape Memory Polyurethane Foams. Journal of polymer science Part B, Polymer physics. 2012;50:724–737. doi: 10.1002/polb.23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhu X, Tam MD, Pierce G, McLennan G, Sands MJ. Advantages and limitations of the Amplatzer vascular plug: a comparison study with conventional coil technique in splenic artery embolization. Journal of Vascular and Interventional Radiology. 2010;21:S78. doi: 10.1007/s00270-010-9957-0. [DOI] [PubMed] [Google Scholar]
- Wilson TS, Bearinger JP, Herberg JL, Marion JE, Wright WJ, Evans CL, Maitland DJ. Shape memory polymers based on uniform aliphatic urethane networks. J Appl Polym Sci. 2007;106:540–551. [Google Scholar]
- Yu YJ, Hearon K, Wilson TS, Maitland DJ. The effect of moisture absorption on the physical properties of polyurethane shape memory polymer foams. Smart materials & structures. 2011:20. doi: 10.1088/0964-1726/20/8/085010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni P, Menegatti E, Galeotti R, Malagoni AM, Tacconi G, Dall'Ara S, Bartolomei I, Salvi F. The value of cerebral Doppler venous haemodynamics in the assessment of multiple sclerosis. Journal of the neurological sciences. 2009;282:21–27. doi: 10.1016/j.jns.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Zweens J, Schiphof P. Permanent catheterization of aorta and pulmonary artery in the dog. Pflugers Archiv : European journal of physiology. 1976;362:201–202. doi: 10.1007/BF00583648. [DOI] [PubMed] [Google Scholar]