Abstract
Spinocerebellar ataxia type 17 (SCA17) is a family member of Autosomal Dominant Cerebellar Ataxias (ADCA) characterized by variable manifestations, including cerebellar ataxia, dementia and psychiatric symptoms. Since the identification of a CAG repeat expansion in the TBP gene in a patient with ataxia in 1999 and then verification of this expansion in SCA17 patients in 2001, several SCA17 rodent models, including both knock-in and transgenic models in mouse and rat, have been established to explore the phenotypic features and pathogenesis of SCA17. These animal models revealed different pathologic changes and phenotypes that are associated with the expression of mutant TBP protein and the CAG repeat lengths. It is important to understand how mutant TBP can cause differential pathological events in SCA17 animal models. In this review, we summarize and compare these animal models for the nature of transgenes and their expression as well as phenotypical features. We also discuss potential directions to use them for future studies.
Keywords: Spinocerebellar Ataxia Type 17, SCA17, Huntington Disease-like 4, HDL4
Introduction
Spinocerebellar Ataxia Type 17 (SCA17) is a neurodegenerative disease caused by expansion of CAA/CAG repeats that are translated to an expanded polyglutamine repeat in TATA-box binding protein (TBP). Based on Harding’s classification (Whaley et al. 2011), SCA17 belongs to Type 1 ADCA (Autosomal Dominant Cerebellar Ataxias/Spinocerebellar ataxias), featuring cerebellar syndrome and other neurologic symptoms such as pyramidal, extrapyramidal, ophthalamoplegia, and dementia. Type 1 ADCA is divided into 21 subtypes, including SCA1-SCA4, SCA8, SCA10, SCA12-SCA14, SCA15/16, SCA17-SCA23, SCA25, SCA27, SCA28 and dentatorubral pallidoluysian atrophy (DRPLA) (Whaley et al. 2011).
In 1999, an expanded CAG repeat in the TBP gene was identified in a 14-year-old Japanese female patient with progressive ataxia and intellectual deterioration (Koide et al. 1999). In 2001, Nakamura et al. verified this abnormal CAG expansion in the TBP gene in four Japanese pedigrees with SCA17 (Nakamura et al. 2001). Since then, more patients with SCA17 with complex clinical features and neuropathological changes were found to associate with this CAG repeat expansion in the TBP gene (Bauer et al. 2004; Bruni et al. 2004; Fujigasaki et al. 2001; Lasek et al. 2006; Rolfs et al. 2003; Silveira et al. 2002; Toyoshima et al. 2004; Zuhlke et al. 2001).
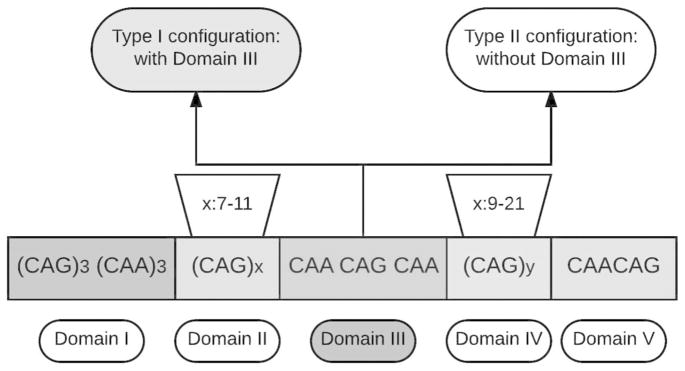
The normal repeat number in the TBP gene varies from 25 to 40, reduced penetrance alleles ranges from 41–48, and full penetrance alleles carry more than 48 CAG/CAA repeats that can lead to SCA17. The configuration of the repeat sequence in TBP is (CAG)3 (CAA)3 (CAG)x CAA CAG CAA (CAG)y CAA CAG in which X ranges from 7 to 11 and Y ranges from 9 to 21 (Fig 1). This domain is divided into five segments, in which the most frequently expansion occurs in domain IV (van Roon-Mom et al. 2005) and the CAA CAG CAA interruption (domain III) works as a stabilizer for transmission between generations (Gao et al. 2008; Maltecca et al. 2003). Therefore, depending on the absence or presence of domain III, two subtypes of TBP gene mutation were determined: Type I with domain III and type II without domain III. Type I is more prevalent than Type II (Gao et al. 2008), and Type II features unstable transmission, leading to intergenerational instability in German and Italian family (Maltecca et al. 2003). As a general transcriptional factor, TBP protein works as a critical part of the preinitiation complex with polymerase II and other molecules, which bind to the TATA box to initiate transcription (Gostout et al. 1993; Roeder 1991).
Figure 1.
A schematic picture showing the genetic configuration of TBP.
The clinical features of SCA17 are broad and variable. Patients with SCA17 present a variety of prominent symptoms in an age-dependent manner. Like other polyQ diseases, most SCA17 cases show late-onset and progressive symptoms. The most frequently observed clinical symptom is cerebellar ataxia, and other clinical symptoms include dementia, psychiatric symptoms, pyramidal signs, abnormal movements, parkinsonism, and epilepsy (Bruni et al. 2004; Koide et al. 1999; Rolfs et al. 2003; Stevanin and Brice 2008). Variable clinical features overlap with those in other neurodegenerative diseases, especially Huntington’s Disease, leading to the difficulty in clinical diagnosis of SCA17 (Stevanin and Brice 2008).
The neuropathological lesions are present mainly in the cerebellum and cortex, with mild alteration in the brain stem, which are consistent with the most frequently observed ataxia symptoms. Within the cerebellum, Purkinje cells show the most severe degeneration, while mild neuronal loss was observed in the dentate nucleus and granular layers (Stevanin and Brice 2008). In the cerebral cortex, abnormal arborization of neuronal dendrites and spongiosis was presented in the motor cortex and visual areas (Bruni et al. 2004; Fujigasaki et al. 2001; Toyoshima et al. 2004). In the brainstem, the locus coeruleus and the substantia nigra were affected mildly (Stevanin and Brice 2008).
Growing clinical reports in SCA17 pedigrees revealed more complex symptoms in SCA17 disease. However, we still have little knowledge about the pathogenesis of SCA17, which hinders the development of effective therapy for the treatment of this disease. Animal models that express mutant TBP are highly valuable for us to understand SCA17 pathogenesis and find its treatment. Over the past 15 years, a number of different animal models of SCA17, including both knock-in and transgenic rodent models with different polyQ repeat numbers in TBP, have been generated and characterized. It would be interesting to compare these animal models to better understand how mutant TBP can elicit different neuropathology and symptoms in different animal models. In this review, we focus on the genetically modified rodent models of SCA17 and discuss their genotypic and phenotypic features, which may help the selection of SCA17 animal models for future studies and the development of SCA17 treatment.
Generation of SCA17 animal models
The identification of the CAG repeat expansion in the TBP gene allows for generating SCA17 animal models via genetically modification approaches. Basically, transgenic approach is to overexpress exogenous mutant TBP in animals to mimic SCA17 neuropathology and behavior phenotypes. Gene targeting is to insert a large CAG repeat in the endogenous TBP gene in mice to create knock-in models that express mutant TBP at the endogenous level. Animal models created by these different approaches can recapitulate some phenotypes of SCA17 patients and also have limitations in mimicking the full range of disease features of SCA17, which will be discussed below.
1. Transgenic rodent models of SCA17
In 2007, Friedman et al. generated the first SCA17 mouse model that expresses human TBP cDNAs with 13, 71 or 105 CAG repeats under the control of the mouse prion promotor (Friedman et al. 2007). These mouse models can recapitulate SCA17 both genotypically and phenotypically in a repeat length dependent manner, as TBP-105Q mice show more severe genotype than TBP-71Q mice (Table 1).
Table 1.
Comparison of transgenic SCA17 rodent models
| TBP-71Q mice | TBP-105Q mice | L7-hTBP mice | TBP-64Q rats | |
|---|---|---|---|---|
| Publication | (Friedman et al. 2007) | (Chang et al. 2011) | (Kelp et al. 2013) | |
| Transgene | human TBP cDNA | |||
| Promoter | Mouse prion promoter | Pcp2/L7 | Mouse prion promoter | |
| CAG repeats | 71 | 105 | 109 | 64 |
| Mutant TBP Expression | Both soluble and aggregated forms in cerebellum, cerebral cortex, striatum, and brain stem (the highest level in cerebellum) | Both soluble form and aggregated form mainly in the cerebellum and brainstem (lower level in the cortex and hippocampus) | Soluble form in cerebellum, cortex, olfactory bulb; aggregated form was not described | |
| mTBP reduces endogenous TBP | 4 months old | No description | No description | 8 months old |
| General Phenotypes | ||||
| Kyphosis | 4 months old | 3 months old | No description | 8 months old |
| Life span | 3 months old | 2.25 months old | No difference | 10 months old |
| Body weight loss | 2.5 months old | 2 months old | No difference | 4 months old |
| Motor deficits | ||||
| Rotarod deficits | Non-accelerating rotarod (1.5 months old) | Accelerating rotarod (2–4 months old) | Accelerating rotarod (8 months old) | |
| Foot-printing assay | No description | 3 months old | No description | |
| Balance beam | No description | No description | 3.5 months old | |
| Ataxia | No description | 2 months old | 5 months old | |
| Clasping | + (NS) | + (NS) | + (NS) | 8 months old |
| Spontaneous seizures | + (NS) | + (NS) | No description | No description |
| Tremor | + (NS) | + (NS) | No description | + (NS) |
| Anxiety | No description | No description | No description | 4 months old-EPM test 7 months old-adaptation test |
| Neuropathology (cerebellum) | ||||
| Gross brain morphology | No difference | No difference | No difference | No description |
| Cerebellum atrophy | No description | No description | + (NS) | No description |
| GFAP Gliosis | 5.5 months old | 2.5 months old | 3 months old | No description |
| Granular layer | No description | Degeneration | No description | No description |
| Purkinje layers | Degeneration | Degeneration | Degeneration | |
| Molecular layer | Loss or disruption of calbindin- positive neurites | Neurite loss and disruption | No description | |
| Neuropathology (other brain regions) | ||||
| Minimal neuritic degeneration in cortex and striatum. | Cell loss in cortex, striatum, brain stem, and hippocampus | No description | ||
The age of mice when a specific phenotype was first observed is indicated in the table.
NS: The age was not mentioned in the original publication.
These transgenic SCA17 mice show severe neurological symptoms and early death. For example, the transgenic mouse model showed early lethality (11.5 weeks for TBP-71Q, and as early as 9 weeks for TBP-105Q). Importantly, these transgenic SCA17 mice display TBP accumulation and aggregation in the brain, an important feature of SCA17 patients. In the TBP-71Q and TBP-105Q mice, both soluble and aggregated forms of mutant TBP can be detected in various brain regions, including the cerebellum, cerebral cortex, striatum and brain stem, though cerebellum has the highest expression level (Table 1). The severe phenotypes of these SCA17 mice are likely due to the overexpression of transgenic mutant TBP driven by the prion promoter.
After the generation of the first SCA17 mouse model, Chang et al. generated another transgenic SCA17 (L7-hTBP) transgenic mouse model. They used a construct carrying the cDNA encoding human TBP (hTBP) with a polyQ (109Q) repeat under the control the Purkinje specific protein (Pcp2/L7) gene promoter (Chang et al. 2011), which can drive transgene expression mainly in the Purkinje cells (Walton et al. 2012). As a result, the expression of mutant TBP occurred mainly in the cerebellum and brainstem in L7-hTBP transgenic mice.
In 2013, Alexandra Kelp et al. generated TBP-64Q transgenic rat model, in which a construct carrying full length human TBP with 64 CAG repeats driven by the murine prion promoter was used. The expression of mutant TBP was mainly found in the cerebellum, with moderate expression in the cortex and olfactory bulb, while very low level of mutant TBP was found in the hypothalamus, brain stem, and striatum. Different from the transgenic mouse models, the transgenic rat model showed that mutant TBP was mainly present in the granular and molecular layer, with a low level in the Purkinje layer (Kelp et al. 2013).
The expression of transgenic mutant TBP in the above rodent models depends on the copy number and chromosomal location of transgenes under different promoters. Also, the repeat numbers in these transgenic mutant TBP models are not the same. Thus, we compare the phenotypes and neuropathology of these transgenic SCA17 rodent models (TBP-71Q, TBP-105Q, L7-hTBP, TBP-64Q rat) in Table 1. In TBP-71Q and TBP-105Q mice, mutant TBP formed aggregates in an age-dependent manner, with the decreased expression of soluble mutant TBP (Friedman et al. 2007). Both TBP-71Q and TBP-105Q mice displayed decreased lifespan and body weight loss, while TBP-105Q mice showed such phenotype earlier than TBP-71Q mice. Both TBP-71Q and TBP-105Q mice were found to have spontaneous seizures and tremor phenotypes. In the L7-hTBP mice, the phenotypes are less severe and hunchback phenotype was not described. For the motor deficits, all the mouse models showed poor performance in rotarod test. They also displayed typical clasping phenotypes, and gait disturbance abnormalities were found in the L7-hTBP mice by foot printing assay. Similar to the transgenic mouse models, the transgenic rat model also displayed severe phenotypes including kyphosis, decreased life span and body weight loss, though the rat model carries a smaller CAG repeat. A wide array of behavior tests have been performed to elucidate both motor and non-motor defects In the SCA17 rat model. For example, the transgenic rat model was evaluated with modified ataxia score test and elevated plus maze (EPM) test, and their ataxia phenotype was quantitatively analyzed.
For the neuropathological characterization, no differences in the gross brain morphology were reported in all transgenic rodent models as compared to their wild type littermates. As SCA17 mainly affects the cerebellum, studies of all these models focused on the neuropathy in the cerebellum. Gliosis and cerebellar degeneration were detected using different methods, including fluoresent staining or electron microscopy (Chang et al. 2011; Friedman et al. 2007). In the TBP-71Q and TBP-105Q mice, degenerated Purkinje cells and axons were observed, while degenerated neurons with nuclear inclusions was observed only in the cerebellar granular layer in TBP-105Q mice. Moreover, the TBP-105Q protein formed prominent nuclear inclusions, whereas the TBP-71Q protein remained largely diffuse in the neuronal nuclei. Because of the larger brain size in rats, positron emission tomography (PET) and diffusion tensor imaging (DTI) data were collected from TBP-64Q transgenic rat model. The highlight of this rat model is that PET and DTI analysis was conducted, confirming the possibility of using DTI result as a potential biomarker for SCA17, which is also an obvious advantage of using the rat model of SCA17.
One significant concern of using transgenic mouse models to study SCA17 pathogenesis is the overexpression of mutant TBP controlled by exogenous promoters, as in the research field of neurodegenerative proteinopathies, it is becoming increasingly appreciated that the overexpression of mutant proteins could lead to artificial phenotypes. This concern is even more justified based on studies showing that overexpression of wild type polyQ proteins caused disease-like phenotypes in animal models as well (Fernandez-Funez et al. 2000; Monks et al. 2007; Nedelsky et al. 2010). Therefore, knock-in models which express mutant TBP driven by its endogenous promoter are more likely to recapitulate toxicity under physiological conditions, and are preferable in terms of studying SCA17 pathological mechanisms. Nonetheless, the relative low cost and short turnaround time makes it possible to generate transgenic models using different model organisms, which is ideal for studying different aspects of SCA17 pathology. Moreover, the rapidly progressive phenotypes commonly found in such models make them good fits for the initial screening of potential therapeutic targets for SCA17 treatment.
2. SCA17 knock-in mouse model
In 2011, a knock-in mouse model of SCA17 was generated and expected to most closely recapitulate SCA17 genetically (Huang et al. 2011). This knock-in SCA17 mouse model utilizes the Cre-loxP technique, which allows for tissue and time specific expression of mutant TBP (Huang et al. 2015; Yang et al. 2014). In the knock-in mouse model, the mouse TBP exon 2 was replaced with human TBP exon 2 carrying 105 CAGs (Huang et al. 2011). Three stop codons that are flanked by two loxP sites were placed in the front of the translation initiation site. The heterozygous floxed (TBP-105Q+/loxp) mice were crossed with transgenic mice expressing Cre under the control of the neuronal nesting promoter. Thus, expression of Cre can remove the stop codons flanked by loxP sites and activate the expression of mutant TBP in neuronal cells in SCA17 knock-in mice (Huang et al. 2011).
Compared to transgenic SCA17 mouse models in which mutant TBP was overexpressed, mutant TBP in the knock-in mice is expressed at the endogenous level. There is diffuse staining of mutant TBP in the nuclei of the cortex, striatum, brain stem and cerebellum, with only small nuclear aggregates found in Purkinje cells (Huang et al. 2011). This knock-in mouse model shared similar but later onset phenotypes as transgenic mouse models (Table 2). Compared with the transgenic models, this knock-in mouse model displayed milder neurological symptoms and neurodegeneration, perhaps because mutant TBP expression is at the endogenous level and is also restricted to neuronal cells.
Table 2.
Comparison of SCA17 knock-in mouse models
| Nestin-TBP KI mice | Inducible TBP KI Mice | Germline-TBP KI mice | Muscle-TBP KI mice | |
|---|---|---|---|---|
| Publication | (Huang et al. 2011) | (Yang et al. 2014) | (Huang et al. 2015) | |
| Transgene | Floxed stop codon that prevents the expression of hTBP exon 2 with 105 CAG | |||
| Promoter-Cre | Nestin-Cre | Tamoxifen-induced Cre-ER | Ella-Cre | Muscle-Cre (CKmm-Cre) |
| CAG repeats | 105 | |||
| Mutant TBP Expression | Neuronal | Ubiquitous expression upon tamoxifen injection | Ubiquitous | Skeletal muscle cells |
| mTBP reduces endogenous TBP | Yes | Yes | Yes | No description |
| General Phenotypes | ||||
| Kyphosis | 22 months old | 14-month-old mice: 60 days after tamoxifen injection | 6 months old | 7 months old |
| Life span | No early death | 14-month-old mice: 40 days after tamoxifen injection | 5–10 months | 6–12 months |
| Body weight Loss | 11 months old | 14-month-old mice: 35 days after tamoxifen injection | 3 months old | 4 months old |
| Motor deficits | ||||
| Accelerating rotarod deficits | 9 months old | 14-month-old mice: 9 days after tamoxifen injection | 3 months old | 3 months old |
| Ataxia | No description | 14 months old | 3 months old | No description |
| Neuropathology | ||||
| Purkinje cell degeneration | 16 months old | 14-month-old mice: 2 months after tamoxifen injection | 3 months old | No description |
| Muscle atrophy | 12 months old | No description | 3 months old | 3 months old |
The age of mice when a specific phenotype was first observed is indicated in the table.
NS: The age was not mentioned in the original publication.
In 2014, Yang, et al. generated an inducible TBP knock-in mice by crossing the floxed TBP mice with Cre-ER transgenic mice that express tamoxifen-inducible Cre throughout the body so that mutant TBP expression can be turned on at different ages by intraperitoneal injection of tamoxifen (Yang et al. 2014). This mouse model was used for research on the age-related effects of mutant TBP. The authors found that the neurological phenotypes as well as cerebellum neuronal degeneration were all age-related processes. The older the mice are, the faster the mutant TBP accumulated in the neuronal cells. In other words, the aged mice showed more severe neurological phenotypes in a shorter period of time after tamoxifen injection, compared with young knock-in mice.
In 2015, Huang et al., reported the generation of germline TBP knock-in mice by crossing the floxed TBP mice with transgenic mice expressing Cre under the control of the EIIa promoter, which drives the expression of Cre in early embryonic cells. Therefore, mutant TBP can be expressed ubiquitously at the endogenous level in the crossed mice (Huang et al. 2015). In this mouse model, the presence of mutant TBP reduced the level of wild type TBP in mouse brains, which was also reported previously in the transgenic mice, indicating that TBP is an important transcriptional regulator and its protein level is tightly controlled in the body. Moreover, in the germline TBP knock-in mice, mutant TBP was found to preferentially accumulate in the nuclei of neuronal and muscle cells and exerted polyQ length dependent toxic effects in the brain and muscle tissues (Huang et al. 2015).
All TBP knock-in mice showed SCA17 related phenotypes, including hunchback or kyphosis, body weight loss, deceased lifespan and motor deficits. However, the germline knock-in mice displayed more severe and earlier onset phenotypes than neuron specific knock-in mice, suggesting an important role of peripheral mutant TBP in the pathogenesis of SCA17. Indeed, the skeletal muscle degeneration appears to be a more significant symptom that can cause early death of SCA17 knock-in mice. Since muscle atrophy could also be due to the degeneration of motor neurons that can result in denervation of the muscle, Huang et al explored whether the muscle degeneration is the primary effect of mutant TBP or secondary to the motor neuron degeneration. They crossed the floxed TBP mice with muscle specific Cre transgenic mice and found that the muscle specific expression of mutant TBP produced the same muscle atrophy phenotype. Also, electron microscopic study and immunohistochemistry staining did not uncover any abnormal structures in neuro-muscular junction (Huang et al. 2015).
The significant muscle degeneration in SCA17 knock-in mice could be polyQ repeat length dependent. In support of this idea, direct expression of mutant TBP with 44Q in muscle cells via viral infection did not elicit obvious muscle phenotypes. Also, juvenile SCA17 patients with >63Q showed symptoms such as muscle weakness and seizure (Koide et al. 1999; Maltecca et al. 2003), supporting the idea that the peripheral or muscle phenotypes of SCA17 patients are polyQ length dependent and explain why most of SCA17 patients carrying 43–48 CAGs do not show an obvious muscle atrophy phenotype. These findings provide strong evidence that mutant TBP protein with a large polyQ repeat could directly affect peripheral tissues and produce more complex phenotypes.
As evidenced by the above studies, knock-in models enable us to understand the molecular mechanisms that are closely relevant to SCA17 pathogenesis, which brings valuable insights into the development of potential therapeutic strategies for SCA17 treatment. Nonetheless, generating knock-in models is time and money consuming. Also due to the endogenous expression of mutant TBP, the toxicity develops slowly and the symptoms normally appear at a much late stage. It is also possible that the rodent brain is less vulnerable than the human brain to polyQ disease proteins. Thus, to ensure that the disease phenotypes could occur within the life span of the mice, an extremely extended CAG repeats (105 repeats) was used, which may not fully recapitulate the conditions of most SCA17 patients with moderate repeat numbers.
Conclusion and Future Perspectives
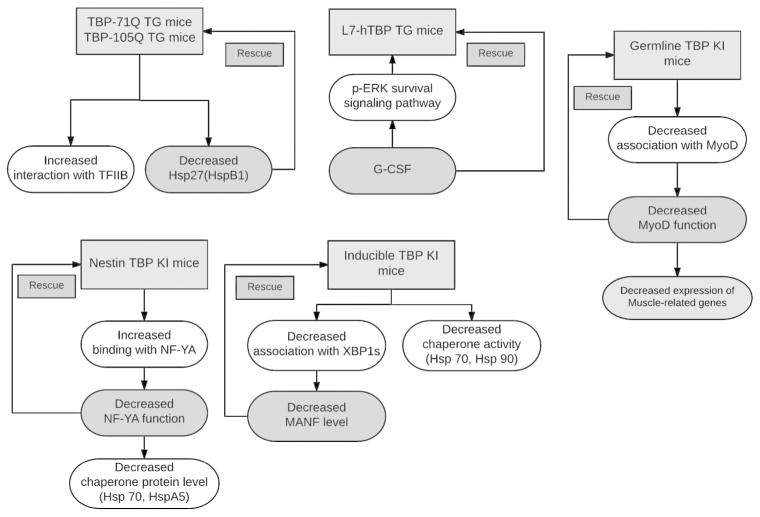
Genetically modified rodent models of SCA17 provide us with valuable tools to further investigate SCA17 pathogenesis and to develop the treatment of SCA17 (Fig 2). Because SCA17 represents a family of polyQ diseases and the function of TBP has been well characterized, investigation of SCA17 will also provide broad insight into other polyQ diseases. Using these SCA17 rodent models, we can address an important issue regarding how a ubiquitously expressed polyQ protein can cause selective pathology in an age dependent manner.
Figure 2.
A brief summary of our current understanding about SCA17 molecular pathogenesis and potential therapeutic options by studying SCA17 mouse models.
The current genetic animal models of SCA17 clearly indicate that the phenotypes are dependent on the expression levels of mutant TBP and the length of CAG/glutamine repeat. It should also be pointed out that phenotype assays are important to reveal the effects of mutant TBP. As motor deficit is the most prominent symptom in patients with SCA17, several assays can be used for evaluating motor coordination deficits, including rotarod test, balance beam, and foot-printing assay. The rotarod test is the commonly used method to test the motor coordination in the mouse models, which can be divided into non-accelerating and accelerating versions (Brooks and Dunnett 2009). The original version for rotarod test is the non-accelerating test with a set speed, which is not suitable for poor coordination animals, as the animals can fall off at the beginning of the test. Thus, the non-accelerating test makes the result a representation of endurance rather than coordination. To overcome this problem, accelerating version is designed, which can better evaluate the motor coordination (Shiotsuki et al. 2010). In addition to motor deficits, psychiatric symptoms are also present in SCA17 patients. In recently established rodent models, especially in the rat model of SCA17, several tests, such as the elevated plus maze test and PhenoMaster system, have been performed to measure non-motor symptoms.
Characterization of pathological changes is also important to reveal the in vivo toxicity of mutant TBP. Identification and quantification of soluble and aggregated forms of mutant TBP in different tissues would help us to understand how mutant TBP mediates tissue or cell-type specific pathology. Such a study relies on the utilization of antibodies to different forms of mutant TBP. Four antibodies were reported for TBP detection, including N-12, 1C2, 1TBP18 and EM192 (Friedman et al. 2008). All three antibodies (N12, 1C2 and 1TBP18) can be used to detect both soluble and aggregated forms of TBP in western blotting and immunostaining (Table 3). The N-12 antibody was used for the detection of the N-terminus of TBP of human origin. The 1C2 antibody was generated against the polyQ region, therefore, can also be used for detecting other polyglutamine disease proteins. The epitope of 1TBP18 is located in the amino acid residues 1–20 of human, mouse and rat TBP. EM192 was developed in the Li Lab at Emory University and had been used for detecting C-terminal fragment of TBP (Friedman et al. 2008).
Table 3.
Frequently used antibodies detecting TBP protein in SCA17 animal models.
| Antibody | Epitope | Soluble form | Aggregates | Western blotting | Immunostaining |
|---|---|---|---|---|---|
| N-12 | N-terminus of human TBP | + | + | + | + |
| 1C2 | Expanded polyglutamine repeats | + | + | + | + |
| 1TBP18 | Amino acid residues 1–20 of TBP | + | + | + | + |
| EM192 | C-terminal of mouse TBP | + | + | + | − |
+ indicates the antibody works for that particular application; − indicates the antibody does not work for that particular application.
The genetically modified SCA17 rodent models will allow us to investigate how tissue-specific expression of mutant TBP contributes to SCA17 symptoms and phenotypes. The relative milder phenotypes of SCA17 mice that express mutant TBP primarily in neuronal cells than those in SCA17 mice that express mutant TBP ubiquitously indicate that the peripheral effects of mutant TBP critically contribute to the progression and severity of SCA17. Although these SCA17 mouse models have revealed the toxic effects of mutant TBP in skeletal muscles, it remains to be investigated whether mutant TBP also affects other types of cells. Understanding this issue would be important to test the idea that polyQ proteins may preferentially affect postmitotic cells, such as neurons and muscle cells. Since SCA17 knock-in mice show much more severe muscle atrophy than other polyQ diseases such as Huntington disease mouse model, it is clear that protein context also defines the specific pathology in each polyQ disease. One possibility for this peripheral phenotype is that a large polyQ repeat can confer additional or unique toxicity or phenotypes that are different from those mediated by intermediate-length polyQ repeats. This possibility should be rigorously tested by generating additional knock-in mouse models that express mutant TBP with different lengths of polyQ tracts. Also, there may be cell-type or tissue specific proteins whose function is differentially affected by TBP with different lengths of polyQ tracts. The current and future SCA17 mouse models that express mutant TBP selectively in different types of cells or tissues should provide us with tools to address this important issue. Finally, since TBP is a well-studied transcription factor, studying SCA17 animal models has the potential to provide mechanistic insights into other polyQ diseases that share a number of common phenotypic and pathological aspects.
Significance Statement.
Spinocerebellar Ataxia Type 17 (SCA17) belongs to the family of neurodegenerative diseases caused by polyglutamine expansion and represents a devastating disease family without effective therapeutic treatments. Over the past two decades, remarkable efforts have been devoted to understand the mechanisms underlying SCA17 pathogenesis, with the hope to eventually find a cure for SCA17. Especially, the generation of various rodent models provides a valuable platform for conducting such research. In the review, we summarize the current rodent models of SCA17, with the aim to facilitate future basic and preclinical studies of SCA17, which could also be informative for other neurodegenerative diseases.
Acknowledgments
This work was supported by the NIH (AG19206 and NS041449 to XJL, AG031153 and NS045016 to SHL). We thank Xiangya Hospital for supporting Yiting Cui’s study at Emory University in the USA.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Authors’ Contributions
Cui, Y and Yang, S drafted the manuscript, Li, X and Li, S reviewed and edited the manuscript.
References
- Bauer P, Laccone F, Rolfs A, Wullner U, Bosch S, Peters H, Liebscher S, Scheible M, Epplen JT, Weber BH, Holinski-Feder E, Weirich-Schwaiger H, Morris-Rosendahl DJ, Andrich J, Riess O. Trinucleotide repeat expansion in SCA17/TBP in white patients with Huntington’s disease-like phenotype. J Med Genet. 2004;41(3):230–232. doi: 10.1136/jmg.2003.015602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SP, Dunnett SB. Tests to assess motor phenotype in mice: a user’s guide. Nat Rev Neurosci. 2009;10(7):519–529. doi: 10.1038/nrn2652. [DOI] [PubMed] [Google Scholar]
- Bruni AC, Takahashi-Fujigasaki J, Maltecca F, Foncin JF, Servadio A, Casari G, D’Adamo P, Maletta R, Curcio SA, De Michele G, Filla A, El Hachimi KH, Duyckaerts C. Behavioral disorder, dementia, ataxia, and rigidity in a large family with TATA box-binding protein mutation. Arch Neurol. 2004;61(8):1314–1320. doi: 10.1001/archneur.61.8.1314. [DOI] [PubMed] [Google Scholar]
- Chang YC, Lin CY, Hsu CM, Lin HC, Chen YH, Lee-Chen GJ, Su MT, Ro LS, Chen CM, Hsieh-Li HM. Neuroprotective effects of granulocyte-colony stimulating factor in a novel transgenic mouse model of SCA17. J Neurochem. 2011;118(2):288–303. doi: 10.1111/j.1471-4159.2011.07304.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Funez P, Nino-Rosales ML, de Gouyon B, She WC, Luchak JM, Martinez P, Turiegano E, Benito J, Capovilla M, Skinner PJ, McCall A, Canal I, Orr HT, Zoghbi HY, Botas J. Identification of genes that modify ataxin-1-induced neurodegeneration. Nature. 2000;408(6808):101–106. doi: 10.1038/35040584. [DOI] [PubMed] [Google Scholar]
- Friedman MJ, Shah AG, Fang ZH, Ward EG, Warren ST, Li S, Li XJ. Polyglutamine domain modulates the TBP-TFIIB interaction: implications for its normal function and neurodegeneration. Nature neuroscience. 2007;10(12):1519–1528. doi: 10.1038/nn2011. [DOI] [PubMed] [Google Scholar]
- Friedman MJ, Wang CE, Li XJ, Li S. Polyglutamine expansion reduces the association of TATA-binding protein with DNA and induces DNA binding-independent neurotoxicity. The Journal of biological chemistry. 2008;283(13):8283–8290. doi: 10.1074/jbc.M709674200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujigasaki H, Martin JJ, De Deyn PP, Camuzat A, Deffond D, Stevanin G, Dermaut B, Van Broeckhoven C, Durr A, Brice A. CAG repeat expansion in the TATA box-binding protein gene causes autosomal dominant cerebellar ataxia. Brain. 2001;124(Pt 10):1939–1947. doi: 10.1093/brain/124.10.1939. [DOI] [PubMed] [Google Scholar]
- Gao R, Matsuura T, Coolbaugh M, Zuhlke C, Nakamura K, Rasmussen A, Siciliano MJ, Ashizawa T, Lin X. Instability of expanded CAG/CAA repeats in spinocerebellar ataxia type 17. Eur J Hum Genet. 2008;16(2):215–222. doi: 10.1038/sj.ejhg.5201954. [DOI] [PubMed] [Google Scholar]
- Gostout B, Liu Q, Sommer SS. “Cryptic” repeating triplets of purines and pyrimidines (cRRY(i)) are frequent and polymorphic: analysis of coding cRRY(i) in the proopiomelanocortin (POMC) and TATA-binding protein (TBP) genes. Am J Hum Genet. 1993;52(6):1182–1190. [PMC free article] [PubMed] [Google Scholar]
- Huang S, Ling JJ, Yang S, Li XJ, Li S. Neuronal expression of TATA box-binding protein containing expanded polyglutamine in knock-in mice reduces chaperone protein response by impairing the function of nuclear factor-Y transcription factor. Brain: a journal of neurology. 2011;134(Pt 7):1943–1958. doi: 10.1093/brain/awr146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Yang S, Guo J, Yan S, Gaertig MA, Li S, Li XJ. Large Polyglutamine Repeats Cause Muscle Degeneration in SCA17 Mice. Cell reports. 2015;13(1):196–208. doi: 10.1016/j.celrep.2015.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelp A, Koeppen AH, Petrasch-Parwez E, Calaminus C, Bauer C, Portal E, Yu-Taeger L, Pichler B, Bauer P, Riess O, Nguyen HP. A novel transgenic rat model for spinocerebellar ataxia type 17 recapitulates neuropathological changes and supplies in vivo imaging biomarkers. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33(21):9068–9081. doi: 10.1523/JNEUROSCI.5622-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide R, Kobayashi S, Shimohata T, Ikeuchi T, Maruyama M, Saito M, Yamada M, Takahashi H, Tsuji S. A neurological disease caused by an expanded CAG trinucleotide repeat in the TATA-binding protein gene: a new polyglutamine disease? Human molecular genetics. 1999;8(11):2047–2053. doi: 10.1093/hmg/8.11.2047. [DOI] [PubMed] [Google Scholar]
- Lasek K, Lencer R, Gaser C, Hagenah J, Walter U, Wolters A, Kock N, Steinlechner S, Nagel M, Zuhlke C, Nitschke MF, Brockmann K, Klein C, Rolfs A, Binkofski F. Morphological basis for the spectrum of clinical deficits in spinocerebellar ataxia 17 (SCA17) Brain. 2006;129(Pt 9):2341–2352. doi: 10.1093/brain/awl148. [DOI] [PubMed] [Google Scholar]
- Maltecca F, Filla A, Castaldo I, Coppola G, Fragassi NA, Carella M, Bruni A, Cocozza S, Casari G, Servadio A, De Michele G. Intergenerational instability and marked anticipation in SCA-17. Neurology. 2003;61(10):1441–1443. doi: 10.1212/01.wnl.0000094123.09098.a0. [DOI] [PubMed] [Google Scholar]
- Monks DA, Johansen JA, Mo K, Rao P, Eagleson B, Yu Z, Lieberman AP, Breedlove SM, Jordan CL. Overexpression of wild-type androgen receptor in muscle recapitulates polyglutamine disease. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(46):18259–18264. doi: 10.1073/pnas.0705501104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Jeong SY, Uchihara T, Anno M, Nagashima K, Nagashima T, Ikeda S, Tsuji S, Kanazawa I. SCA17, a novel autosomal dominant cerebellar ataxia caused by an expanded polyglutamine in TATA-binding protein. Human molecular genetics. 2001;10(14):1441–1448. doi: 10.1093/hmg/10.14.1441. [DOI] [PubMed] [Google Scholar]
- Nedelsky NB, Pennuto M, Smith RB, Palazzolo I, Moore J, Nie Z, Neale G, Taylor JP. Native functions of the androgen receptor are essential to pathogenesis in a Drosophila model of spinobulbar muscular atrophy. Neuron. 2010;67(6):936–952. doi: 10.1016/j.neuron.2010.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder RG. The complexities of eukaryotic transcription initiation: regulation of preinitiation complex assembly. Trends Biochem Sci. 1991;16(11):402–408. doi: 10.1016/0968-0004(91)90164-q. [DOI] [PubMed] [Google Scholar]
- Rolfs A, Koeppen AH, Bauer I, Bauer P, Buhlmann S, Topka H, Schols L, Riess O. Clinical features and neuropathology of autosomal dominant spinocerebellar ataxia (SCA17) Annals of neurology. 2003;54(3):367–375. doi: 10.1002/ana.10676. [DOI] [PubMed] [Google Scholar]
- Shiotsuki H, Yoshimi K, Shimo Y, Funayama M, Takamatsu Y, Ikeda K, Takahashi R, Kitazawa S, Hattori N. A rotarod test for evaluation of motor skill learning. J Neurosci Methods. 2010;189(2):180–185. doi: 10.1016/j.jneumeth.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Silveira I, Miranda C, Guimaraes L, Moreira MC, Alonso I, Mendonca P, Ferro A, Pinto-Basto J, Coelho J, Ferreirinha F, Poirier J, Parreira E, Vale J, Januario C, Barbot C, Tuna A, Barros J, Koide R, Tsuji S, Holmes SE, Margolis RL, Jardim L, Pandolfo M, Coutinho P, Sequeiros J. Trinucleotide repeats in 202 families with ataxia: a small expanded (CAG)n allele at the SCA17 locus. Arch Neurol. 2002;59(4):623–629. doi: 10.1001/archneur.59.4.623. [DOI] [PubMed] [Google Scholar]
- Stevanin G, Brice A. Spinocerebellar ataxia 17 (SCA17) and Huntington’s disease-like 4 (HDL4) Cerebellum. 2008;7(2):170–178. doi: 10.1007/s12311-008-0016-1. [DOI] [PubMed] [Google Scholar]
- Toyoshima Y, Yamada M, Onodera O, Shimohata M, Inenaga C, Fujita N, Morita M, Tsuji S, Takahashi H. SCA17 homozygote showing Huntington’s disease-like phenotype. Annals of neurology. 2004;55(2):281–286. doi: 10.1002/ana.10824. [DOI] [PubMed] [Google Scholar]
- van Roon-Mom WM, Reid SJ, Faull RL, Snell RG. TATA-binding protein in neurodegenerative disease. Neuroscience. 2005;133(4):863–872. doi: 10.1016/j.neuroscience.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Walton JC, Schilling K, Nelson RJ, Oberdick J. Sex-dependent behavioral functions of the Purkinje cell-specific Galphai/o binding protein, Pcp2(L7) Cerebellum. 2012;11(4):982–1001. doi: 10.1007/s12311-012-0368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley NR, Fujioka S, Wszolek ZK. Autosomal dominant cerebellar ataxia type I: a review of the phenotypic and genotypic characteristics. Orphanet J Rare Dis. 2011;6:33. doi: 10.1186/1750-1172-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Huang S, Gaertig MA, Li XJ, Li S. Age-dependent decrease in chaperone activity impairs MANF expression, leading to Purkinje cell degeneration in inducible SCA17 mice. Neuron. 2014;81(2):349–365. doi: 10.1016/j.neuron.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuhlke C, Hellenbroich Y, Dalski A, Kononowa N, Hagenah J, Vieregge P, Riess O, Klein C, Schwinger E. Different types of repeat expansion in the TATA-binding protein gene are associated with a new form of inherited ataxia. Eur J Hum Genet. 2001;9(3):160–164. doi: 10.1038/sj.ejhg.5200617. [DOI] [PubMed] [Google Scholar]