Proton pump inhibitors (PPIs) are a widely used category of drug for past 20 years. Originally requiring a prescription, PPIs were used to treat peptic ulcer and gastroesophageal reflux disease. PPIs now are readily available over the counter, and their use is often unmonitored. Over utilization of PPIs for inappropriate indications and their use for durations beyond that indicated in original recommendations have been reported in both hospital and ambulatory settings.1,2 Over the past few years, the safety of long-term PPI use has come into question with observational studies reporting increased prevalence of dementia, myocardial infarction, and renal failure in people who use long-term PPIs.3–6 However, no causative mechanism for the adverse effects of PPI has been established.
Following their recent report of association of PPI use with myocardial infarction in the general population,5 in this issue of Circulation Research, Yepuri et al7 used a series of in vitro studies using human microvascular endothelial cells (EC) treated with clinically relevant concentrations of the PPI, esomeprazole. They showed reduced lysosomal cathepsin-B and phosphatase, and increased lysosomal protein aggregates in ECs treated with esomeprazole versus controls, indicating impaired lysosomal proteostasis. Treatment with esomeprazole increased production of EC superoxide anions, decreased eNOS (endothelial nitric oxide synthase) and iNOS (inducible nitric oxide synthase) expression, and reduced nitric oxide (NO) production. In matrigel assays, treatment with esomeprazole reduced microvascular EC proliferation and network formation. Finally, the authors show that esomeprazole treatment accelerated EC senescence as seen by visual morphology. This was corroborated with a pattern of gene expression changes consistent with increased expression of genes involved in EC to mesenchymal cell transition and telomerase shortening. Together, these data show the first evidence of a causative role of PPIs across a spectrum of EC injury, providing a potential unifying pathway for vascular dysfunction in the adverse clinical associations noted with long-term PPI use (Figure).
Figure. Summary of predicted pathophysiology leading to atherosclerotic vascular diseases that can account as the potential unifying factor in the clinical associations noted with long-term proton pump inhibitor (PPI) use.
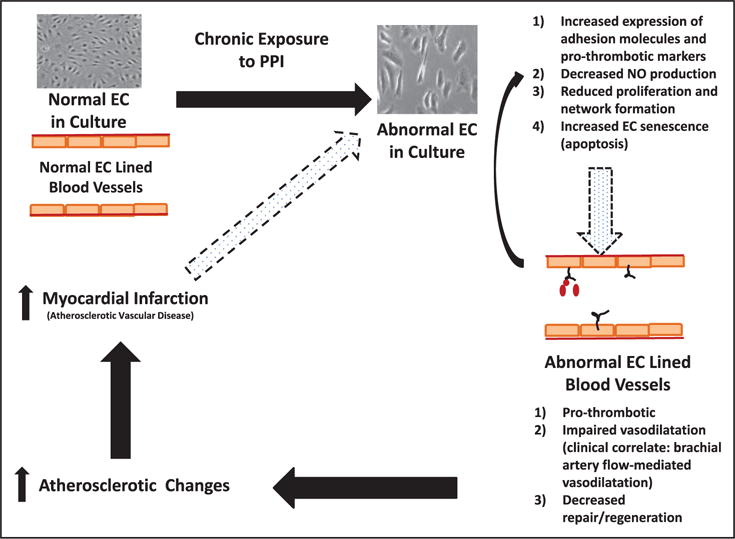
It is important to note the limitation that there are gaps in translating conclusions from observations noted in one type of endothelial cells (EC) in culture to in vivo vascular dysfunction. NO indicates nitric oxide.
Cells in culture are conceptually used to model what happens to the endothelium in a blood vessel wall. A healthy endothelium is a key regulator of normal vascular homeostasis. The healthy endothelium is able to respond adaptively to physical and chemical signals by promoting an anti-inflammatory and thromboresistant vascular surface and by regulating vascular tone by interactions with smooth muscle cells.8 NO is a key endothelium-derived signaling molecule that mediates vasodilatation and a cascade of anti-inflammatory signals.9 Much like the changes from normal EC to abnormal EC in culture, when considered within a blood vessel, endothelial dysfunction can be considered as a switch from a normal phenotype to an abnormal phenotype with impaired NO release and a proinflammatory state with activation of several chemokine/cytokine receptors and adhesion molecules. In most clinically relevant situations, this abnormal versus normal endothelium demonstrate varying degrees of impaired vasoreactivity, increased thombogenicity, and reduced capacity to repair. This creates the milieu for atherosclerotic plaque formation/progression, leading to atherosclerotic vascular diseases, including acute coronary syndromes.10 The concept that a translational highway can take us from EC in a dish to intact blood vessels, however, may not be that simple. There are several areas of caution in traveling this path.
The first potential roadblock comes from drawing the pathway from adverse effects based on observations from one type of EC in culture. Cell culture experiments are well-suited to test specific questions and to validate, or exclude, specific hypotheses regarding an agent, pathway, or condition on a specific (endothelial) cell type. Extrapolating findings from one EC type to a complex disease process may place one on an unstable road. First, even within the human body, ECs are heterogeneous depending on vessel size and location (eg, aortic, venous, carotid, coronary versus other microvasculatures).11 In addition, characteristics of macrovascular ECs can differ from those of microvascular ECs. In in vitro settings, ECs can differ based on origin, the growth conditions used, the presence or absence of serum, the presence or absence of hypoxia, and even just the extent of confluence of the cells in the culture. As such, the generalized applicability of the findings by Yepuri et al7 across different EC types and conditions needs to be determined.
In making the connection from PPI effects on cultured EC to in vivo vascular dysfunction, perhaps the biggest gap is the lack of data showing that in vivo vascular reactivity is altered by chronic PPI use. Ghebremariam et al12 did report that PPIs increase endogenous inhibitor of NO synthase which results in attenuated endothelium-dependent vasodilatation in isolated mouse aorta and in ex vivo human saphenous vein. However, in a pilot study by the same authors, there was no effect of PPI on in vivo flow-mediated vasodilatation as measured by brachial artery flow when subjects were on a PPI for 4 weeks.13 Could the effects on in vivo vascular function be different in patients with longer term PPI use? This is a possibility that should be tested, though the variables in such a potential trial are a bit daunting. Of course, it is also prudent to consider that the brachial artery flow is a measurement of predominantly the macrovascular response, whereas the in vitro findings are in microvascular ECs. Still, the absence of a direct link from altered EC NO production to the brachial-artery vasoreactivity findings raises concern about the pathway (Figure). Circulating endothelial progenitor cell count,14 early senescence, and impaired function15 have implications in prognosis of cardiovascular diseases. As such, another potential study would be to isolate endothelial progenitor cells from patients before and after PPI use and evaluate for evidence for impaired endothelial progenitor cell viability and impaired endothelial progenitor cell function in culture. Such studies would add confidence that the path from cell to the clinical outcomes is well-grooved before invoking wide-spread changes based solely on in vitro findings.
Lysosomes are not limited to ECs. In addition to potential diverse effects across different ECs, it is also important to consider effects on other cell types (ie, vascular smooth muscle or fibroblasts) that play key roles in atherosclerosis. There are examples of stimuli which may injure EC but have beneficial effects on VSMCs and vice versa.16 Studies in nonendothelial cells will help delineate the pathophysiology further. Finally, Yepuri et al7 showed that treatment with PPI impairs the proliferation and network formation in microvascular ECs. In the clinical context of an adult, these in vitro measures are classically relevant to a regenerative/repair process of the endothelium in response to vascular injury. To that extent, it will be important to elucidate the effects of PPI on ECs in the context of cellular injury, such as hypoxia and metabolic deprivation.
PPIs are so commonly used that even small degrees of incremental risk for vascular injury need to be considered strongly. The findings by Yepuri et al7 suggest that a journey from an in vitro system to a unifying clinical outcome is a possibility. As for any planned travel, one needs to verify that the road is intact for the journey.
Acknowledgments
Sources of Funding
This work was supported by the following grants: NHLBI 1R01HL121635-01A1 (B.H. Annex) and American Heart Association 15SDG25490007 (S. Hazarika).
Footnotes
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
Circulation Research is available at http://circres.ahajournals.org
Disclosures
None.
References
- 1.Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ. 2008;336:2–3. doi: 10.1136/bmj.39406.449456.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naunton M, Peterson GM, Bleasel MD. Overuse of proton pump inhibitors. J Clin Pharm Ther. 2000;25:333–340. doi: 10.1046/j.1365-2710.2000.00312.x. [DOI] [PubMed] [Google Scholar]
- 3.Fusaro M, Noale M, Tripepi G, Giannini S, D’Angelo A, Pica A, Calò LA, Miozzo D, Gallieni M. Long-term proton pump inhibitor use is associated with vascular calcification in chronic kidney disease: a cross-sectional study using propensity score analysis. Drug Saf. 2013;36:635–642. doi: 10.1007/s40264-013-0062-6. [DOI] [PubMed] [Google Scholar]
- 4.Gomm W, von Holt K, Thomé F, Broich K, Maier W, Fink A, Doblhammer G, Haenisch B. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73:410–416. doi: 10.1001/jamaneurol.2015.4791. [DOI] [PubMed] [Google Scholar]
- 5.Shah NH, LePendu P, Bauer-Mehren A, Ghebremariam YT, Iyer SV, Marcus J, Nead KT, Cooke JP, Leeper NJ. Proton Pump Inhibitor Usage and the Risk of Myocardial Infarction in the General Population. PLoS One. 2015;10:e0124653. doi: 10.1371/journal.pone.0124653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shih CJ, Chen YT, Ou SM, Li SY, Chen TJ, Wang SJ. Proton pump inhibitor use represents an independent risk factor for myocardial infarction. Int J Cardiol. 2014;177:292–297. doi: 10.1016/j.ijcard.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 7.Yepuri G, Sukhovershin R, Nazari-Shafti TZ, Petrascheck M, Ghebre YT, Cooke JP. Proton pump inhibitors accelerate endothelial senescence. Circ Res. 2016;118:e36–e42. doi: 10.1161/CIRCRESAHA.116.308807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Triggle CR, Ding H, Anderson TJ, Pannirselvam M. The endothelium in health and disease: a discussion of the contribution of non-nitric oxide endothelium-derived vasoactive mediators to vascular homeostasis in normal vessels and in type II diabetes. Mol Cell Biochem. 2004;263:21–27. doi: 10.1023/B:MCBI.0000041845.62061.c9. [DOI] [PubMed] [Google Scholar]
- 9.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 10.Gimbrone MA, Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aird WC. Spatial and temporal dynamics of the endothelium. J Thromb Haemost. 2005;3:1392–1406. doi: 10.1111/j.1538-7836.2005.01328.x. [DOI] [PubMed] [Google Scholar]
- 12.Ghebremariam YT, LePendu P, Lee JC, Erlanson DA, Slaviero A, Shah NH, Leiper J, Cooke JP. Unexpected effect of proton pump inhibitors: elevation of the cardiovascular risk factor asymmetric dimethylarginine. Circulation. 2013;128:845–853. doi: 10.1161/CIRCULATIONAHA.113.003602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghebremariam YT, Cooke JP, Khan F, Thakker RN, Chang P, Shah NH, Nead KT, Leeper NJ. Proton pump inhibitors and vascular function: a prospective cross-over pilot study. Vasc Med. 2015;20:309–316. doi: 10.1177/1358863X14568444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aragona CO, Imbalzano E, Mamone F, Cairo V, Lo Gullo A, D’Ascola A, Sardo MA, Scuruchi M, Basile G, Saitta A, Mandraffino G. Endothelial progenitor cells for diagnosis and prognosis in cardiovascular disease. Stem Cells Int. 2016;2016:8043792. doi: 10.1155/2016/8043792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yiu KH, Tse HF. Specific role of impaired glucose metabolism and diabetes mellitus in endothelial progenitor cell characteristics and function. Arterioscler Thromb Vasc Biol. 2014;34:1136–1143. doi: 10.1161/ATVBAHA.114.302192. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Cheng Y, Yang J, Xu L, Zhang C. Cell-specific effects of miR-221/222 in vessels: molecular mechanism and therapeutic application. J Mol Cell Cardiol. 2012;52:245–255. doi: 10.1016/j.yjmcc.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


