Abstract
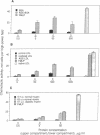
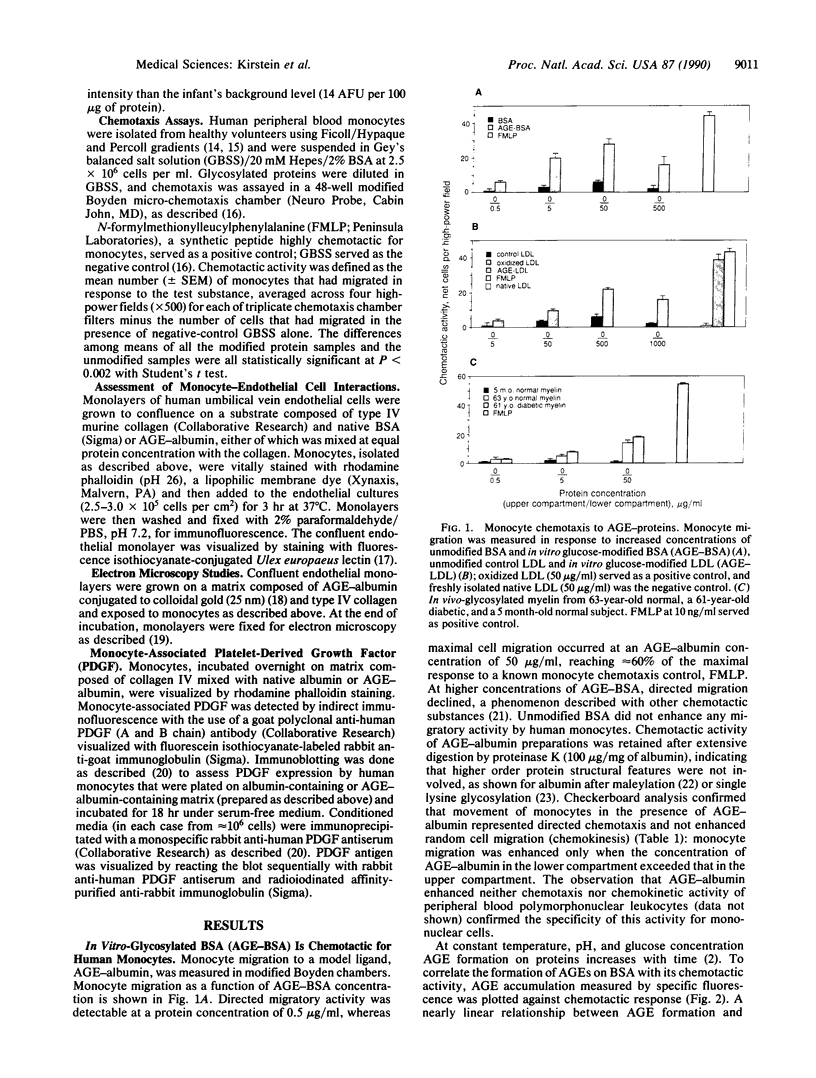
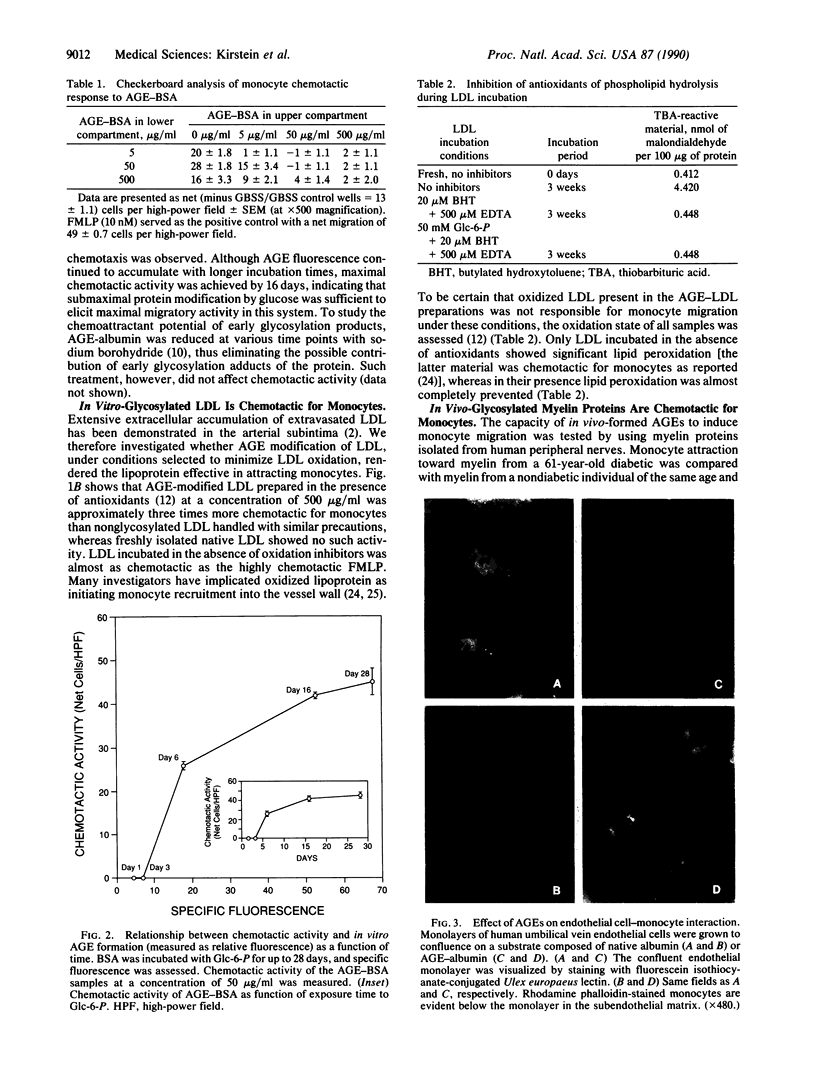
Diabetes and aging are commonly accompanied by arterio- and atherosclerosis. Infiltration of the arterial subendothelial intima by macrophages/monocytes is an important early event preceding the development of atheromatous lesions; these macrophages are known to produce mitogenic factors in early atherosclerotic lesions. It has been previously shown that, over time, vascular matrix accumulates proteins nonenzymatically modified by advanced glycosylation end products (AGEs). In view of the fact that macrophages/monocytes have AGE-specific receptors associated with the expression of several growth factors, we investigated the possibility that AGEs mediate initial monocyte-vessel wall interactions that occur before overt formation of vascular lesions. This study demonstrates that (i) in vitro- and in vivo-formed AGEs are chemotactic for human blood monocytes, (ii) sub-endothelial AGEs can selectively induce monocyte migration across an intact endothelial cell monolayer, and (iii) subsequent monocyte interaction with AGE-containing matrix results in the expression of platelet-derived growth factor. These results support the existing hypothesis that in vivo-forming glucose-derived protein adducts can act as signals for the normal turnover of senescent tissue protein by means of the AGE-specific receptor system. Time-dependent glucose-induced deposition of AGEs on matrix proteins may promote monocyte infiltration into the subendothelium. Subsequent AGE-triggered macrophage activation and consequent elaboration of proliferative factors may normally coordinate remodeling but may also lead to the diverse pathogenic changes typical of arterio- and atherosclerosis in diabetic or aging populations.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brownlee M., Cerami A., Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988 May 19;318(20):1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chung B. H., Wilkinson T., Geer J. C., Segrest J. P. Preparative and quantitative isolation of plasma lipoproteins: rapid, single discontinuous density gradient ultracentrifugation in a vertical rotor. J Lipid Res. 1980 Mar;21(3):284–291. [PubMed] [Google Scholar]
- Esposito C., Gerlach H., Brett J., Stern D., Vlassara H. Endothelial receptor-mediated binding of glucose-modified albumin is associated with increased monolayer permeability and modulation of cell surface coagulant properties. J Exp Med. 1989 Oct 1;170(4):1387–1407. doi: 10.1084/jem.170.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk W., Goodwin R. H., Jr, Leonard E. J. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33(3):239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. Am J Pathol. 1981 May;103(2):191–200. [PMC free article] [PubMed] [Google Scholar]
- Haberland M. E., Rasmussen R. R., Fogelman A. M. Receptor recognition of maleyl-albumin induces chemotaxis in human monocytes. J Clin Invest. 1986 Sep;78(3):827–831. doi: 10.1172/JCI112647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley D. A., Chien S. Colloidal gold: a pluripotent receptor probe. Proc Soc Exp Biol Med. 1983 Oct;174(1):1–11. doi: 10.3181/00379727-174-41697. [DOI] [PubMed] [Google Scholar]
- Holthöfer H., Virtanen I., Kariniemi A. L., Hormia M., Linder E., Miettinen A. Ulex europaeus I lectin as a marker for vascular endothelium in human tissues. Lab Invest. 1982 Jul;47(1):60–66. [PubMed] [Google Scholar]
- Koenig R. J., Cerami A. Hemoglobin A Ic and diabetes mellitus. Annu Rev Med. 1980;31:29–34. doi: 10.1146/annurev.me.31.020180.000333. [DOI] [PubMed] [Google Scholar]
- Kohn R. R., Cerami A., Monnier V. M. Collagen aging in vitro by nonenzymatic glycosylation and browning. Diabetes. 1984 Jan;33(1):57–59. doi: 10.2337/diab.33.1.57. [DOI] [PubMed] [Google Scholar]
- Quinn M. T., Parthasarathy S., Fong L. G., Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci U S A. 1987 May;84(9):2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Masuda J., Raines E. W., Gown A. M., Katsuda S., Sasahara M., Malden L. T., Masuko H., Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. 1990 May 25;248(4958):1009–1012. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Schiffmann E., Corcoran B. A., Wahl S. M. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaklai N., Garlick R. L., Bunn H. F. Nonenzymatic glycosylation of human serum albumin alters its conformation and function. J Biol Chem. 1984 Mar 25;259(6):3812–3817. [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P., Parthasarathy S., Leake D. S., Witztum J. L., Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D., Brett J., Harris K., Nawroth P. Participation of endothelial cells in the protein C-protein S anticoagulant pathway: the synthesis and release of protein S. J Cell Biol. 1986 May;102(5):1971–1978. doi: 10.1083/jcb.102.5.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timonen T., Saksela E. Isolation of human NK cells by density gradient centrifugation. J Immunol Methods. 1980;36(3-4):285–291. doi: 10.1016/0022-1759(80)90133-7. [DOI] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Cerami A. Excessive nonenzymatic glycosylation of peripheral and central nervous system myelin components in diabetic rats. Diabetes. 1983 Jul;32(7):670–674. doi: 10.2337/diab.32.7.670. [DOI] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Cerami A. High-affinity-receptor-mediated uptake and degradation of glucose-modified proteins: a potential mechanism for the removal of senescent macromolecules. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5588–5592. doi: 10.1073/pnas.82.17.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Cerami A. Recognition and uptake of human diabetic peripheral nerve myelin by macrophages. Diabetes. 1985 Jun;34(6):553–557. doi: 10.2337/diab.34.6.553. [DOI] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Manogue K. R., Dinarello C. A., Pasagian A. Cachectin/TNF and IL-1 induced by glucose-modified proteins: role in normal tissue remodeling. Science. 1988 Jun 10;240(4858):1546–1548. doi: 10.1126/science.3259727. [DOI] [PubMed] [Google Scholar]