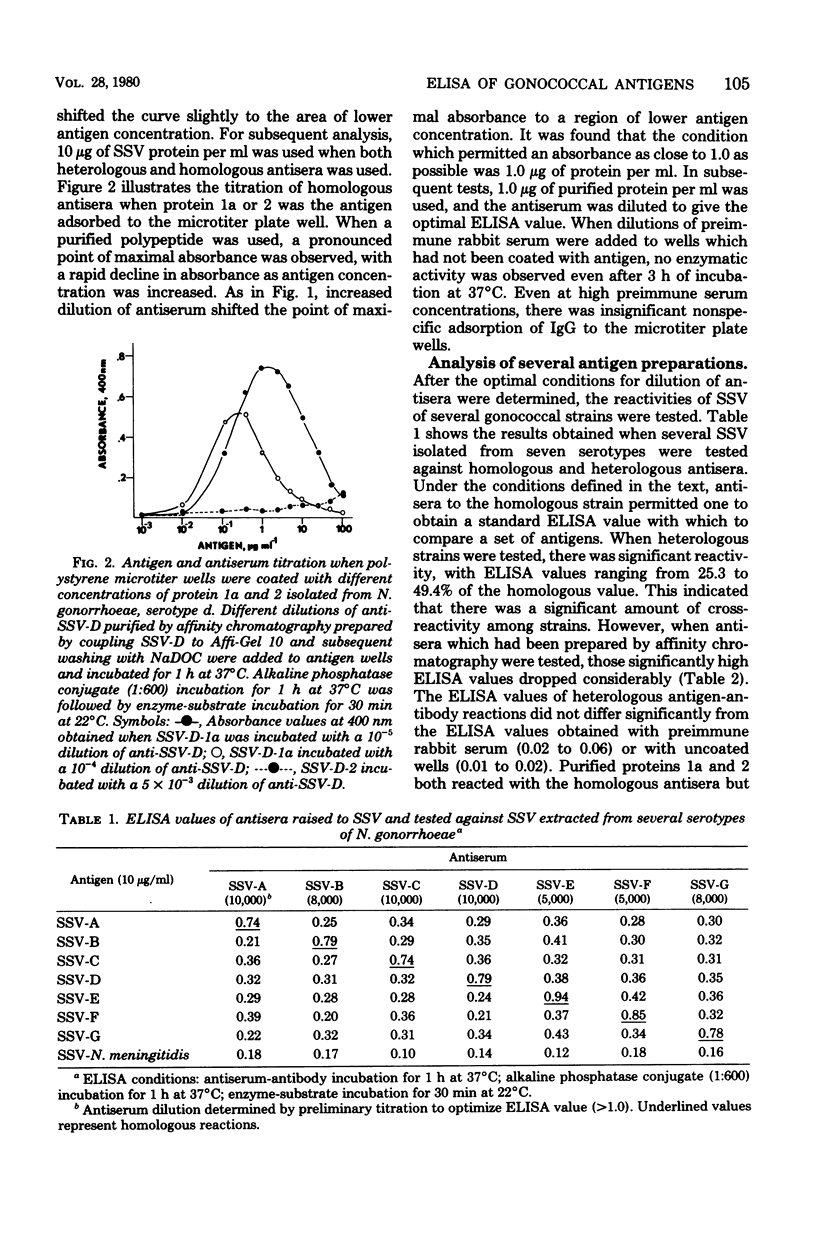
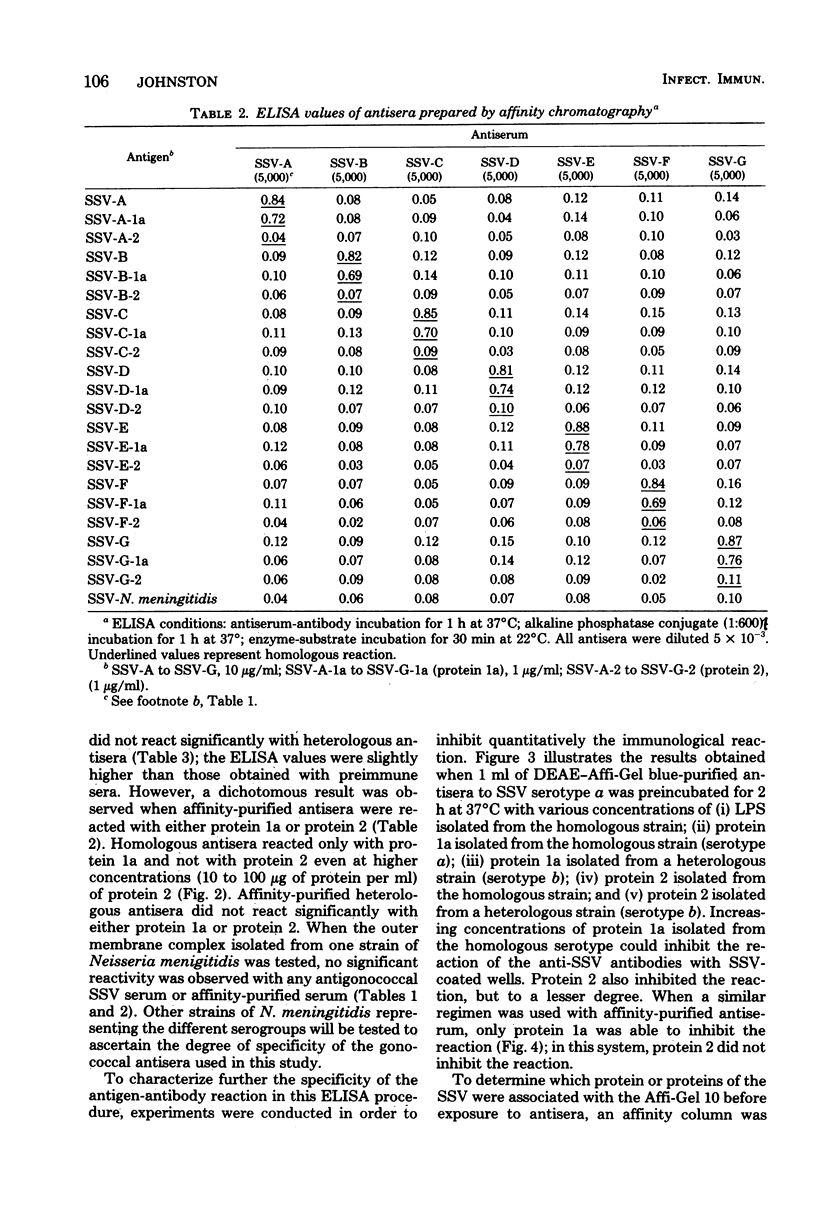
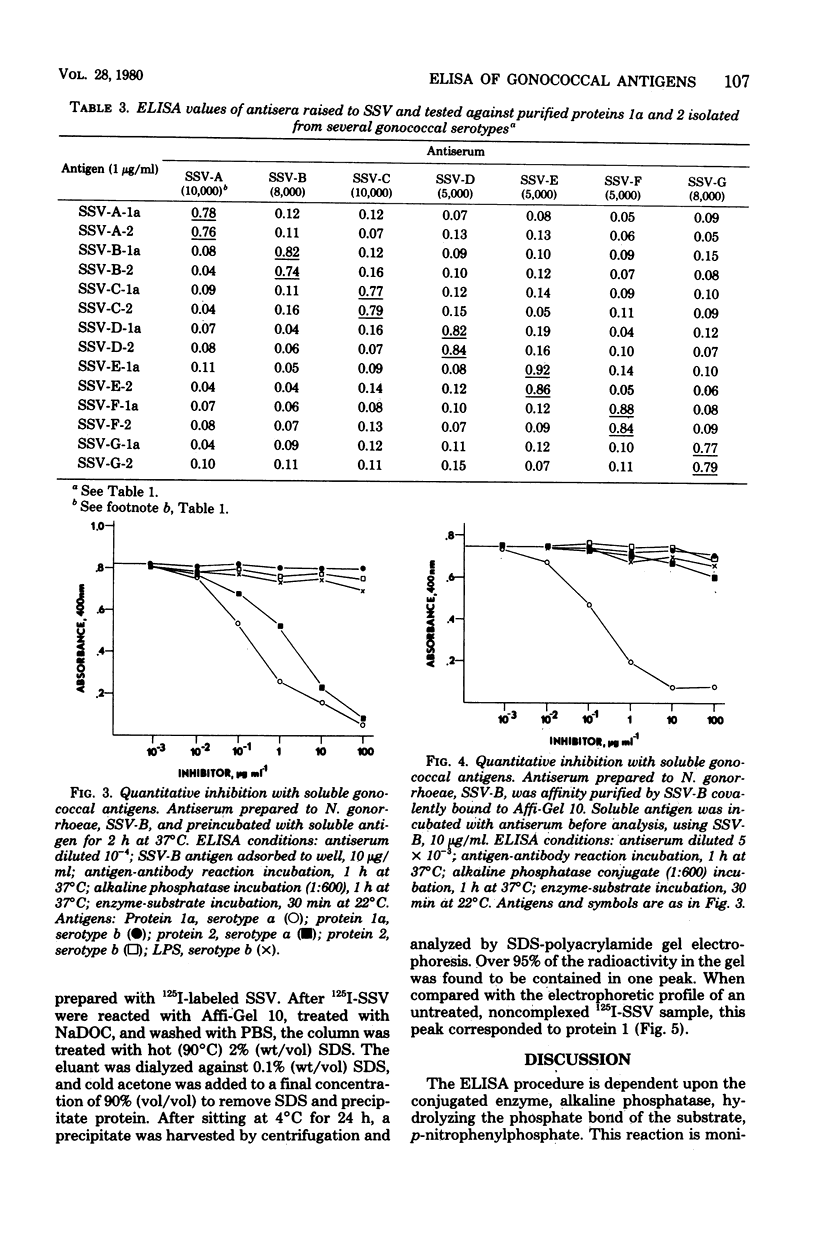
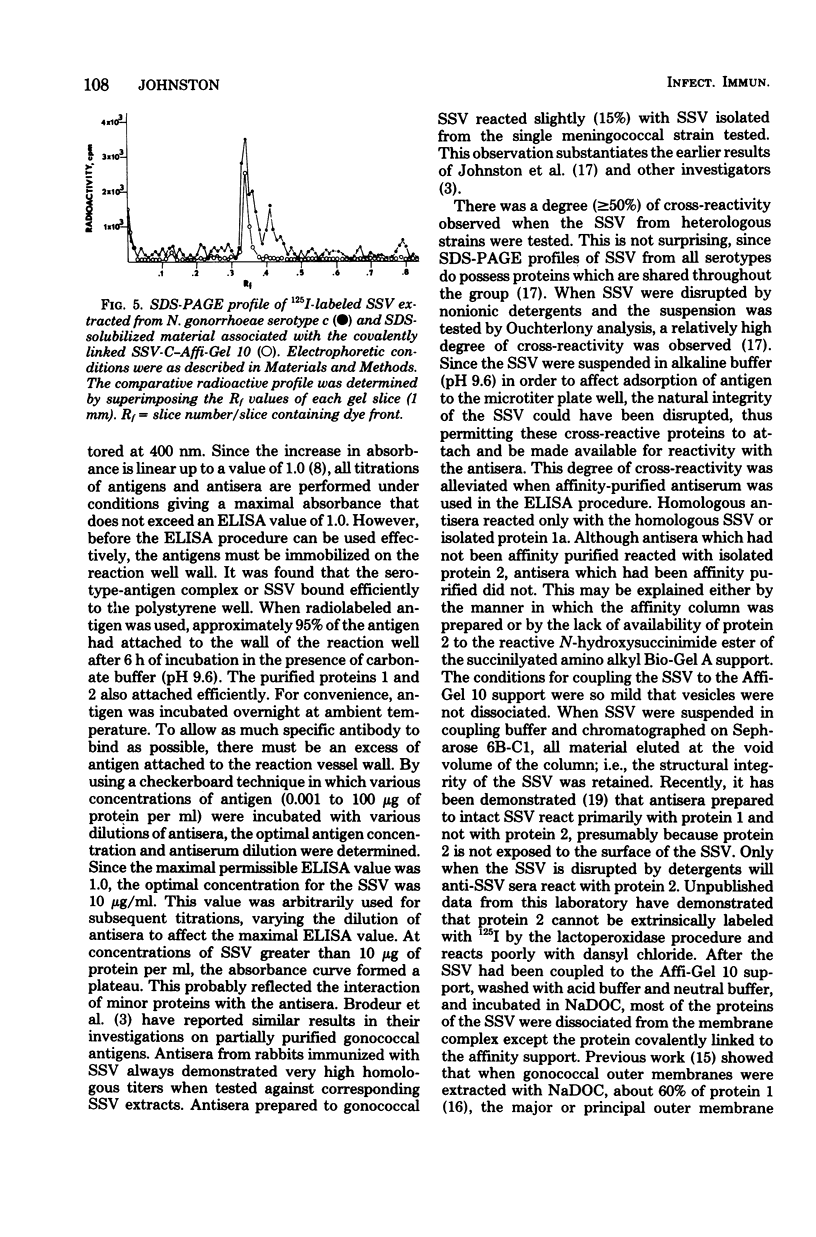
Abstract
An indirect enzyme-linked immunoassay (ELISA) has been developed to analyze the antigenic profile of the outer membrane serotype complex of Neisseria gonorrhoeae. Antisera raised in rabbits to serotype-specific vesicles (SSV) reacted primarily with homologous SSV; however, there was significant cross-reactivity (less than 50%) with heterologous SSV. N. meningitidis SSV cross-reacted with all antigonococcal SSV but at a lower degree (less than 20%). Preimmune sera did not cross-react significantly with all antigonoccoccal SSV. The sensitivity of the ELISA was enhanced when the integral SSV proteins 1a and 2 were used as adsorbed antigen. Heterologous anti-SSV cross-reacted slightly, having ELISA values less than 15% of the homologous reaction. Antisera prepared by immunoabsorbent affinity columns were highly specific. Homologous affinity anti-SSV reacted only with proteins 1a and 2. The reaction of immune sera was inhibited by homologous proteins 1a and 2; lipopolysaccharide and proteins 1a and 2 isolated from heterologous serotypes did not inhibit the reaction. The reaction of affinity-purified antisera could be inhibited only by homologous protein 1a. By the use of affinity-purified antisera, a specific and highly sensitive ELISA was developed to analyze the antigenic profile of strains of N. gonorrhoeae.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apicella M. A. Antigenically distinct populations of Neisseria gonorrhoeae: isolation and characterization of the responsible determinants. J Infect Dis. 1974 Dec;130(6):619–625. doi: 10.1093/infdis/130.6.619. [DOI] [PubMed] [Google Scholar]
- Apicella M. A. Serogrouping of Neisseria gonorrhoeae: identification of four immunologically distinct acidic polysaccharides. J Infect Dis. 1976 Oct;134(4):377–383. doi: 10.1093/infdis/134.4.377. [DOI] [PubMed] [Google Scholar]
- Brodeur B. R., Ashton F. E., Diena B. B. Enzyme-linked immunosorbent assays for the detection of Neisseria gonorrhoeae specific antibodies. Can J Microbiol. 1978 Nov;24(11):1300–1305. doi: 10.1139/m78-211. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M. Antigenic heterogeneity of gonococcal pili. J Exp Med. 1975 Jun 1;141(6):1470–1475. doi: 10.1084/jem.141.6.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson H. E., Lindberg A. A., Hammarström S., Ljunggren A. Quantitation of Salmonella O-antibodies in human sera by enzyme-linked immunosorbent assay (ELISA). Int Arch Allergy Appl Immunol. 1975;48(4):485–494. doi: 10.1159/000231336. [DOI] [PubMed] [Google Scholar]
- Carlsson H. E., Lindberg A. A., Hammarström S. Titration of antibodies to salmonella O antigens by enzyme-linked immunosorbent assay. Infect Immun. 1972 Nov;6(5):703–708. doi: 10.1128/iai.6.5.703-708.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten W., Henning U. Cell envelope and shape of Escherichia coli K12. Isolation and preliminary characterization of the major ghost-membrane proteins. Eur J Biochem. 1974 Sep 1;47(2):343–352. doi: 10.1111/j.1432-1033.1974.tb03699.x. [DOI] [PubMed] [Google Scholar]
- Glynn A. A., Ison C. Serological diagnosis of gonorrhoea by an enzyme-linked immunosorbent assay (Elisa). Br J Vener Dis. 1978 Apr;54(2):97–102. doi: 10.1136/sti.54.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckels J. E. The surface of Neisseria gonorrhoeae: isolation of the major components of the outer membrane. J Gen Microbiol. 1977 Apr;99(2):333–341. doi: 10.1099/00221287-99-2-333. [DOI] [PubMed] [Google Scholar]
- Ichihara S., Mizushima S. Characterization of major outer membrane proteins O-8 and O-9 of Escherichia coli K-12. Evidence that structural genes for the two proteins are different. J Biochem. 1978 Apr;83(4):1095–1100. doi: 10.1093/oxfordjournals.jbchem.a131998. [DOI] [PubMed] [Google Scholar]
- Jodal U., Ahlstedt S., Carlsson B., Hanson L. A., Lindberg U., Sohl A. Local antibodies in childhood urinary tract infection: a preliminary study. Int Arch Allergy Appl Immunol. 1974;47(4):537–546. doi: 10.1159/000231248. [DOI] [PubMed] [Google Scholar]
- Johnston K. H., Gotschlich E. C. Isolation and characterization of the outer membrane of Neisseria gonorrhoeae. J Bacteriol. 1974 Jul;119(1):250–257. doi: 10.1128/jb.119.1.250-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Mizushima S. Effects of heating in dodecyl sulfate solution on the conformation and electrophoretic mobility of isolated major outer membrane proteins from Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1411–1422. doi: 10.1093/oxfordjournals.jbchem.a131414. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry M. B., Daoust V. The lipopolysaccharides of Neisseria gonorrhoeae colony types 1 and 4. Can J Biochem. 1975 May;53(5):623–629. doi: 10.1139/o75-084. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Van Alphen L., Havekes L., Lugtenberg B. Major outer membrane protein d of Escherichia coli K12. Purification and in vitro activity of bacteriophages k3 and f-pilus mediated conjugation. FEBS Lett. 1977 Mar 15;75(1):285–290. doi: 10.1016/0014-5793(77)80104-x. [DOI] [PubMed] [Google Scholar]
- Van Weemen B. K., Schuurs A. H.W.M. Immunoassay using antigen-enzyme conjugates. FEBS Lett. 1971 Jun 24;15(3):232–236. doi: 10.1016/0014-5793(71)80319-8. [DOI] [PubMed] [Google Scholar]
- Veldkamp J., Visser A. M. Application of the enzyme-linked immunosorbent assay (ELISA) in the serodiagnosis of syphilis. Br J Vener Dis. 1975 Aug;51(4):227–231. doi: 10.1136/sti.51.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voller A., Bidwell D. E., Bartlett A. Enzyme immunoassays in diagnostic medicine. Theory and practice. Bull World Health Organ. 1976;53(1):55–65. [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



