Abstract
Eccrine sweat glands help to maintain homoeostasis, primarily by stabilizing body temperature. Derived from embryonic ectoderm, millions of eccrine glands are distributed across human skin and secrete litres of sweat per day. Their easy accessibility has facilitated the start of analyses of their development and function. Mouse genetic models find sweat gland development regulated sequentially by Wnt, Eda and Shh pathways, although precise subpathways and additional regulators require further elucidation. Mature glands have two secretory cell types, clear and dark cells, whose comparative development and functional interactions remain largely unknown. Clear cells have long been known as the major secretory cells, but recent studies suggest that dark cells are also indispensable for sweat secretion. Dark cell-specific Foxa1 expression was shown to regulate a Ca2+-dependent Best2 anion channel that is the candidate driver for the required ion currents. Overall, it was shown that cholinergic impulses trigger sweat secretion in mature glands through second messengers – for example InsP3 and Ca2+ – and downstream ion channels/transporters in the framework of a Na+-K+-Cl− cotransporter model. Notably, the microenvironment surrounding secretory cells, including acid–base balance, was implicated to be important for proper sweat secretion, which requires further clarification. Furthermore, multiple ion channels have been shown to be expressed in clear and dark cells, but the degree to which various ion channels function redundantly or indispensably also remains to be determined.
Keywords: Best2, Ca2+, clear cell, dark cell, ectodysplasin, FoxA1, InsP3R2, Itpk, Nkcc1, Shh, Wnt
Introduction
Two major types of sweat glands, apocrine and eccrine, are known in humans. Apocrine glands are a phylogenetic remnant of mammalian scent glands. They are larger but are non-thermoregulatory restricted into a few areas, notably the axillae and external genitalia (1). They secrete turbid fluid containing water, proteins, lipids and odour precursors with a DNA variant in the ABCC11 required for odour formation (2). By contrast, eccrine glands are tiny but very numerous (3). Millions are distributed across the human skin and directly open to the skin surface. Eccrine glands form a thermoregulatory organ and secrete primarily water that contains electrolytes. We focus on the eccrine glands in this review.
An individual can secrete up to 4 l of eccrine sweat in an hour (3), cooling down body temperature as necessary. Evaporation of sweat from the skin surface effectively dissipates heat generated by physical activity, fever or hot environments. Dysfunction of sweat glands, seen characteristically in the elderly, is linked to the heat stroke that kills about 1000 people each year in the United States (www.cdc.gov/mmwr). By contrast, overactive sweat glands can cause local hyperhidrosis, a chronic condition affecting the quality of life of about 2.8% of the US population (4).
Eccrine glands also have additional roles in humans. They secrete moisturizing factors such as lactate, urea, sodium and potassium to maintain skin hydration (5). Further, secreted sweat mixed with sebum on the skin surface forms a moisturizing lipid layer (6). Recent studies have further demonstrated that sweat glands secrete several antimicrobial peptides, including dermcidin, cathelicidin and lactoferrin (7–10), which help to control skin flora and fight skin infections. Furthermore, sweat also contains secreted IgA and cytokines, including IL-1 and IL-31, which likely contribute to immune defense and the extent of inflammatory reactions (11–14).
Thus, increasing evidence suggests critical involvement of eccrine glands in skin homoeostasis as well as thermoregulation. Here, we review recent findings on the mechanism of eccrine gland development and secretion. Other aspects, including sweat gland-related diseases and therapeutic approaches, are covered in extensive reviews (1,3,15–21).
Morphological features of eccrine sweat glands
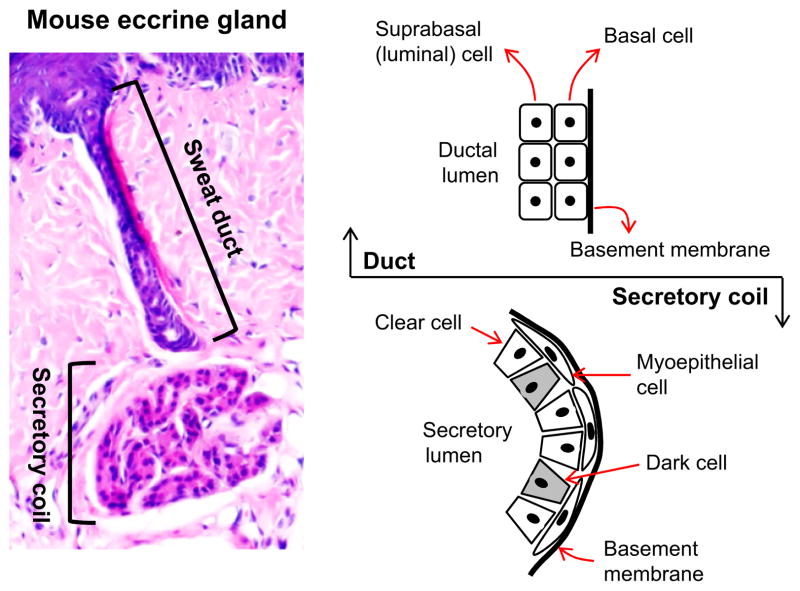
Each eccrine gland is a single tubular structure, about 3–5 mm in length in humans with a secretory coil below a relatively straight sweat duct (Fig. 1) (17). The secretory coil contains three types of cells – dark, clear and myoepithelial – identifiable by conventional microscopy in humans. Dark and clear cells are secretory cells, whereas myoepithelial cells support the glands mechanically and create a major niche for gland stem cells (3,18,22). Dark cells contain cytoplasmic electron-dense granules and secrete periodic acid–Schiff (PAS)-positive diastase-resistant glycoproteins as well as the novel antimicrobial dermcidin (7,23). Notably, dark cells have relatively few mitochondria and membrane villi and were long thought to play a minor role in sweat secretion (3) (but see below). By contrast, clear cells have many mitochondria and membrane villi and have major secretory function, extending to excessive sweat production in patients with local hyperhidrosis (24).
Figure 1.
A mouse eccrine sweat gland and cellular constituents are depicted. As labelled, the eccrine gland comprises a secretory coil in the deep dermis and a relatively straight duct open to the skin surface (left panel). The secretory coil contains three types of cells, clear, dark and myoepithelial, all derived from embryonic ectoderm. Clear and dark cells are secretory cells, and myoepithelial cells form a major niche for sweat gland stem/progenitor cells (right lower panel). The sweat duct consists of suprabasal (luminal) and basal layers where ions partially reabsorbed (right upper panel).
Among other markers, S100 and CA II are expressed only in clear cells, CGRP mainly in dark cells, Krt5/Krt14 in myoepithelial cells and Krt8/Krt18 in both dark and clear cells (25–28). These proteins are thus useful for immunostaining to distinguish the different cell types (Table 1). In addition, several ion channels, pumps and cotransporters were identified in human dark and/or clear cells. The NKCC1, Na-K-ATPase, NHE1, CFTR and AQP5 are all expressed in the secretory coil (29–36) (Table 1).
Table 1.
Cellular constituents and known key regulators of human eccrine sweat glands
| Anatomical structures | Cellular constituents | Key proteins |
|---|---|---|
| Sweat duct | Basal cells | CFTR, ENaC, Na+-K+-ATPase, NKCC1 (weak), NHE1, K1, K10, K5, K14 |
| Suprabasal (luminal) cells | CFTR, NHE1, K1b, K10, K19, K77 | |
| Secretory portion | Dark cells | NKCC1, Na+-K+-ATPase, NHE1, CFTR, AQP5, CGRP, K8, K18 |
| Clear cells | NKCC1, Na+-K+-ATPase, NHE1, CFTR, AQP5, S100, CA II, K8, K18 | |
| Myoepithelial cells | K5, K14 |
The sweat duct connects the secretory coil to the skin surface. The duct itself consists of two layers of cuboidal cells, suprabasal (luminal) and basal (Fig. 1). The basal cells strongly express several ion channels and transporters including CFTR, ENaC, Na-K-ATPase and more weakly NKCC1 and NHE1 (3,29–31,37), suggesting they are involved in the partial reabsorption of ions during the excretion process (Table 1). Lesions in the chloride channel CFTR cause cystic fibrosis, an autosomal recessive life-threatening disease affecting lungs, pancreases and sweat glands (38).
Regulation of eccrine sweat gland development
Origin and timing
Like all skin appendages, eccrine glands are derived from embryonic ectoderm. The eccrine glands form everywhere in human skin apart from a few areas such as lips and external auditory canals. In most other mammals, eccrine glands are located only in ventral paw skin, typically clustered in contact areas such as dome-shaped footpads. Accordingly, humans cool down body temperature by sweating, whereas other mammals generally conserve heat by hair coat and dissipate heat in alternative less efficient ways such as panting, increased saliva secretion and elevated blood flow in less hairy skin. In this sense, expanded skin coverage by sweat glands (eccrine) is an evolutionary marker, with the sole known exception of ungulates, in which apocrine rather than eccrine glands increase to numbers comparable to our species. In humans, eccrine glands begin to form around the 4th gestational month in palmoplantar skin and at the 5th month across the rest of the body, maturing morphologically before term. In mice, eccrine gland formation in ventral paw skin begins around E16.5–E17.5 and is completed around postnatal day 21 (39,40).
Dermal signalling centre
Skin appendage formation is characteristically regulated by reciprocal signalling interactions between ectoderm and mesenchyme in general, and hair follicles, teeth, mammary glands and salivary glands all have clearly visible dermal papillae or dermal condensations below epidermal structures (41,42). A long-standing puzzle in morphological features of developing eccrine glands was the lack of a corresponding histologically discernible dermal condensation or any dermal signalling centre (43). However, we have found that mouse eccrine gland germs are nevertheless surrounded by clustered mesenchymal cells (dermal sheath) during development, although the sheath was small (40). The small size likely accounts for the failure of conventional detection, but the notion is thus supported that eccrine gland formation requires an interaction of ectoderm with mesenchyme. The identity and function of eccrine gland mesenchymal cells remain to be characterized.
Involvement of Wnt–Eda–Shh cascade and Bmp
Signalling pathways are correlated with morphological stages in sweat gland formation. A Wnt/β-catenin–Eda/NF-kB–Shh cascade functions in a genetic relay for eccrine gland development. The process starts with K14-positive progenitor cells in epidermis, as seen in mouse models (22). Morphogenetic pathways similar to those seen for other skin appendages are involved in the regulation of early-stage eccrine gland development (40). Wnt signalling is required for eccrine gland induction. As in hair follicles, uniform Wnt activity can be detected in dermis before any morphological indication of eccrine gland induction, but declines quickly as the gland germs start to form. Instead, at this stage, strong Wnt activity appears in the basal layer of epidermis, gradually concentrating in the gland germs. Wnt is then continuously active at the tip of growing ducts until it disappears as sweat ducts start to coil. Consistent with its requirement for induction, inactivation of Wnt signalling by β-catenin knockout in mouse skin epidermis results in total absence of eccrine gland induction, and overexpression of Dkk4, a Wnt antagonist, significantly blocks eccrine gland induction (40).
Directly activated by Wnt, the Eda pathway regulates the next stage of development. When the cytokine Eda or its receptor Edar or adaptor Edaradd is inactive, Lef1-positive pregerms still form but never develop to full germs (40,44). Furthermore, sweat ducts fail to form if Eda is selectively inactivated after the germ formation stage (45). By contrast, supplementation of the Eda-A1 isoform rescues eccrine gland formation in Eda-null Tabby mice (46).
The next stage of secretory portion formation requires Shh signalling. Unlike its early involvement in hair follicle development (47,48), Shh inactivation does not affect eccrine gland germ or subsequent duct formation, but the secretory coil formation remains blocked at a rudimentary stage (40,49).
This signalling cascade, however, has additional actors. One additional critical morphogen is Bmp: overexpression of the Bmp antagonist noggin in skin epidermis led to hair follicle formation in mouse footpads, which are otherwise the exclusive site for eccrine sweat glands (50). In a recent study, eccrine glands were shown to fail to form in Bmpr1a knockout mice (51) although detailed analysis was not included. Suppression of the Bmp pathway was reported to promote hair follicle formation (52). However, eccrine gland formation may still require active Bmp signalling, a possibility that requires further experimental testing.
Innervation
Innervation, an obvious indispensable requirement for regulation of sweat secretion, develops along with the sweat glands. In normal mouse development, sympathetic innervation starts in developing eccrine glands at postnatal day 1 (P1) – the early secretory coil formation stage – and transition to cholinergic innervation can be detected at P6, when secretory coil formation is far along (53). Sympathetic nerve fibres were found distributed in the ‘correct’ location even when eccrine glands were missing, although they disappeared after a short period of about 2 weeks (54). The mature glands are then surrounded by postganglionic sympathetic nerve endings, although the principal final neurotransmitter is acetylcholine (3). Notably, nascent fibres failed to acquire cholinergic status in the absence of association with developing eccrine glands (54). Thus, the final transition to cholinergic response likely requires humoral factors released by developing eccrine glands (55–57). Notably, NGF transgenic mice showed a lack of eccrine gland innervation, although sympathetic axons reached the footpads, implicating NGF as a participant in innervation (58). A further indication of its importance in eccrine gland innervation comes from findings that mutations in NGF receptor TrkA led to apoptosis of primary afferent and postganglionic sympathetic nerves during development, resulting in congenital insensitivity to pain with anhidrosis (59).
Eccrine sweat gland progenitor/stem cells
Sweat gland progenitor/stem cells were recently identified from developing and mature mouse eccrine glands by lineage tracing (22). Eccrine gland formation starts with K14+ multipotent bud progenitors in the basal layer of embryonic ectoderm. The K14+ bud progenitors then transition to transient multipotent K14+ basal progenitors and K18+/lowK14 suprabasal progenitors as development proceeds. Eventually, they form four unipotent adult progenitor/stem cell populations in mature glands: basal duct, suprabasal duct, myoepithelial, and glandular luminal progenitors (22). Consistent with their regenerative potential, the basal and suprabasal duct progenitors were shown to contribute to wound healing of epidermal sweat duct and skin epidermis. Further, myoepithelial and glandular luminal progenitors replenished their own descendants only when localized injury was made, suggesting their unipotent characteristics. However, some unipotent progenitors likely regain multipotent status when localized in foreign environments. The myoepithelial and basal duct progenitors, but not luminal or suprabasal duct progenitors, were shown to regenerate de novo sweat glands in mammary and shoulder fat pads and stratified epidermis in back skin (22). Among the four adult progenitor populations, myoepithelial and basal duct progenitors, but not luminal and suprabasal duct progenitors, form large colonies and can be cultured long term (22). Notably, the myoepithelial progenitors were shown to be the major ‘label-retaining cells’ (22,51) and can regenerate sweat glands and hair follicles in foreign environments (51). Therefore, myoepithelial progenitors may possess greater ‘stemness’ and greater potential to regenerate sweat glands or skin epidermis.
Human eccrine sweat gland epithelial cells have also been shown to regenerate epidermis during skin wound healing (60,61). Human eccrine gland stem cells have not yet been characterized in detail, but myoepithelial cells were suggested to be the progenitor cells (62).
Mechanism of sweat secretion
Neuronal control
Sweat secretion in mature eccrine glands is controlled by the central nerve system. Several types of sweating are regulated at different levels of neuronal centres (16). Cortical sweating responds to emotions and is seen on palms and soles. Medullary sweating responds to spicy food and is found on the face. But hypothalamic sweating, the thermoregulatory mediator, responds to elevated body temperature. It is systemic and predominant. The thermosensitive neurons (warm-sensitive and cold-sensitive) are located in the preoptic area of the hypothalamus, and local heating of that area induces sweating (63). Eccrine sweat glands respond to both core (internal) and peripheral (skin) temperatures. The internal temperature is much more efficient than skin temperature for both sweat induction and sweating rate (64,65); this may reflect the steadier internal signal compared to external cold or hot objects or conditions.
Innervation by cholinergic sympathetic nerves is consistent with reports that denervation, like postganglionic sympathectomy, abruptly abrogates sweating and is widely used clinically to treat local hyperhidrosis (66). Sweating can occur when periglandular cholinergic, α-adrenergic or β-adrenergic nerves are activated, but cholinergic sweat secretion is the major route and also accounts for more than 70% of sweating capacity in isolated human eccrine sweat glands analysed in vitro with pharmacological agonists (Table 2) (3). In fact, inhibition of beta-adrenergic nerve activity had no detectable effect on sweat secretion in people during heat acclimation (67). Furthermore, eccrine glands express adrenergic α1 and β2 receptors particularly during the transition of noradrenergic to cholinergic innervation at early developmental stages, but they decline sharply at adult stages, consistent with the ineffective sweating induced by adrenergic agonists (68,69). Therefore, adrenergic sweating in physiological conditions is questionable and of unknown significance.
Table 2.
Pharmacological reagents commonly used for studies of sweat secretion
| Pharmacological reagents | Function |
|---|---|
| Methacholine | Muscarinic receptor agonist |
| Pilocarpine | Muscarinic receptor agonist |
| Carbachol | Muscarinic and nicotinic receptor agonist |
| Atropine | Muscarinic receptor antagonist |
| Phenylephrine | α-adrenergic receptor agonist |
| Isoproterenol | β-adrenergic receptor agonist |
| Forskolin | cAMP inducer |
| Bumetanide | NKCC1 inhibitor |
| Ouabain | Na+/K+-ATPase (Na+/K+ pump) inhibitor |
| Amiloride | ENaC sodium channel inhibitor |
| Ba2+ | K+ channel inhibitor |
| Niflumic acid | Ca2+-activated Cl− channel inhibitor |
| NPPB | Cl− channel inhibitor |
| DIDS | exchanger inhibitor |
| A23187 | Ca2+ carrier (ionophore) |
Released acetylcholine (Ach) functions in eccrine glands through its receptor Chrm3, and not via M1 or M2 types (70,71). Chrm3 is expressed in the secretory cells as well as in myoepithelial cells, and blocking Chrm3 with specific antagonists inhibited sweat secretion (72,73). Some Chrm3 mutations cause the prune belly-like syndrome in human (OMIM#100100) (74), and Chrm3 mutant mice showed similar phenotypes, including decreased salivary secretion (75). The secretory function of eccrine glands in Chrm3 mutant patients and mice has not been documented in detail, but they are predicted to be hypohidrotic (75) (Table 3).
Table 3.
A list of genes and their animal models suggested in sweat secretion
| Genes | Genotype | Gross phenotype | Sweat gland phenotype | References |
|---|---|---|---|---|
| Foxa1 | cKO (skin) | None | Anhidrosis | (94) |
| Best2 | KI | None | Hypohidrosis | (94,112) |
| Itpr2 (InsP3R2) | KO | None | Hypohidrosis | (81) |
| Aqp5 | KO | None | Hypohidrosis; Normal sweating | (32,33) |
| Chrm3 | KO | Reduced body weight, dilated pupils | Not analysed | (75,113) |
| Slc12a2 (Nkcc1) | KO | Deafness, head-shaking, growth retardation, circling | Not analysed | (114–116) |
| Atp1a1 (Na+-K+ pump) | KO | KO perinatal lethal. Heterozygotes viable | Not analysed | (117) |
| Cftr | KO | KO poor growth, decreased survival, intestinal obstruction | Not analysed | (118–122) |
| cKO | ||||
| Scnn1a (ENaC) | KO | KO perinatal lethal | Not analysed | (123) |
| cKO | ||||
| Slc9a1 (Nhe1) | KO | 2/3 of progeny die before weaning | Not analysed | (124) |
Two neuropeptides have been suggested as further regulators of sweat secretion. Galanin increased the chloride current in NCL-SG3 sweat gland cells, and a GalR3 antagonist blocked the current (76); and both galanin and its receptors GalR2 and GalR3 are expressed in human eccrine glands. Galanin knockout sweat glands, however, showed normal innervation and normal response to sudorific agents, and thermal stimulation rather increased the number of active eccrine glands compared to wild-type mice (72). Furthermore, galanin transgenic mice showed normal sweating (77), and therefore, any substantive galanin function in sweat secretion remains uncertain. A second neuropeptide, CGRP, is also itself expressed in dark cells, and enhanced acetylcholine-induced sweating in humans when applied dermally (78), but no further studies of its mechanism or relative importance have been reported.
Ca2+ requirement
Ca2+ is unequivocally required for sweat secretion (79). The immediate response to cholinergic input on eccrine glands is a sharp increase of cytosolic Ca2+. This is accomplished from two sources: influx from extracellular interstitial fluid and release of intracellular Ca2+ stores. Intracellular Ca2+ release is mediated at least in part by InsP3. When acetylcholine activates the Chrm3 receptor, InsP3 is produced from PIP2 in the secretory cells, most likely through phospholipase C (PLC) action, which then promotes intracellular Ca2+ release from the endoplasmic reticulum (3) [similar to observations in kidney epithelial cells (80)]. A recent genetic study showed that mutations in InsP3R2 blocked Ca2+ release in the secretory cells and resulted in severe hypohidrosis both in patients and in a knockout mouse model (81). Thus, an Ach-Chrm3-PLC-InsP3-InsP3R2 pathway is instrumental for intracellular Ca2+ mobilization. It remains to be seen whether elaborated InsP3 and increased Ca2+ in activated secretory cells can spread to neighbouring secretory cells, as they do in airway epithelial cells (82), to trigger sweat secretion.
The other source of Ca2+, influx from extracellular fluid, is also prerequisite for sweat secretion. Muscarinic sweating was completely blocked when Ca2+ was removed from the culture medium of isolated monkey eccrine glands (79), and local chelation of Ca2+ in humans resulted in a significant decrease in cholinergic sensitivity (83). The mechanism of Ca2+ influx into sweat secretory cells is poorly understood, but involvement of voltage-gated Ca2+ channels has been suggested (79,83). An intriguing candidate effector to be explored as a second messenger is InsP4, derived from InsP3 by Itpks. InsP4 (and not InsP3) was reported to induce Ca2+ influx into neurons, involving voltage-gated calcium channels (84–86), and we have found that Itpks are expressed in mouse eccrine glands (work in progress). Speculatively, a balance between InsP3 and InsP4 in secretory cells could thus vary the relative extent of intracellular Ca2+ release and extracellular Ca2+ influx to optimize sweat secretion (Fig. 2a).
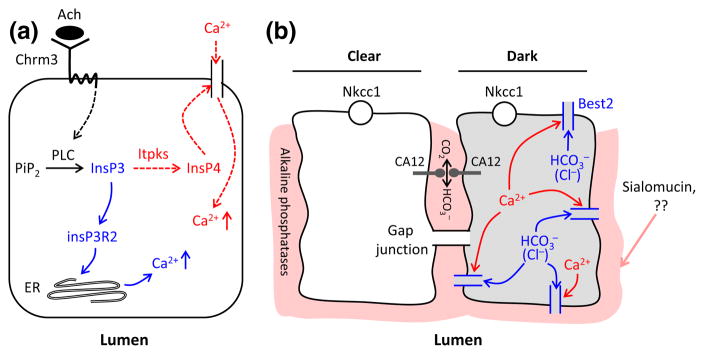
Figure 2.
Working models for calcium action and dark cell function in sweat secretion. Panel a: A possible cascade of Ca2+ release and influx. Acetylcholine binds to Chrm3 receptor and activates phospholipase C (PLC) to generate InsP3 from PIP2. InsP3 in turn activates InsP3R2 in the endoplasmic reticulum to release Ca2+ from reserves (blue). At the same time, InsP3 can be phosphorylated by Itpks to generate InsP4, which may induce Ca2+ influx from extracellular interstitium (red). These events most likely occur in clear cells (81), but it remains to be seen whether InsP3 and P4 spread to dark cells. Panel b: Possible involvement of dark cells in sweat secretion. Dark cell-specific Best2 may be a and Cl− channel (see text). Elevated Ca2+ ions (red) activate Best2 (blue) and could secrete and/or Cl− into the extracellular region. may then affect acid–base balance and the functions of channels and transporters in intercellular canaliculi and at the luminal surface, interacting with proteins such as acidic sialomucin. CA XII may also be involved in the formation of a pH gradient and thereby affect the function of alkaline phosphatases. In parallel, secretion may create a chemical gradient in dark cells that spreads to clear cells through gap junctions, and could thereby maximize sweat secretion in cooperation with clear cells.
InsP4 in conjunction with InsP3 was further shown to activate Ca2+-dependent K+ channels to induce an outward K+ current in lacrimal acinar cells, and also enhanced Ca2+-activated chloride current in pancreatic cells (87,88). Given that K+ and Cl− efflux is critical for sweat secretion as well, InsP4/InsP3 may function similarly on Ca2+-activated K+ and Cl− channels in sweat glands. It may be relevant that elevation of Ca2+ in the sweat secretory cells, induced by a calcium ionophore, does not itself induce sweat secretion. Rather, sweating was induced when Ca2+ concentration was extremely high, but at even higher levels, above those that muscarinic agents can attain, sweating again failed (89). This would be consistent with a requirement for increased production of InsP3/InsP4 by muscarinic stimulation not only for elevation of Ca2+ concentration in secretory cells, but also for effective Ca2+ action on K+ and Cl− channels in sweat secretion.
Na+-K+-Cl− cotransporter model
Cholinergic sweat secretion has been correlated with a Na+-K+-Cl− cotransporter model, as has also been suggested for secretory processes in salivary glands and pancreas (3). In this model, acetylcholine release from periglandular sympathetic nerves activates K+ and Cl− channels of secretory cells, most likely via the action of second messengers (79,89), leading to efflux of K+ ions into the interstitium and Cl− ions into the secretory lumen. The chemical gradient formed by K+ and Cl− efflux would subsequently activate the Nkcc1 cotransporter, the major ionic force, resulting in influx of Na+, K+ and Cl− ions into secretory cells in an electrically neutral way. Indeed, when Nkcc1 was inhibited, sweat secretion ceased abruptly (90). Excessive Na+ ions entering the secretory cells would then be successively pumped out to the interstitium, drawn into the secretory lumens by Cl− ions – plausibly through tight junctions between secretory cells – and followed by water – perhaps through AQP5 water channel (32,33) – to form nearly isotonic primary sweat. Partial reabsorption of NaCl in the sweat duct at the subsequent excretion stage results in final hypotonic sweat.
The Na+-K+-Cl− cotransporter model can explain most features of cholinergic sweat secretion. However, among many missing details, the identity of the ion channels involved and their relative roles remain conjectural. Several candidate ion channels have recently been identified in sweat glands. TMEM16A, a CaCC, and its splice isoform, TMEM16A(acΔe3), are expressed in human sweat glands and contribute to Cl− secretion in NCL-SG3 cells (91). The acΔe3 isoform was implicated in basal sweat secretion because it was active even in the absence of Ca2+ influx. We have recently identified a number of K+ and Cl− channels from mouse sweat glands, and Ca2+-activated Kcnn4 and pH-sensitive Kcnk5 channels were suggested to be partially required for sweat secretion (Cui CY & Schlessinger D, unpublished data).
Involvement of dark cells and Best2 in sweat secretion
Unlike clear cells, involvement of dark cells in sweat secretion is not well characterized. We summarize the available information and outline some alternative possibilities as follows. It was suggested that dark, clear and myoepithelial cells, all derived from embryonic ectoderm, require one another to maintain their identity, as they lose their morphological characteristics quickly when cultured in vitro (3). Consistent with this phenomenon, the origin of NCL-SG3, the only cell line derived from human sweat glands, was unidentifiable and in fact was shown to respond to β-adrenergic rather than the expected physiological cholinergic stimulation (92). Notably, as in clear cells, the dark cells were also shown to change cellular ion concentrations drastically upon methacholine stimulation (93), and at least some dark cells were shown to have many mitochondria and membrane villi (3). Thus, in addition to secreting glycoproteins and antimicrobial dermcidin, dark cells may play an important role for sweat secretion, assisting clear cells. Recent findings of a Foxa1–Best2 cascade have more decisively suggested involvement of dark cells in sweat secretion (94). Both Foxa1 and Best2 were expressed exclusively in mouse dark cells, and Foxa1 knockout mice showed anhidrosis, while Best2 knockout mice showed severe hypohidrosis (Table 3). Best2 was shown to function in HEK-293 cells as a CaCC that is also permeable to other anions including (95,96). However, it functions exclusively as a channel in vivo in mucin-producing goblet colon cells (97). It is still to be determined whether Best2 is a channel or a Cl− channel, or transports both as in cell culture system in glycoprotein-producing dark cells (95,96). But if it is a channel as in goblet cells, increased Ca2+ ions in dark cell cytosol, induced by cholinergic inputs, could activate Best2 to transport , which may contribute to formation of a proper extracellular or intracellular microenvironment to foster secretion.
Coincidentally, dark cells are known to secrete sialomucin, an acidic adhesive glycoprotein, which is confined to the intercellular canaliculi and luminal surface of secretory cells, the critical structures for sweat secretion (98). It is conceivable that the microenvironment, including acid–base balance, at ion channel/transporter sites, is important for sweat secretion. We suggest as a working model that ions secreted by Best2 channels may be involved in generating a balanced extracellular environment by cooperating with acidic proteins such as sialomucin (Fig. 2b). Consistent with the notion of acid–base regulatory involvement, pH-sensitive alkaline phosphatases were detected in the intercellular canaliculi of sweat gland secretory cells (99), and inactivation of alkaline phosphatases resulted in Cl− secretion through the CFTR chloride channels in human airway epithelial cells (100). More recently, a missense mutation in the CA XII in an autosomal recessively inherited family was shown to cause excessive Na+ secretion in sweat (101). CA XII is a transmembrane protein with an extracellular active site that interconverts carbon dioxide and bicarbonate. CA XII was suggested to be broadly expressed in sweat glands (102) and could thus affect the extracellular microenvironment for sweat secretion and reabsorption. In addition, it was previously shown that inhibition of the (or Na+/H+) exchange alone, without blocking of Best2 channels or other transporters, partially reduced K+ efflux and sweat secretion in isolated monkey sweat glands (3,90).
An alternative possible mode of action for Best2 is suggested by the demonstrated effects of cytosolic pH on ion channel activity. K+ current was increased when intracellular pH was elevated in rabbit colon cells or rat pancreatic B cells (103,104). Similarly, basic cytosolic pH activated and acidic pH inhibited ENaC sodium channel in human sweat ducts (105). Therefore, secretion or influx of by Best2 may affect intracellular acid–base balance and ion channels/transporters in the secretory coil. Intracellular or extracellular acid–base changes by Best2 may also affect pH-sensitive ion channels in sweat glands – for example Kcnk5, which is localized in dark cells in mice (Cui CY & Schlessinger D, unpublished data). The hypohidrosis seen in Best2 knockout mice might then result from an inadequate extracellular or intracellular microenvironment, and the anhidrosis found in Foxa1 knockout mice could be due to lack of Best2 as well as significant reduction of Nkcc1 expression in sweat glands (94).
The routes to intracellular regulation of sweat secretion are of course not limited to direct effects on ion channels. For example, PAR-2 is also expressed in NCL-SG3 cells, and activation of PAR-2 by trypsin-like serine proteases increased Ca2+ concentrations in cytosol and enhanced chloride secretion (106). In addition, histamine was recently shown to inhibit sweat secretion by suppressing phosphorylation of GSK3β, which is a target for phosphorylation by acetylcholine (107,108). This may hint at an uncharacterized mechanism involving glycogen levels in sweat secretion.
Major open questions
We have described first steps in the analysis of sweat gland development and function that raise many questions. We suggest four that are ripe for investigation, primarily in the mouse model system. First, what is the regulatory network regulating the secretory machinery? Genetic and epigenetic regulation of secretory function should be assessed (109). Foxa1 is the first transcription factor identified in sweat glands, known to regulate Best2 anion channels, but many more must be characterized. Also missing from present formulations is the ‘fine-tuning’ of regulation of gene expression by a cohort of non-coding RNAs in developing and mature sweat glands (110,111). Large-scale genomic and proteomic profiling should increasingly provide a full spectrum of gene expression and its variation. Second, dark cells are implicated in sweat secretion, but what is the detailed process – including the origins, interdependency, and functional interactions of clear and dark cells? For example, as an alternative to be tested, chemical gradients formed by secretion in dark cells by Best2 could then spread to clear cells through gap junctions to maximize sweat secretion (Fig. 2b). A technical challenge to the analyses is presented by the lack of known reliable cell membrane markers that could permit isolation of the cell types for direct analyses. The hurdle could be resolved with extended analyses of cell composition. Third, what second messengers mediate Ca2+ influx from extracellular fluid upon cholinergic impulses? Fourth, which of the many ion channels in eccrine sweat glands are indispensable, and how and in which cells? An adjunct to the identification of critical components for questions 3 and 4 could be targeted analyses of mouse genetic models and human diseases, which have largely ignored in the examination of sweat glands (Table 3).
Perspectives
As mentioned above, signalling pathways for development and ion channels/transporters for secretion are known to overlap for several exocrine glands. Although the specific fate decisions and secretions of each gland obviously require specific study, the structurally comparably simple eccrine sweat glands could provide a model for features of dynamics of other exocrine glands as well. Answers to the major open questions posed above can fill in many critical details for comparative analysis. Furthermore, they are the prelude to further analyses of the way in which eccrine gland dynamics – from the impulse provided by sympathetic nerves to the composition and amount of sweat produced – change with genetic variation and with age. Such analyses can provide a route to understand congenital eccrine gland agenesis, local hyperhidrosis, and ageing-related hypohidrosis.
Acknowledgments
Cui CY and Schlessinger D designed the study, reviewed the references and wrote the manuscript. This work was supported by Intramural Research Program of National Institute on Aging.
Abbreviations
- AQP5
aquaporin-5
- CaCC
Ca2+-activated chloride channel
- CA II
carbonic anhydrase 2
- CA XII
carbonic anhydrase 12
- CFTR
cystic fibrosis transmembrane conductance regulator
- CGRP
calcitonin gene-related peptide
- Chrm3
acetylcholine muscarinic receptor M3
- ENaC
epithelial sodium channel
- InsP3
inositol 1,4,5-trisphosphate
- InsP3R2
InsP3 receptor type 2
- InsP4
inositol 1,3,4,5-tetrakisphosphate
- Itpks
InsP3 kinases
- Na-K-ATPase
Na+-K+ pump
- NGF
nerve growth factor
- NHE1
Na+/H+ exchanger
- NKCC1
Na+-K+-Cl− cotransporter 1
- PAR-2
proteinase-activated receptor 2
Footnotes
Conflict of interests
The authors have declared no conflicting interests.
References
- 1.Saga K. Prog Histochem Cytochem. 2002;37:323–386. doi: 10.1016/s0079-6336(02)80005-5. [DOI] [PubMed] [Google Scholar]
- 2.Martin A, Saathoff M, Kuhn F, et al. J Invest Dermatol. 2010;130:529–540. doi: 10.1038/jid.2009.254. [DOI] [PubMed] [Google Scholar]
- 3.Sato K, Kang WH, Saga K, et al. J Am Acad Dermatol. 1989;20:537–563. doi: 10.1016/s0190-9622(89)70063-3. [DOI] [PubMed] [Google Scholar]
- 4.Grunfeld A, Murray CA, Solish N. Am J Clin Dermatol. 2009;10:87–102. doi: 10.2165/00128071-200910020-00002. [DOI] [PubMed] [Google Scholar]
- 5.Watabe A, Sugawara T, Kikuchi K, et al. J Dermatol Sci. 2013;72:177–182. doi: 10.1016/j.jdermsci.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Shelmire JB. J Invest Dermatol. 1959;32:471– 472. doi: 10.1038/jid.1959.77. [DOI] [PubMed] [Google Scholar]
- 7.Schittek B, Hipfel R, Sauer B, et al. Nat Immunol. 2001;2:1133–1137. doi: 10.1038/ni732. [DOI] [PubMed] [Google Scholar]
- 8.Niyonsaba F, Suzuki A, Ushio H, et al. Br J Dermatol. 2009;160:243–249. doi: 10.1111/j.1365-2133.2008.08925.x. [DOI] [PubMed] [Google Scholar]
- 9.Murakami M, Ohtake T, Dorschner RA, et al. J Invest Dermatol. 2002;119:1090–1095. doi: 10.1046/j.1523-1747.2002.19507.x. [DOI] [PubMed] [Google Scholar]
- 10.Park JH, Park GT, Cho IH, et al. Exp Dermatol. 2011;20:369–371. doi: 10.1111/j.1600-0625.2010.01218.x. [DOI] [PubMed] [Google Scholar]
- 11.Okada T, Konishi H, Ito M, et al. J Invest Dermatol. 1988;90:648–651. doi: 10.1111/1523-1747.ep12560807. [DOI] [PubMed] [Google Scholar]
- 12.Sato K, Sato F. Am J Physiol. 1994;266:R950– R959. doi: 10.1152/ajpregu.1994.266.3.R950. [DOI] [PubMed] [Google Scholar]
- 13.Dai X, Okazaki H, Hanakawa Y, et al. PLoS One. 2013;8:e67666. doi: 10.1371/journal.pone.0067666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Udey MC, Nagao K. Mucosal Immunol. 2008;1:470–474. doi: 10.1038/mi.2008.37. [DOI] [PubMed] [Google Scholar]
- 15.Shibasaki M, Wilson TE, Crandall CG. J Appl Physiol (1985) 2006;100:1692–1701. doi: 10.1152/japplphysiol.01124.2005. [DOI] [PubMed] [Google Scholar]
- 16.Wilke K, Martin A, Terstegen L, et al. Int J Cosmet Sci. 2007;29:169–179. doi: 10.1111/j.1467-2494.2007.00387.x. [DOI] [PubMed] [Google Scholar]
- 17.Quinton PM. Physiology (Bethesda) 2007;22:212–225. doi: 10.1152/physiol.00041.2006. [DOI] [PubMed] [Google Scholar]
- 18.Lu C, Fuchs E. Cold Spring Harb Perspect Med. 2014;4:a015222. doi: 10.1101/cshperspect.a015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Zee HH, Laman JD, Boer J, et al. Exp Dermatol. 2012;21:735–739. doi: 10.1111/j.1600-0625.2012.01552.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang C, Chen Y, Fu X. Cytotherapy. 2015;17:526–535. doi: 10.1016/j.jcyt.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Wilson TE, Metzler-Wilson K. Exp Dermatol. 2015;24:177–178. doi: 10.1111/exd.12595. [DOI] [PubMed] [Google Scholar]
- 22.Lu CP, Polak L, Rocha AS, et al. Cell. 2012;150:136–150. doi: 10.1016/j.cell.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munger BL. J Biophys Biochem Cytol. 1961;11:385–402. doi: 10.1083/jcb.11.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bovell DL, MacDonald A, Meyer BA, et al. Exp Dermatol. 2011;20:1017–1020. doi: 10.1111/j.1600-0625.2011.01361.x. [DOI] [PubMed] [Google Scholar]
- 25.Heller DS, Haefner HK, Hameed M, et al. J Reprod Med. 2002;47:695–700. [PubMed] [Google Scholar]
- 26.Zancanaro C, Merigo F, Crescimanno C, et al. J Anat. 1999;194(Pt 3):433–444. doi: 10.1046/j.1469-7580.1999.19430433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langbein L, Rogers MA, Praetzel S, et al. J Invest Dermatol. 2005;125:428–444. doi: 10.1111/j.0022-202X.2005.23860.x. [DOI] [PubMed] [Google Scholar]
- 28.Langbein L, Reichelt J, Eckhart L, et al. Cell Tissue Res. 2013;354:793–812. doi: 10.1007/s00441-013-1716-5. [DOI] [PubMed] [Google Scholar]
- 29.Nejsum LN, Praetorius J, Nielsen S. Am J Physiol Cell Physiol. 2005;289:C333–C340. doi: 10.1152/ajpcell.00228.2004. [DOI] [PubMed] [Google Scholar]
- 30.Reddy MM, Quinton PM. J Membr Biol. 2009;231:65–78. doi: 10.1007/s00232-009-9205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato F, Sato K. J Histochem Cytochem. 2000;48:345–354. doi: 10.1177/002215540004800304. [DOI] [PubMed] [Google Scholar]
- 32.Nejsum LN, Kwon TH, Jensen UB, et al. Proc Natl Acad Sci U S A. 2002;99:511–516. doi: 10.1073/pnas.012588099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song Y, Sonawane N, Verkman AS. J Physiol. 2002;541:561–568. doi: 10.1113/jphysiol.2001.020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang M, Zeng S, Zhang L, et al. Acta Histochem. 2014;116:1374–1381. doi: 10.1016/j.acthis.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Zhang X, Zeng S, et al. Acta Histochem. 2014;116:1237–1243. doi: 10.1016/j.acthis.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Saga K, Sato K. J Histochem Cytochem. 1988;36:1023–1030. doi: 10.1177/36.8.2839572. [DOI] [PubMed] [Google Scholar]
- 37.Reddy MM, Light MJ, Quinton PM. Nature. 1999;402:301–304. doi: 10.1038/46297. [DOI] [PubMed] [Google Scholar]
- 38.Quinton PM, Reddy MM. Nature. 1992;360:79–81. doi: 10.1038/360079a0. [DOI] [PubMed] [Google Scholar]
- 39.Tafari AT, Thomas SA, Palmiter RD. J Neurosci. 1997;17:4275–4281. doi: 10.1523/JNEUROSCI.17-11-04275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cui CY, Yin M, Sima J, et al. Development. 2014;141:3752–3760. doi: 10.1242/dev.109231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Millar SE. J Invest Dermatol. 2002;118:216– 225. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- 42.Mikkola ML, Millar SE. J Mammary Gland Biol Neoplasia. 2006;11:187–203. doi: 10.1007/s10911-006-9029-x. [DOI] [PubMed] [Google Scholar]
- 43.Headon DJ. J Invest Dermatol. 2009;129:817–819. doi: 10.1038/jid.2008.426. [DOI] [PubMed] [Google Scholar]
- 44.Cui CY, Schlessinger D. Cell Cycle. 2006;5:2477–2483. doi: 10.4161/cc.5.21.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cui CY, Kunisada M, Esibizione D, et al. J Invest Dermatol. 2009;129:984–993. doi: 10.1038/jid.2008.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui CY, Durmowicz M, Ottolenghi C, et al. Hum Mol Genet. 2003;12:2931–2940. doi: 10.1093/hmg/ddg325. [DOI] [PubMed] [Google Scholar]
- 47.St-Jacques B, Dassule HR, Karavanova I, et al. Curr Biol. 1998;8:1058–1068. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- 48.Michno K, Boras-Granic K, Mill P, et al. Dev Biol. 2003;264:153–165. doi: 10.1016/s0012-1606(03)00401-9. [DOI] [PubMed] [Google Scholar]
- 49.Kunisada M, Cui CY, Piao Y, et al. Hum Mol Genet. 2009;18:1769–1778. doi: 10.1093/hmg/ddp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Plikus M, Wang WP, Liu J, et al. Am J Pathol. 2004;164:1099–1114. doi: 10.1016/S0002-9440(10)63197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leung Y, Kandyba E, Chen YB, et al. PLoS One. 2013;8:e74174. doi: 10.1371/journal.pone.0074174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Botchkarev VA, Botchkareva NV, Roth W, et al. Nat Cell Biol. 1999;1:158–164. doi: 10.1038/11078. [DOI] [PubMed] [Google Scholar]
- 53.Schütz B, von Engelhardt J, Gördes M, et al. Neuroscience. 2008;156:310–318. doi: 10.1016/j.neuroscience.2008.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guidry G, Landis SC. J Neurosci. 1995;15:7565–7574. doi: 10.1523/JNEUROSCI.15-11-07565.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Habecker BA, Tresser SJ, Rao MS, et al. Dev Biol. 1995;167:307–316. doi: 10.1006/dbio.1995.1025. [DOI] [PubMed] [Google Scholar]
- 56.Francis NJ, Asmus SE, Landis SC. Dev Biol. 1997;182:76–87. doi: 10.1006/dbio.1996.8464. [DOI] [PubMed] [Google Scholar]
- 57.Habecker BA, Landis SC. Science. 1994;264:1602–1604. doi: 10.1126/science.8202714. [DOI] [PubMed] [Google Scholar]
- 58.Guidry G, Landis SC, Davis BM, et al. J Comp Neurol. 1998;393:231–243. doi: 10.1002/(sici)1096-9861(19980406)393:2<231::aid-cne7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 59.Indo Y. Expert Rev Neurother. 2010;10:1707– 1724. doi: 10.1586/ern.10.154. [DOI] [PubMed] [Google Scholar]
- 60.Pontiggia L, Biedermann T, Böttcher-Haberzeth S, et al. J Invest Dermatol. 2014;134:1735– 1742. doi: 10.1038/jid.2014.30. [DOI] [PubMed] [Google Scholar]
- 61.Rittié L, Sachs DL, Orringer JS, et al. Am J Pathol. 2013;182:163–171. doi: 10.1016/j.ajpath.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kurata R, Futaki S, Nakano I, et al. Cell Struct Funct. 2014;39:101–112. doi: 10.1247/csf.14009. [DOI] [PubMed] [Google Scholar]
- 63.Boulant JA. Fed Proc. 1981;40:2843–2850. [PubMed] [Google Scholar]
- 64.Nadel ER, Mitchell JW, Saltin B, et al. J Appl Physiol. 1971;31:828–833. doi: 10.1152/jappl.1971.31.6.828. [DOI] [PubMed] [Google Scholar]
- 65.Nadel ER, Bullard RW, Stolwijk JA. J Appl Physiol. 1971;31:80–87. doi: 10.1152/jappl.1971.31.1.80. [DOI] [PubMed] [Google Scholar]
- 66.Drott C, Göthberg G, Claes G. J Am Acad Dermatol. 1995;33:78–81. doi: 10.1016/0190-9622(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 67.Martinez R, Jones D, Hodge D, et al. Auton Neurosci. 2012;169:113–115. doi: 10.1016/j.autneu.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 68.Habecker BA, Malec NM, Landis SC. J Neurosci. 1996;16:229–237. doi: 10.1523/JNEUROSCI.16-01-00229.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sato K, Cavallin S, Sato KT, et al. Clin Sci (Lond) 1994;86:133–139. doi: 10.1042/cs0860133. [DOI] [PubMed] [Google Scholar]
- 70.Torres NE, Zollman PJ, Low PA. Brain Res. 1991;550:129–132. doi: 10.1016/0006-8993(91)90415-r. [DOI] [PubMed] [Google Scholar]
- 71.Grant MP, Landis SC, Siegel RE. J Neurosci. 1991;11:3763–3771. doi: 10.1523/JNEUROSCI.11-12-03763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vilches JJ, Wynick D, Kofler B, et al. Neuropeptides. 2012;46:151–155. doi: 10.1016/j.npep.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 73.Landis SC. Life Sci. 1999;64:381–385. doi: 10.1016/s0024-3205(98)00578-5. [DOI] [PubMed] [Google Scholar]
- 74.Weber S, Thiele H, Mir S, et al. Am J Hum Genet. United States: 2011 The American Society of Human Genetics. Elsevier Inc; 2011. Muscarinic Acetylcholine Receptor M3 Mutation Causes Urinary Bladder Disease and a Prune-Belly-Like Syndrome; pp. 668–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matsui M, Motomura D, Karasawa H, et al. Proc Natl Acad Sci U SA. 2000;97:9579– 9584. doi: 10.1073/pnas.97.17.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bovell DL, Holub BS, Odusanwo O, et al. Exp Dermatol. 2013;22:141–143. doi: 10.1111/exd.12067. [DOI] [PubMed] [Google Scholar]
- 77.Holmberg K, Kuteeva E, Brumovsky P, et al. Neuroscience. 2005;133:59–77. doi: 10.1016/j.neuroscience.2005.01.062. [DOI] [PubMed] [Google Scholar]
- 78.Schlereth T, Dittmar JO, Seewald B, et al. J Physiol. 2006;576:823–832. doi: 10.1113/jphysiol.2006.116111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sato K, Sato F. Am J Physiol. 1981;241:C113– C120. doi: 10.1152/ajpcell.1981.241.3.C113. [DOI] [PubMed] [Google Scholar]
- 80.Hirose K, Kadowaki S, Tanabe M, et al. Science. 1999;284:1527–1530. doi: 10.1126/science.284.5419.1527. [DOI] [PubMed] [Google Scholar]
- 81.Klar J, Hisatsune C, Baig SM, et al. J Clin Invest. 2014;124:4773–4780. doi: 10.1172/JCI70720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sneyd J, Wetton BT, Charles AC, et al. Am J Physiol. 1995;268:C1537–C1545. doi: 10.1152/ajpcell.1995.268.6.C1537. [DOI] [PubMed] [Google Scholar]
- 83.Metzler-Wilson K, Sammons DL, Ossim MA, et al. Exp Physiol. 2014;99:393–402. doi: 10.1113/expphysiol.2013.076547. [DOI] [PubMed] [Google Scholar]
- 84.Hashii M, Nakashima S, Yokoyama S, et al. Biochem J. 1996;319(Pt 2):649–656. doi: 10.1042/bj3190649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsubokawa H, Oguro K, Robinson HP, et al. J Physiol. 1996;497(Pt 1):67–78. doi: 10.1113/jphysiol.1996.sp021750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Szinyei C, Behnisch T, Reiser G, et al. J Physiol. 1999;516(Pt 3):855–868. doi: 10.1111/j.1469-7793.1999.0855u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morris AP, Gallacher DV, Irvine RF, et al. Nature. 1987;330:653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- 88.Ho MW, Shears SB, Bruzik KS, et al. Am J Physiol. 1997;272:C1160–C1168. doi: 10.1152/ajpcell.1997.272.4.C1160. [DOI] [PubMed] [Google Scholar]
- 89.Sato K, Sato F. Am J Physiol. 1988;254:C310– C317. doi: 10.1152/ajpcell.1988.254.2.C310. [DOI] [PubMed] [Google Scholar]
- 90.Sato F, Sato K. J Physiol. 1987;393:195– 212. doi: 10.1113/jphysiol.1987.sp016819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ertongur-Fauth T, Hochheimer A, Buescher JM, et al. Exp Dermatol. 2014;23:825–831. doi: 10.1111/exd.12543. [DOI] [PubMed] [Google Scholar]
- 92.Lee CM, Dessi J. J Cell Sci. 1989;92(Pt 2):241–249. doi: 10.1242/jcs.92.2.241. [DOI] [PubMed] [Google Scholar]
- 93.Saga K, Sato K. J Membr Biol. 1989;107:13– 24. doi: 10.1007/BF01871079. [DOI] [PubMed] [Google Scholar]
- 94.Cui CY, Childress V, Piao Y, et al. Proc Natl Acad Sci U S A. 2012;109:1199–1203. doi: 10.1073/pnas.1117213109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun H, Tsunenari T, Yau KW, et al. Proc Natl Acad Sci U S A. 2002;99:4008–4013. doi: 10.1073/pnas.052692999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qu Z, Hartzell HC. Am J Physiol Cell Physiol. 2008;294:C1371–C1377. doi: 10.1152/ajpcell.00398.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yu K, Lujan R, Marmorstein A, et al. J Clin Invest. 2010;120:1722–1735. doi: 10.1172/JCI41129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Constantine VS, Mowry RW. J Invest Dermatol. 1966;46:536–541. doi: 10.1038/jid.1966.81. [DOI] [PubMed] [Google Scholar]
- 99.Saga K, Morimoto Y. J Histochem Cytochem. 1995;43:927–932. doi: 10.1177/43.9.7642965. [DOI] [PubMed] [Google Scholar]
- 100.Becq F, Jensen TJ, Chang XB, et al. Proc Natl Acad Sci U S A. 1994;91:9160–9164. doi: 10.1073/pnas.91.19.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Muhammad E, Leventhal N, Parvari G, et al. Hum Genet. 2011;129:397–405. doi: 10.1007/s00439-010-0930-4. [DOI] [PubMed] [Google Scholar]
- 102.Ivanov S, Liao SY, Ivanova A, et al. Am J Pathol. 2001;158:905–919. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Duffey ME, Devor DC. Am J Physiol. 1990;258:C336–C343. doi: 10.1152/ajpcell.1990.258.2.C336. [DOI] [PubMed] [Google Scholar]
- 104.Cook DL, Ikeuchi M, Fujimoto WY. Nature. 1984;311:269–271. doi: 10.1038/311269a0. [DOI] [PubMed] [Google Scholar]
- 105.Reddy MM, Wang XF, Quinton PM. J Membr Biol. 2008;225:1–11. doi: 10.1007/s00232-008-9126-4. [DOI] [PubMed] [Google Scholar]
- 106.Bovell DL, Santic R, Kofler B, et al. Exp Dermatol. 2008;17:505–511. doi: 10.1111/j.1600-0625.2007.00659.x. [DOI] [PubMed] [Google Scholar]
- 107.Matsui S, Murota H, Takahashi A, et al. J Invest Dermatol. 2014;134:326–334. doi: 10.1038/jid.2013.323. [DOI] [PubMed] [Google Scholar]
- 108.Murota H, Matsui S, Ono E, et al. J Dermatol Sci. 2015;77:3–10. doi: 10.1016/j.jdermsci.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 109.Botchkarev VA, Gdula MR, Mardaryev AN, et al. J Invest Dermatol. 2012;132:2505–2521. doi: 10.1038/jid.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Botchkareva NV. Cell Cycle. 2012;11:468–474. doi: 10.4161/cc.11.3.19058. [DOI] [PubMed] [Google Scholar]
- 111.Kretz M, Siprashvili Z, Chu C, et al. Nature. 2013;493:231–235. doi: 10.1038/nature11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bakall B, McLaughlin P, Stanton JB, et al. Invest Ophthalmol Vis Sci. 2008;49:1563–1570. doi: 10.1167/iovs.07-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yamada M, Miyakawa T, Duttaroy A, et al. Nature. 2001;410:207–212. doi: 10.1038/35065604. [DOI] [PubMed] [Google Scholar]
- 114.Pace AJ, Lee E, Athirakul K, et al. J Clin Invest. 2000;105:441–450. doi: 10.1172/JCI8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Delpire E, Lu J, England R, et al. Nat Genet. 1999;22:192–195. doi: 10.1038/9713. [DOI] [PubMed] [Google Scholar]
- 116.Flagella M, Clarke LL, Miller ML, et al. J Biol Chem. 1999;274:26946–26955. doi: 10.1074/jbc.274.38.26946. [DOI] [PubMed] [Google Scholar]
- 117.James PF, Grupp IL, Grupp G, et al. Mol Cell. 1999;3:555–563. doi: 10.1016/s1097-2765(00)80349-4. [DOI] [PubMed] [Google Scholar]
- 118.Hodges CA, Cotton CU, Palmert MR, et al. Genesis. 2008;46:546–552. doi: 10.1002/dvg.20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.O’Neal WK, Hasty P, McCray PB, et al. Hum Mol Genet. 1993;2:1561–1569. doi: 10.1093/hmg/2.10.1561. [DOI] [PubMed] [Google Scholar]
- 120.Dorin JR, Dickinson P, Alton EW, et al. Nature. 1992;359:211–215. doi: 10.1038/359211a0. [DOI] [PubMed] [Google Scholar]
- 121.Rozmahel R, Wilschanski M, Matin A, et al. Nat Genet. 1996;12:280–287. doi: 10.1038/ng0396-280. [DOI] [PubMed] [Google Scholar]
- 122.Zeiher BG, Eichwald E, Zabner J, et al. J Clin Invest. 1995;96:2051–2064. doi: 10.1172/JCI118253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hummler E, Mérillat AM, Rubera I, et al. Genesis. 2002;32:169–172. doi: 10.1002/gene.10041. [DOI] [PubMed] [Google Scholar]
- 124.Bell SM, Schreiner CM, Schultheis PJ, et al. Am J Physiol. 1999;276:C788–C795. doi: 10.1152/ajpcell.1999.276.4.C788. [DOI] [PubMed] [Google Scholar]