Abstract
Scope
Nutrigenomics is a rapidly expanding field that elucidates the link between diet-genome interactions. Recent evidence demonstrates that regulation of the epigenome, and in particular inhibition of HDACs, impact pathogenetic mechanisms involved in chronic disease. Few studies, to date, have screened libraries of bioactive compounds that act as epigenetic modifiers. This study screened a library of 131 natural compounds to determine bioactive compounds that inhibit Zn-dependent HDAC activity.
Methods and results
Using class-specific HDAC substrates, we screened 131 natural compounds for HDAC activity in bovine cardiac tissue. From this screen, we identified 18 bioactive compound HDAC inhibitors. Using our class-specific HDAC substrates, we next screened these 18 bioactive compounds against recombinant HDAC proteins. Consistent with inhibition of HDAC activity, these compounds were capable of inhibiting activity of individual HDAC isoforms. Lastly, we report that treatment of H9c2 cardiac myoblasts with bioactive HDAC inhibitors was sufficient to increase lysine acetylation as assessed via immunoblot.
Conclusion
This study provided the first step in identifying multiple bioactive compound HDAC inhibitors. Taken together, this report sets the stage for future exploration of these bioactive compounds as epigenetic regulators to potentially ameliorate chronic disease.
Keywords: acetylation, bioactive compounds, dietary HDAC inhibitors, HDACs, histone deacetylases
1 Introduction
Nutritional genomics, or nutrigenomics, is a rapidly emerging field that explores the link between diet-gene interactions [1]. Epigenetics is defined by heritable changes in gene expression that does not involve alterations in DNA sequence. Dynamic changes in the nucleosomal landscape, in part, via post-translational modification (PTM) of histone tails plays a central role in regulating DNA accessibility and thus gene transcription [2]. Diet can greatly impact the epigenetic landscape by regulating PTMs; therefore, epigenetics has emerged as a topic of interest in the field of nutrigenomics. Accumulating evidence has shown that dietary bioactive food components affect epigenetic marks and that these alterations impact pathogenetic mechanisms involved in heart disease [1, 3, 4].
Histone acetylation provides a critical mechanism for epigenetic control of gene expression [5]. Histone acetyltransferases (HAT) and histone deacetylases (HDACs) govern the addition or removal of acetyl groups to lysine residues. Historically, HDACs have been studied in the context of chromatin, where they deacetylate histones and alter electrostatic properties of chromatin in a manner that favors gene repression [5]. Eighteen HDACs are encoded by distinct genes and grouped into four classes: class I (HDACs 1, 2, 3 and 8), IIa (HDACs 4, 5, 7 and 9), IIb (HDACs 6 and 10) and IV (HDAC 11) HDACs require zinc for catalysis, whereas class III HDACs (SirT1-7), also known as sirtuins, require nicotinamide adenine dinucleotide (NAD+) for catalytic activity [6] (Fig. 1).
Figure 1. Schematic representation of histone deacetylases (HDACs).
Zinc-dependent HDACs fall into three categories (Class I, II, IV; represented in blue), where class II are subdivided into IIa and IIb. Class III HDACs (Sirtuins; represented in green) are NAD+-dependent.
HDAC inhibitors have been shown to block cardiac hypertrophy, fibrosis and inflammation in animal models of myocardial infarction, transverse aortic constriction and hypertension [7–9]. More specifically, these beneficial effects on cardiac structure and function have been attributed to inhibition of Zn-dependent HDACs [10, 11]. Recent reports highlight diet and dietary bioactive compounds as regulators of HDAC activity and cardiac health [1, 3]. Most of these studies have typically focused on a single bioactive food compound in the regulation of disease. These studies have provided crucial insight into the importance for diet in regulation of epigenetic marks critical for the control of gene expression. Given the wide spread implications for diet in the regulation of the epigenome, we screened a library of bioactive compounds against Zn-dependent HDAC activity. In this report, we screened a natural compound library, containing 131 bioactive compounds using an enzymatic HDAC assay previously established [12] that enables quantitative examination of class I, IIa, and IIb Zn-dependent HDAC activity in tissue or cell homogenates. We selected a pre-designed natural product compound library from SelleckChem; this limited selection bias yet allowed for the screening of unidentified as well as previously identified HDAC inhibitors to further substantiate our results. Using this assay, we report that 18 of the 131 bioactive compounds screened dose-dependently inhibited HDAC activity and consistent with these findings increased lysine acetylation. This report marks a first step in identifying multiple dietary bioactive compounds that function as HDAC inhibitors to potentially ameliorate chronic diseases and in particular cardiac dysfunction.
2 Materials and Methods
2.1. HDAC activity assays
Bovine heart tissue was procured from the University of Nevada, Wolf Pack Meats, with care and handling of all animals conducted and approved by the University of Nevada, Reno, Institutional Animal Care and Use Committee (#00676). HDAC activity assays were completed as previously described [12]. Each substrate is based on ε-N-acylated lysine, derivatized on the carboxyl group with amino methylcoumarin (AMC) [13]. Heart tissue lysate was prepared in PBS (pH 7.4) containing 0.5% Triton X-100, 300 mM NaCl and protease/phosphatase inhibitor cocktail (Thermo Fisher) using a Bullet Blender homogenizer (Next Advance). Tissue was clarified by centrifugation prior to determination of protein concentration using a BCA Protein Assay Kit (Pierce). Tissue (30 µg protein/well) was then diluted into PBS buffer for a total volume of 100 µl/well in a 96-well plate. For concentration-response determination, tissue was dosed with increasing semi-log scale concentrations of dietary compounds (SelleckChem Natural Product Library; L1400) for 2 hrs. Class-specific HDAC substrates were added (5 µl of 1 mM DMSO stock solutions), and plates returned to the 37 °C incubator for 2 hrs. Developer/stop solution was added (50 µl per well of PBS with 1.5% Triton X-100, 3 µM TSA, and 0.75 mg/ml trypsin) and plates incubated at 37 °C for 20 min. Subsequent to deacylation, trypsin is used to release AMC, resulting in increased fluorescence. AMC fluorescence was measured via BioTek Synergy plate reader, with excitation and emission filters of 360 nm and 460 nm, respectively. Background signals from buffer blanks were subtracted, and GraphPad Prism used to calculate IC50 values for each compound.
To monitor recombinant HDAC activity, 50 ng of recombinant HDAC1 (Reaction Biology; KDA-21-365), HDAC2 (Reaction Biology; KDA-21-277), HDAC3 (Reaction Biology; KDA-22-278), HDAC4 (Reaction Biology; KDA-21-279), HDAC5 (Reaction Biology; KDA-21-280), HDAC6 (Biovision; 7534-10), HDAC7 (Reaction Biology; KDA-21-281), HDAC9 (Sigma; SRP5268-10UG), 200 ng of recombinant HDAC8 (Reaction Biology; KDA-21-285), 500 ng recombinant HDAC10 (abcam; ab80283) or PBS blank was added to 96-well plates. Recombinant proteins were incubated at 37 °C for 2 hrs, with identified dietary HDAC inhibitors (50 µM) screened above, prior to addition of the cell-permeable fluorescent HDAC substrate. After two hours, stop buffer was added and incubated for 20 min at 37 °C. Deacetylated substrates are susceptible to trypsin cleavage, resulting in increased fluorescence. Deacetylase activity was measured via BioTek Synergy plate reader as described above and data quantified using GraphPad Prism.
2.3. Cells and cell culture
H9c2 cells (ATCC; CRL-1446) were cultured in DMEM supplemented with 10% fetal bovine serum (FBS). At 90% confluence, H9c2 cells were treated with emodin (2 hrs; SelleckChem; S2295), gossypol (2 hrs; SelleckChem; S2303), luteolin (2 hrs; SelleckChem; S2320), quercetin dihydrate (2 hrs; SelleckChem; S2347), TSA (2 hrs; Sigma T8552), or DMSO.
2.4. Immunoblotting
Cell monolayers were washed with ice-cold PBS and scraped into ice-cold lysis buffer containing PBS (pH 7.4), 0.5% Triton X-100, 300 mM NaCl and protease/phosphatase inhibitor cocktail (Thermo Fisher). Cultured cells were sonicated prior to clarification by centrifugation. Protein concentrations were determined using a BCA Protein Assay Kit (Pierce). Proteins were resolved by SDS-polyacrylamide gel electrophoresis (PAGE), transferred to nitrocellulose membranes (BioRad) and probed with antibodies for acetyl-lysine (Cell Signaling Technology; 9441), Acetyl-H3K9/14 (Cell Signaling Technology; 9677), Total H3 (Cell Signaling Technology; 4499). HRP-conjugated secondary antibodies (Southern Biotech) were used at a concentration of 1:2000. Proteins were detected using a SuperSignal West Pico chemiluminescence system (Thermo Scientific) and a ChemiDoc XRS+ imager (BioRad). Densitometry was performed using Image J software and statistical analyses conducted via GraphPad Prism software.
2.5. Statistical analyses
Statistical analysis was completed by ANOVA followed by post-hoc testing (Tukey's test was used for post-hoc analysis unless otherwise noted) using GraphPad Prism software. Statistical significance (defined as p<0.05) is reported as applicable.
3 Results
3.1. Catalytic activity of class-specific HDAC substrates
Histone deacetylase activity can be determined via fluorescent substrates that contain acetyl-lysine moieties [14]. These substrates become susceptible to trypsin cleavage upon HDAC deacetylation and thus releases the 7-amino-4-methlcoumarin (AMC) fluorophore [15]. Moreover, these substrates can be used to measure recombinant or endogenous (i.e. tissue/cell lysate) HDAC activity. Class specific substrates for class I, IIa and IIb (Fig. 1) HDACs have previously been described [12, 13, 16]. To assess selectivity of these substrates in our model, we treated heart tissue (30 µg) with well-established class-specific and pan-HDAC inhibitors; structures illustrated for TSA (pan-HDAC inhibitor), MGCD0103 (class I HDAC inhibitor), DPAH (Class IIa HDAC inhibitor) and Tubastatin A (Class IIb/HDAC6 inhibitor) (Fig.2). As shown (Fig. 2A), treatment with the pan-HDAC inhibitor TSA significantly decreased catalytic HDAC activity of all substrates. Further analysis demonstrated that MGCD only inhibited class I HDAC activity (Fig. 2B), while DPAH only inhibited class IIa HDAC activity (Fig. 2C) and TubA only inhibited class IIb HDAC activity (Fig. 2D); confirming the class selectivity of these substrates.
Figure 2. HDAC fluorogenic substrate specificity in bovine cardiac tissue.
Bovine cardiac tissue was treated for 2 hours with pan- or class-specific HDAC inhibitors prior to incubation with cell permeable fluorogenic HDAC substrates for 2 hours and developer solution for 20 minutes. A) Trichostatin A (TSA) acted as a pan-HDAC inhibitor, while B) MGCD0103 (MGCD; Class I), C) DPAH (Class IIa) and D) Tubastatin A (TubA; Class IIb) worked as class-specific HDAC inhibitors.
3.2. Select bioactive food compounds alter catalytic HDAC activity in the heart
In these experiments, we postulated that bioactive food compounds act as epigenetic modifiers that can inhibit HDAC activity. Using the class-selective HDAC substrates as described above, we thus screened a natural product library (SelleckChem) containing 131 bioactive compounds against HDAC activity in bovine cardiac tissue. We observed dose-dependent inhibition of 18 bioactive compounds from the library screen (Table 1), whereas a majority of the compounds did not alter HDAC activity even at the highest doses used for these studies (supplemental Table 1). Many of the bioactive compounds act as pan-HDAC inhibitors and thus attenuated class I, IIa, IIb HDAC activity (Table 1). This is reflected in Figure3, where we observed dose-dependent pan-HDAC inhibition of non-flavonoids (Fig. 3A&B), emodin and gossypol, and flavonoids (Fig. 3C&D), luteolin and quercetin dihydrate. We did note, however, substrate specificity for a few of the compounds; where for instance apigenin and palmatine only inhibit class I HDAC activity while hematoxylin targeted class IIb HDACs for inhibition (Table 1). Non-flavonoid and flavonoid compound structures were illustrated in Fig.3 for emodin, gossypol, luteolin and quercetin dihydrate; provided as a representative compound structures for dietary HDAC inhibitors (Fig.3).
Table 1.
Bioactive compounds found to inhibit HDAC activity in bovine cardiac tissue.
| IC50 HDAC Activity [M] | |||
|---|---|---|---|
|
|
|||
| Bioactive Food Compound | Class I HDAC | Class IIa HDAC | Class IIb HDAC |
| Apigenin | 2.7 × 10−5 | - | - |
| Baicalein | 4.5 × 10−5 | 1.0 × 10−4 | - |
| Baicalin | 5.2 × 10−5 | 6.7 × 10−5 | 8.4 × 10−5 |
| Berberine HCl | 2.0 × 10−5 | - | 6.9 × 10−5 |
| Caffeic acid | 8.1 × 10−5 | 1.1 × 10−4 | 1.2 × 10−4 |
| Dihydromyricetin | 3.6 × 10−5 | 9.5 × 10−5 | 2.6 × 10−5 |
| Emodin | 2.2 × 10−5 | 3.2 × 10−5 | 2.9 × 10−5 |
| EGCG | 3.0 × 10−3 | 9.0 × 10−5 | 2.3 × 10−5 |
| Gossypol | 1.1 × 10−5 | 1.3 × 10−5 | 1.0 × 10−5 |
| Hematoxylin | - | - | 8.9 × 10−5 |
| Indirubin | 1.5 × 10−5 | - | 1.5 × 10−5 |
| Kaempferol | 3.9 × 10−5 | 4.8 × 10−5 | 2.9 × 10−5 |
| Luteolin | 1.7 × 10−5 | 1.8 × 10−5 | 1.8 × 10−5 |
| Morin hydrate | 4.5 × 10−5 | 6.8 × 10−5 | - |
| Myricetin | 2.0 × 10−5 | 1.1 × 10−5 | 2.7 × 10−5 |
| Myricitrin | 2.5 × 10−5 | 1.4 × 10−5 | 2.0 × 10−5 |
| Palmatine | 2.7 × 10−5 | - | - |
| Quercetin dihydrate | 3.6 × 10−5 | 3.6 × 10−5 | 3.7 × 10−5 |
No Inhibition (−)
[M] = Molar
Figure 3. Identification of bioactive HDAC inhibitors in bovine cardiac tissue.
Bovine cardiac tissue were treated with increasing concentrations of indicated non-flavonoids (A&B) and flavonoids (C&D) for 2 hours. Bovine cardiac tissue was subsequently incubated with fluorogenic HDAC substrates for 2 hours prior to addition of stop solution for 20 minutes. Fluorescence was assessed via BioTek Synergy plate reader and demonstrated pan-HDAC inhibition of all compounds.
3.3. Bioactive food compounds selectively inhibit recombinant HDAC activity
Data above demonstrated that 18 out of 131 bioactive compounds suppressed HDAC activity, yet it is unclear which HDAC is being targeted for inhibition. Using our HDAC activity assay, we further screened the 18 bioactive compounds at a chosen dose of 50 µM against all recombinant class I and II HDACs. This dose was chosen as it closely reflected a dose at or above the determined IC50 for most of the screened compounds (Table 1); this was to ensure an adequate inhibitor concentration. In keeping with our screening assay above, the bioactive compounds were found to potently inhibit recombinant HDAC activity and pan-HDAC inhibitors targeted most of the class I, IIa, and IIb HDACs for inhibition (Table 2). Similar to Figure 3, pan-HDAC inhibition by non-flavonoids (Fig. 4A&B) and flavonoids (Fig. 4C&D) were adequate in suppressing activity of most of the recombinant HDACs. We further noted that emodin was only one of six compounds capable of inhibiting HDAC6 activity and one of two compounds able to inhibit HDAC7 activity (Fig. 4 & Table 2). Quercetin dihydrate was one of only two compounds to significantly inhibit HDAC4 activity. Lastly, we observed no inhibition by caffeic acid (50 µM) on any of the recombinant HDACs screened. However, caffeic acid was the least potent inhibitor of HDAC activity as determined by dose-response curves, where IC50 values were greater than 80 µM (Table 1).
Table 2.
Recombinant HDAC activity in response to bioactive compounds
| % HDAC activity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Bioactive Food Compound |
Class I HDACs |
Class IIa HDACs |
Class IIb HDACs |
|||||||
|
| ||||||||||
| 1 | 2 | 3 | 8 | 4 | 5 | 7 | 9 | 6 | 10 | |
| Apigenin | 76.4 | 90.4 | 72.7 | 76.5 | - | - | - | - | - | - |
| Baicalein | 64.7 | 48.6 | 59.2 | 58.7 | - | 76.6 | - | 66.1 | - | - |
| Baicalin | 89.0 | 72.9 | 81.7 | 78.3 | - | 81.8 | - | 91.5 | 86.2 | 84.7 |
| Berberine HCl | 50.5 | 70.3 | 48.3 | 46.8 | - | - | 60.9 | 45.0 | ||
| Caffeic acid | - | - | - | - | - | - | - | - | - | - |
| Dihydromyricetin | 90.0 | 73.9 | 82.5 | 81.9 | - | 73.4 | 94.0 | 92.5 | - | 86.3 |
| Emodin | 35.8 | 35.0 | 35.1 | 32.3 | 92.8 | 31.4 | 53.1 | 34.7 | 38.8 | 35.8 |
| EGCG | 89.0 | 72.9 | 81.7 | 89.1 | 82.5 | - | - | - | - | 91.2 |
| Gossypol | 42.6 | 15.1 | 42.1 | 40.7 | - | 51.5 | - | 44.5 | - | 42.6 |
| Hematoxylin | - | - | - | - | - | - | - | - | 81.8 | - |
| Indirubin | 61.5 | 58.7 | 56.6 | 58.4 | - | - | - | - | 65.7 | 67.0 |
| Kaempferol | 72.8 | 71.7 | 66.9 | 68.7 | - | 73.5 | - | 70.6 | - | 71.6 |
| Luteolin | 60.7 | 49.5 | 51.4 | 52.8 | - | 59.5 | - | 52.3 | - | 55.9 |
| Morin hydrate | 62.3 | 43.3 | 56.0 | 49.3 | - | 56.7 | - | 56.1 | - | - |
| Myricetin | 68.0 | - | 59.9 | 60.2 | - | 65.1 | - | 77.0 | - | 63.4 |
| Myricitrin | 75.1 | - | 67.7 | 65.4 | 93.5 | 68.3 | - | 69.3 | 87.2 | 67.0 |
| Palmatine | 44.8 | 17.5 | 47.1 | 40.9 | - | - | - | - | - | - |
| Quercetin dihydrate | 72.7 | - | 63.6 | 64.0 | 89.8 | 78.2 | - | 70.8 | - | 68.0 |
Recombinant proteins incubated with 50 µM bioactive compounds
No Inhibition (−)
Figure 4. Bioactive compounds inhibit recombinant HDAC activity.
Recombinant HDACs were incubated with non-flavonoids (A&B) and flavonoids (C&D) for 2 hours prior to the addition of cell permeable HDAC substrates. Non-flavonoids A) emodin and B) gossypol as well as flavonoids C) luteolin and D) quercetin dihydrate inhibited activity of most class I, IIa, and IIb HDAC isoforms.
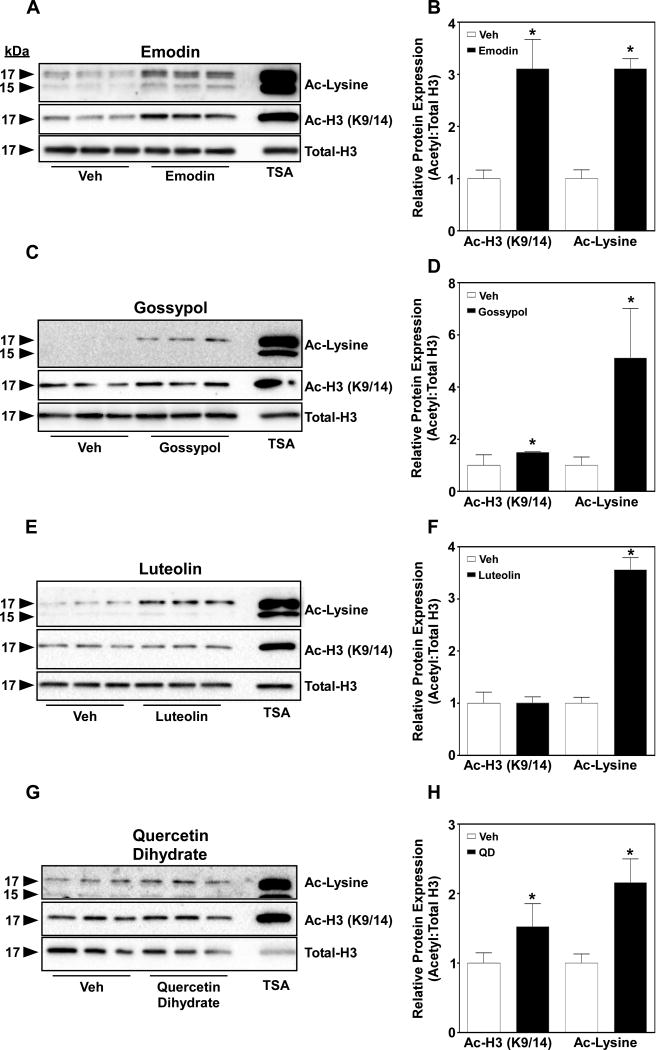
3.4. Dietary HDAC inhibitors enhance lysine acetylation of rat cardiac myocytes
Experiments outlined above clearly demonstrate that 18 of 131 bioactive compounds inhibit HDAC activity. Thus, we would postulate that treatment of cells with these identified bioactive HDAC inhibitors would increase lysine acetylation in rat cardiac myocytes. To test this postulate, we treated H9c2 myocytes for 2 hours with 50 µM of our bioactive HDAC inhibitors shown above (Fig. 3 & Fig. 4) and examined lysine and histone acetylation of whole cell extract via immunoblot. As hypothesized, we observed a significant increase in lysine acetylation (Ac-lys) for all compounds (Fig. 5). However, acetylation of histone 3 on lysine 9/14 (Ac-H3 K9/14) was only increased with treatment of emodin, gossypol and quercetin dihydrate, but not luteolin; suggesting target and tissue specificity for these bioactive compounds. TSA, a potent and well described pan-HDAC inhibitor [17], was used as a positive control for analysis of lysine acetylation. Lastly, we noted no discernible toxicity or cell death of any bioactive HDAC inhibitor in H9c2 cells treated for 2 hours.
Figure 5. Bioactive compound HDAC inhibitors increased lysine acetylation in H9c2 cells.
H9c2 cells were incubated with indicated bioactive compounds for 2 hours prior to protein harvest. Cells were lysed and acetyl-lysine (Ac-Lysine), acetyl-histone 3 (Ac-H3 K9/14) and total histone 3 (Total-H3) were assessed via immunoblot. Acetylation of lysine increased for all compounds (A, C, E & G), whereas Ac-H3 (K9/14) only increased for A) emodin, B) gossypol and G) quercetin dihydrate. Acetyl proteins were normalized to total H3 and quantitation performed (B, D, F & H) via Image J software.
4 Discussion
The findings in this report highlight a screening approach to determine bioactive compounds that inhibit HDAC activity. Using class-specific fluorescent substrates specific for Zn-dependent HDACs, we report that 18 out of 131 bioactive compounds inhibit HDAC activity in cardiac tissue. Moreover, the findings of this study define the individual HDAC(s) inhibited by these bioactive compounds. Consistent with HDAC inhibition, we further demonstrate an increase in lysine acetylation. Taken together, our data demonstrates that many dietary compounds can regulate the dynamic control of the epigenetic landscape and suggest that further exploration for the epigenetic impact of diet on gene regulation in chronic disease is needed.
Small molecule Zn-dependent HDAC inhibitors block cardiac remodeling and inflammation in response to myocardial infarction and hypertension [7–9]. Findings from this report highlight 18 dietary compounds that inhibit Zn-dependent HDAC activity in cardiac tissue (Table 1) and increase lysine acetylation in cardiac myoblasts (Fig.5). Based on these data, we would postulate a cardioprotective role for these compounds in the heart. Indeed, reports have highlighted a role for some of our identified compounds (e.g. EGCG, luteolin, kaempferol) as cardioprotective [18–21]. Luteolin for instance protects against ischemia/reperfusion-induced myocardial injury as well as angiotensin II-induced cardiac remodeling [20, 21]. In addition to this, some of our compounds have previously been implicated in the regulation of post-translational modifications (PTMs) that include phosphorylation, acetylation and methylation in other chronic disease states [4, 22, 23]. Quercetin is a prominent flavanol that has been studied for its anti-carcinogenic and anti-hyperlipidemic actions [24–26]; recent reports demonstrate biological actions of quercetin via inhibition of DNA methyl transferase 1 (DNMT1) subsequently inhibiting methylation [26] or activation of NAD+-dependent HDACs (i.e., Sirtuins) [23, 27]. We further report that quercetin dihydrate acts as a pan-HDAC inhibitor (Fig. 3&4; Table 1&2). Collectively, our findings would argue that future research into the cardioprotective actions of these dietary compounds should examine their role as epigenetic modifiers of the nucleosomal landscape; with our data highlighting HDACs as targets of importance.
While our data supports a role for diet in the epigenetic regulation of heart health, our findings are translatable to other epigenetic diseases including cancer. Indeed, small molecule HDAC inhibitors Vorinostat and Romidepsin are currently approved by the US Food and Drug Administration (FDA) for the treatment of advanced cutaneous T cell lymphoma[10, 22], with more HDAC inhibitor monotherapy and combination therapy trials currently underway according to clinicaltrials.gov. Current nutrigenomic studies have demonstrated the importance of diet as chemopreventative and chemotherapeutic. More specifically, flavonoids have been identified as chemotherapeutic agents; these studies demonstrate that chemotherapeutic actions of flavonoids work in part by regulating histone modifications via alterations in acetylation and/or methylation [4, 22]. Compounds highlighted in this report including epigallocatechin gallate (EGCG), kaempferol, quercetin, luteolin and apigenin (Fig. 1; Table 1), have been shown to regulate multiple post-translational marks that include methylation and acetylation in order to block cancer proliferation and metastasis or promote cancer apoptosis [22]. With regards to our findings, EGCG, kaempferol, quercetin, and apigenin have previously been shown to regulate the Zn-dependent HDACs [1, 28, 29]. We report for the first time pan-HDAC inhibition by myricetin (Table 1), which has previously been shown to act as an anti-proliferative agent for the treatment of cancer [30, 31]. Thus, we would speculate that anti-carcinogenic actions of myricetin are mediated in part through HDAC inhibition. In addition to myricetin, we report new evidence that flavonoids, baicalein, baicalin, dihydromyricetin, morin hydrate and myricitrin (Fig.3&4; Table 1&2) also inhibit Zn-dependent HDAC activity as well as which HDACs these compounds target (Table 2). Combined, our findings uncover newly identified flavonoid HDAC inhibitors; thus, future examination of these dietary compounds in the epigenetic regulation of cancer prevention, cardioprotection and other disease states would be of significant interest to the field of nutrigenomics.
While flavonoids comprise a large group of compounds examined in this study, we also highlight non-flavonoid compounds in the regulation of HDAC activity (Fig. 3&4; Table 1&2). This is important, as flavonoids and non-flavonoids are present in the human diet. Similar to flavonoids, we report several previously undescribed non-flavonoid HDAC inhibitors, including berberine hydrochloride, gossypol and palmatine (Table 1 & Fig. 1). Palmatine and gossypol have reported anti-inflammatory and anti-oxidant actions via mechanisms involving reduced inflammatory gene expression and inhibition of inflammatory signaling cascades [32, 33]. Not surprisingly, HDAC inhibitors block inflammation in various disease models including cardiac disease [10]. Therefore, identification of these compounds as pan-HDAC inhibitors create future avenues of research to elucidate epigenetic mechanisms for these non-flavanoid dietary compounds in cardiac and other chronic disease states.
Given the role for Zn-dependent HDACs in a variety of pathophysiological processes that include cardiac disease, cancer and inflammation, it is not surprising that there has been an increased emphasis on identifying and developing small molecule HDAC inhibitors in the pharmaceutical industry and in academic labs [10]. Structure-function studies using substrate peptides and inhibitors have revealed that HDAC inhibitors can be structurally grouped into at least four chemical classes: hydroxamic acids, short chain fatty acids, benzamides and cyclic peptides. Relative potencies and selectivity profiles differ between and within these classes [34]; where hydroxamic acids produce potent (low nanomolar) pan-HDAC inhibitors, short chain fatty acids weak (millimolar) HDAC inhibitors, while benzamides and cyclic peptides are generally selective for HDACs 1, 2 and 3. Additional studies have shown that HDAC inhibitors act as competitive inhibitors; meaning that they act within the HDAC catalytic site. Indeed, TSA and sodium butyrate have be shown to dock in the catalytic site of HDACs −1, −2 and −3 [35]. Of note, competitive inhibition of HDACs by TSA does not alter HDAC1 and −2 protein-protein interactions with repressor complex Sin3a or transcription factor Sp1 [35]. Thus, structure-function studies have allowed for the development of pan-, class- and isoform-specific HDAC inhibitors. This is apparent in Fig.2, where HDAC inhibitor structure impacts HDAC class selectivity; TSA the first natural hydroxamate acts as a pan-HDAC inhibitor [36], MGCD0103 selective against class I HDACs [37], DPAH selective against class IIa HDACs [38] and tubstatin A selective against HDAC6/class IIb HDACs, [39]. Lastly, given the widespread implications for diet to regulate epigenetic gene expression, bioactive food compounds that inhibit HDAC activity have also become of immense interest in industry and Academia. Thus, it would be interesting to speculate further about dietary HDAC inhibitors; using molecular modeling tools investigators could delineate how the structure of our bioactive compounds (Fig.3) as well as other food compounds regulate HDAC activity via docking at the catalytic site.
Concluding Remarks
The emergence of nutrigenomics has highlighted the vast potential for bioactive compounds in the prevention and treatment of epigenetic diseases including heart disease and cancer [1, 3]. As such, studies such as ours, provide novel insights into bioactive compounds that regulate the epigenome, and in particular histone acetylation via inhibition of HDAC activity. Of note, our study did not examine the role for these bioactive compounds in the regulation of other epigenetic marks (e.g. methylation), nor did we examine the impact of these dietary compounds on other ‘writer’ (e.g. HATs) or ‘eraser’ (e.g. DNMT inhibitors) proteins. This is important as compounds like EGCG have been shown to function as both HAT and HDAC inhibitors [22], suggesting diverse epigenetic actions for dietary compounds in the regulation of disease. In addition, our study did not examine the impact of dietary HDAC inhibitors with regard to biological function e.g. cardiac hypertrophy. However, this study provides the quintessential first step into unraveling newly identified natural food compounds as dietary HDAC inhibitors using screening based technologies. As, broad spectrum HDAC inhibitors are currently being studied for the treatment of various epigenetic diseases [17, 40], our findings that 18 out of 131 bioactive compounds inhibit HDAC activity has relevant potential for preventative and/or therapeutic applications.
Supplementary Material
Acknowledgments
This work is supported by the USDA National Institute of Food and Agriculture (Hatch-NEV00727) to B.S.F. Core facilities used for Research reported in this publication was supported by National Institute of General Medical Sciences of the National Institutes of Health under grant number P20 GM103554. We would like to thank Dr. M. Stratton for thoughtful input into the manuscript.
Abbreviations
- HDACs
Histone deacetylase
- TSA
Trichostatin A
- AMC
7-amino-4-methlcoumarin
- EGCG
epigallocatechin gallate
- DNMT1
DNA methyl transferase 1
Footnotes
Author contributions:
B.S.F. supervised and designed the studies, L.D.G., J.E.L., A.J.B. and S.R. performed the experiments and analyzed data; L.D.G. and B.S.F. wrote the manuscript.
Conflict of Interest:
The authors declare no conflicts of interest.
References
- 1.Vahid F, Zand H, Nosrat-Mirshekarlou E, Najafi R, Hekmatdoost A. The role dietary of bioactive compounds on the regulation of histone acetylases and deacetylases: a review. Gene. 2015;562:8–15. doi: 10.1016/j.gene.2015.02.045. [DOI] [PubMed] [Google Scholar]
- 2.Voss TC, Hager GL. Dynamic regulation of transcriptional states by chromatin and transcription factors. Nat Rev Genet. 2014;15:69–81. doi: 10.1038/nrg3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szarc vel Szic K, Ndlovu MN, Haegeman G, Vanden Berghe W. Nature or nurture: let food be your epigenetic medicine in chronic inflammatory disorders. Biochem Pharmacol. 2010;80:1816–1832. doi: 10.1016/j.bcp.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 4.Busch C, Burkard M, Leischner C, Lauer UM, et al. Epigenetic activities of flavonoids in the prevention and treatment of cancer. Clin Epigenetics. 2015;7:64. doi: 10.1186/s13148-015-0095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choudhary C, Kumar C, Gnad F, Nielsen ML, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 6.Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Biol. 2004;338:17–31. doi: 10.1016/j.jmb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Granger A, Abdullah I, Huebner F, Stout A, et al. Histone deacetylase inhibition reduces myocardial ischemia-reperfusion injury in mice. FASEB J. 2008;22:3549–3560. doi: 10.1096/fj.08-108548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kee HJ, Sohn IS, Nam KI, Park JE, et al. Inhibition of histone deacetylation blocks cardiac hypertrophy induced by angiotensin II infusion and aortic banding. Circulation. 2006;113:51–59. doi: 10.1161/CIRCULATIONAHA.105.559724. [DOI] [PubMed] [Google Scholar]
- 9.Kong Y, Tannous P, Lu G, Berenji K, et al. Suppression of class I and II histone deacetylases blunts pressure-overload cardiac hypertrophy. Circulation. 2006;113:2579–2588. doi: 10.1161/CIRCULATIONAHA.106.625467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKinsey TA. Isoform-selective HDAC inhibitors: closing in on translational medicine for the heart. J Mol Cell Cardiol. 2011;51:491–496. doi: 10.1016/j.yjmcc.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson BS, McKinsey TA. Non-sirtuin histone deacetylases in the control of cardiac aging. J Mol Cell Cardiol. 2015;83:14–20. doi: 10.1016/j.yjmcc.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemon DD, Horn TR, Cavasin MA, Jeong MY, et al. Cardiac HDAC6 catalytic activity is induced in response to chronic hypertension. J Mol Cell Cardiol. 2011;51:41–50. doi: 10.1016/j.yjmcc.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heltweg B, Dequiedt F, Marshall BL, Brauch C, et al. Subtype selective substrates for histone deacetylases. J Med Chem. 2004;47:5235–5243. doi: 10.1021/jm0497592. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann K, Brosch G, Loidl P, Jung M. A non-isotopic assay for histone deacetylase activity. Nucleic Acids Res. 1999;27:2057–2058. doi: 10.1093/nar/27.9.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riester D, Wegener D, Hildmann C, Schwienhorst A. Members of the histone deacetylase superfamily differ in substrate specificity towards small synthetic substrates. Biochem Biophys Res Commun. 2004;324:1116–1123. doi: 10.1016/j.bbrc.2004.09.155. [DOI] [PubMed] [Google Scholar]
- 16.Lahm A, Paolini C, Pallaoro M, Nardi MC, et al. Unraveling the hidden catalytic activity of vertebrate class IIa histone deacetylases. Proc Natl Acad Sci U S A. 2007;104:17335–17340. doi: 10.1073/pnas.0706487104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKinsey TA. Therapeutic potential for HDAC inhibitors in the heart. Annu Rev Pharmacol Toxicol. 2012;52:303–319. doi: 10.1146/annurev-pharmtox-010611-134712. [DOI] [PubMed] [Google Scholar]
- 18.Knekt P, Kumpulainen J, Jarvinen R, Rissanen H, et al. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002;76:560–568. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- 19.Xuan F, Jian J. Epigallocatechin gallate exerts protective effects against myocardial ischemia/reperfusion injury through the PI3K/Akt pathway-mediated inhibition of apoptosis and the restoration of the autophagic flux. Int J Mol Med. 2016;38:328–336. doi: 10.3892/ijmm.2016.2615. [DOI] [PubMed] [Google Scholar]
- 20.Bian C, Xu T, Zhu H, Pan D, et al. Luteolin Inhibits Ischemia/Reperfusion-Induced Myocardial Injury in Rats via Downregulation of microRNA-208b-3p. PLoS One. 2015;10:e0144877. doi: 10.1371/journal.pone.0144877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakayama A, Morita H, Nakao T, Yamaguchi T, et al. A Food-Derived Flavonoid Luteolin Protects against Angiotensin II-Induced Cardiac Remodeling. PLoS One. 2015;10:e0137106. doi: 10.1371/journal.pone.0137106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim E, Bisson WH, Lohr CV, Williams DE, et al. Histone and Non-Histone Targets of Dietary Deacetylase Inhibitors. Curr Top Med Chem. 2016;16:714–731. doi: 10.2174/1568026615666150825125857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dashwood RH. Frontiers in polyphenols and cancer prevention. J Nutr. 2007;137:267S–269S. doi: 10.1093/jn/137.1.267S. [DOI] [PubMed] [Google Scholar]
- 24.Aherne SA, O'Brien NM. Dietary flavonols: chemistry, food content, and metabolism. Nutrition. 2002;18:75–81. doi: 10.1016/s0899-9007(01)00695-5. [DOI] [PubMed] [Google Scholar]
- 25.Odbayar TO, Badamhand D, Kimura T, Takashi Y, et al. Comparative studies of some phenolic compounds (quercetin, rutin, and ferulic acid) affecting hepatic fatty acid synthesis in mice. J Agric Food Chem. 2006;54:8261–8265. doi: 10.1021/jf061135c. [DOI] [PubMed] [Google Scholar]
- 26.Lee WJ, Shim JY, Zhu BT. Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol Pharmacol. 2005;68:1018–1030. doi: 10.1124/mol.104.008367. [DOI] [PubMed] [Google Scholar]
- 27.Gilbert ER, Liu D. Flavonoids influence epigenetic-modifying enzyme activity: structure - function relationships and the therapeutic potential for cancer. Curr Med Chem. 2010;17:1756–1768. doi: 10.2174/092986710791111161. [DOI] [PubMed] [Google Scholar]
- 28.Pandey M, Kaur P, Shukla S, Abbas A, et al. Plant flavone apigenin inhibits HDAC and remodels chromatin to induce growth arrest and apoptosis in human prostate cancer cells: in vitro and in vivo study. Mol Carcinog. 2012;51:952–962. doi: 10.1002/mc.20866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berger A, Venturelli S, Kallnischkies M, Bocker A, et al. Kaempferol, a new nutrition-derived pan-inhibitor of human histone deacetylases. J Nutr Biochem. 2013;24:977–985. doi: 10.1016/j.jnutbio.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Jose J, Dhanya AT, Haridas KR, Sumesh Kumar TM, et al. Structural characterization of a novel derivative of myricetin from Mimosa pudica as an anti-proliferative agent for the treatment of cancer. Biomed Pharmacother. 2016;84:1067–1077. doi: 10.1016/j.biopha.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Wang G, Wang JJ, Du L, Fei L, To ST. Inhibitory Kinetics and Mechanism of Flavonoids Extracted from Cotinus coggygria Scop. Against Glioblastoma Cancer. Nutr Cancer. 2016;68:1357–1368. doi: 10.1080/01635581.2016.1225105. [DOI] [PubMed] [Google Scholar]
- 32.Huo M, Gao R, Jiang L, Cui X, et al. Suppression of LPS-induced inflammatory responses by gossypol in RAW 264.7 cells and mouse models. Int Immunopharmacol. 2013;15:442–449. doi: 10.1016/j.intimp.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Kwon OJ, Kim MY, Shin SH, Lee AR, et al. Antioxidant and Anti-Inflammatory Effects of Rhei Rhizoma and Coptidis Rhizoma Mixture on Reflux Esophagitis in Rats. Evid Based Complement Alternat Med. 2016;2016:2052180. doi: 10.1155/2016/2052180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradner JE, West N, Grachan ML, Greenberg EF, et al. Chemical phylogenetics of histone deacetylases. Nat Chem Biol. 2010;6:238–243. doi: 10.1038/nchembio.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sekhavat A, Sun JM, Davie JR. Competitive inhibition of histone deacetylase activity by trichostatin A and butyrate. Biochem Cell Biol. 2007;85:751–758. doi: 10.1139/o07-145. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida M, Kijima M, Akita M, Beppu T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem. 1990;265:17174–17179. [PubMed] [Google Scholar]
- 37.Fournel M, Bonfils C, Hou Y, Yan PT, et al. MGCD0103, a novel isotype-selective histone deacetylase inhibitor, has broad spectrum antitumor activity in vitro and in vivo. Mol Cancer Ther. 2008;7:759–768. doi: 10.1158/1535-7163.MCT-07-2026. [DOI] [PubMed] [Google Scholar]
- 38.Tessier P, Smil DV, Wahhab A, Leit S, et al. Diphenylmethylene hydroxamic acids as selective class IIa histone deacetylase inhibitors. Bioorg Med Chem Lett. 2009;19:5684–5688. doi: 10.1016/j.bmcl.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Butler KV, Kalin J, Brochier C, Vistoli G, et al. Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A. J Am Chem Soc. 2010;132:10842–10846. doi: 10.1021/ja102758v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zwergel C, Valente S, Jacob C, Mai A. Emerging approaches for histone deacetylase inhibitor drug discovery. Expert Opin Drug Discov. 2015;10:599–613. doi: 10.1517/17460441.2015.1038236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.