Abstract
Cell walls from Streptococcus mutans were prepared by conventional technique and subjected to a series of extraction procedures involving classical protein solvents. The extracted walls contained several non-peptidoglycan amino acids and were also amenable to radiolabeling with [125I]sodium iodide and chloramine T. The cell walls could be chemically modified with tetranitromethane and diazo-1H-tetrazole, suggesting the presence of tyrosine or histidine or both. Flourescence spectra of the walls revealed the presence of either tyrosine or tryptophan. Several proteases, including pronase, trypsin, subtilisin, and proteinase K, removed some of the label from the walls. In contrast, treatment of the walls with salts or denaturants did not result in the solubilization of label. When the walls were solubilized with mutanolysin and subjected to chromatography, three peaks of radioactivity with apparent molecular weights of 73,000, 39,000, and 9,600 were observed. Wall digests subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed a single band of radioactivity corresponding to an apparent molecular weight of 79,000. Isoelectric focusing of labeled wall digest gave rise to two major bands of radioactivity with isoelectric points of approximately 2.4 and 5.6. The results suggest that the cell wall of S. mutans contains tightly and possibley covalently bound polypeptide molecules. We propose that the cell wall polypeptides of S. mutans serve as factors in the attachment of the bacteria to smooth surfaces.
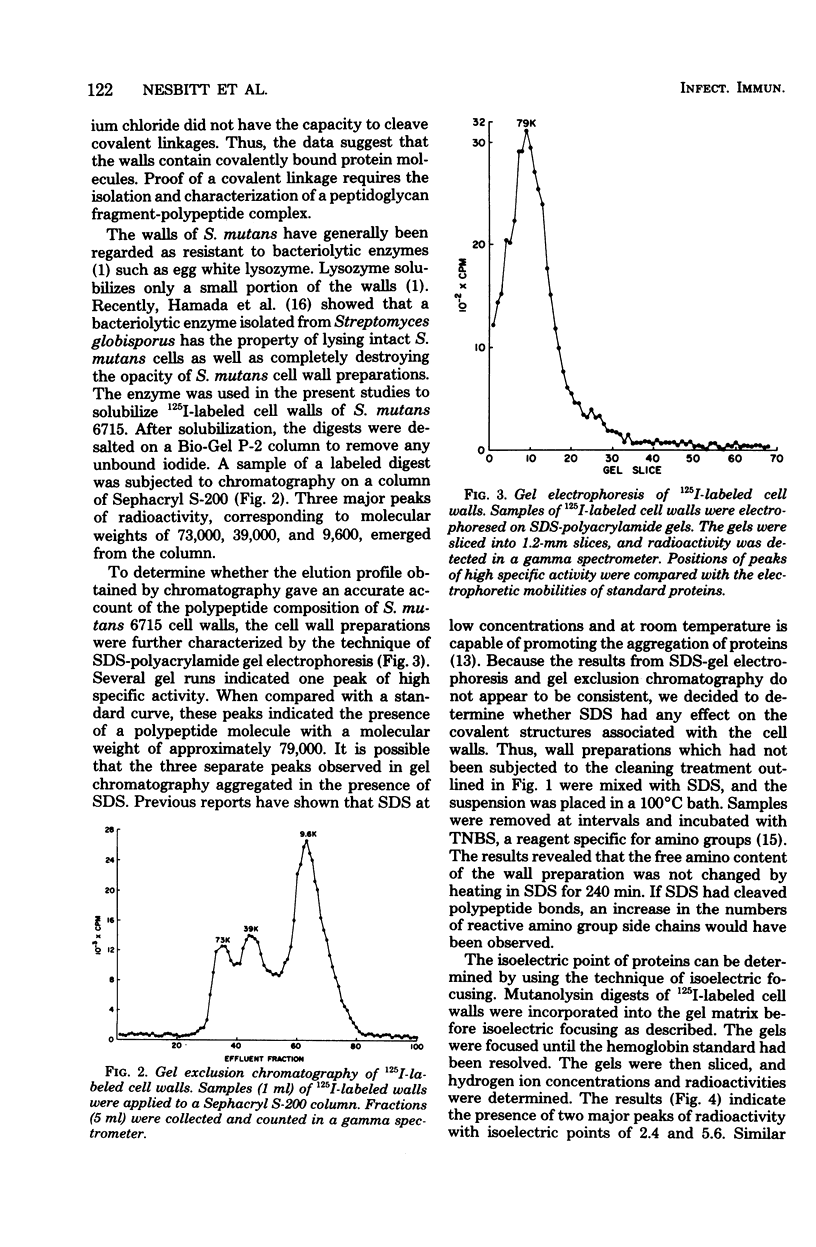
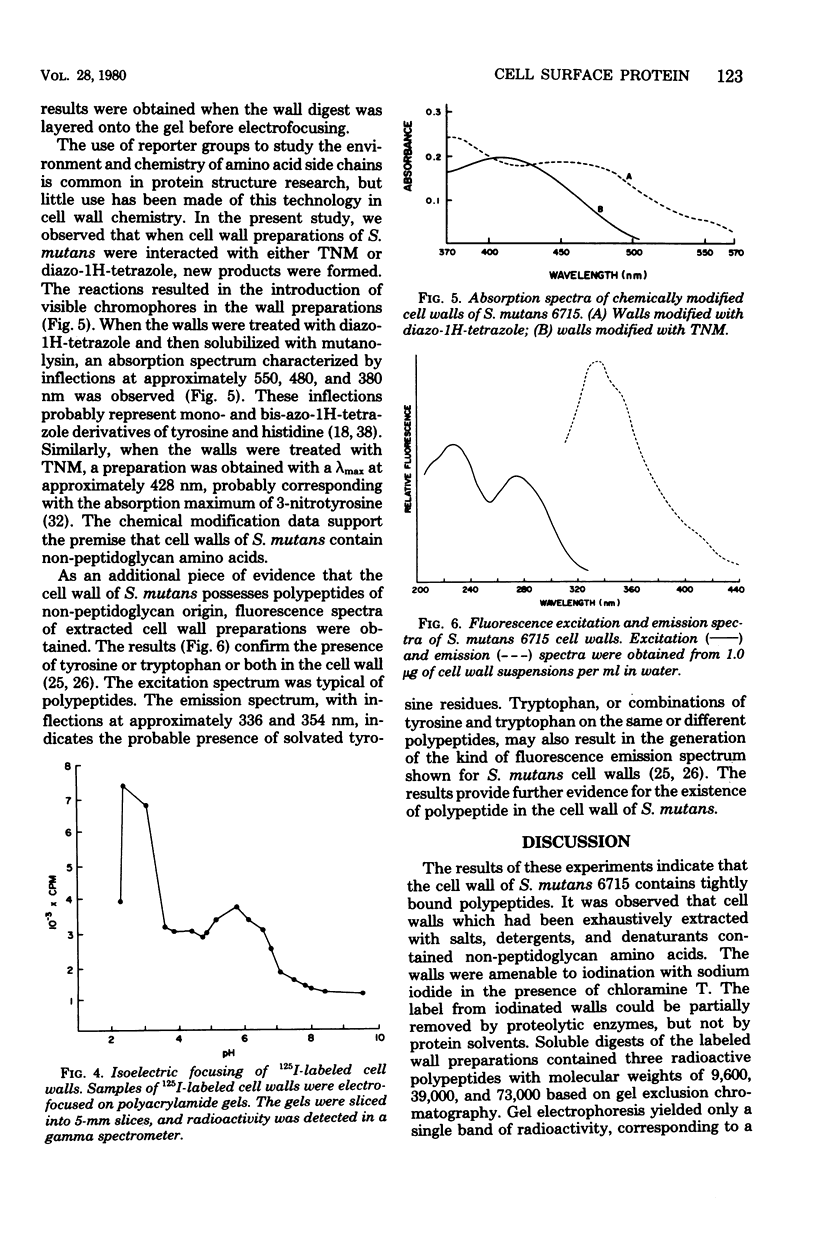
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bleiweis A. S., Craig R. A., Zinner D. D., Jablon J. M. Chemical composition of purified cell walls of cariogenic streptococci. Infect Immun. 1971 Jan;3(1):189–191. doi: 10.1128/iai.3.1.189-191.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta. 1975 Oct 31;415(3):335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- Cooper H. R., Chorpenning F. W., Rosen S. Preparation and chemical composition of the cell walls of Streptococcus mutans. Infect Immun. 1975 Apr;11(4):823–828. doi: 10.1128/iai.11.4.823-828.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle R. J., Bello J., Roholt O. A. Probable protein crosslinking with tetranitromethane. Biochim Biophys Acta. 1968 Jun 26;160(2):274–276. doi: 10.1016/0005-2795(68)90103-7. [DOI] [PubMed] [Google Scholar]
- Doyle R. J., Streips U. N., Fan V. S., Brown W. C., Mobley H., Mansfield J. M. Cell wall protein in Bacillus subtilis. J Bacteriol. 1977 Jan;129(1):547–549. doi: 10.1128/jb.129.1.547-549.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker D. B., Shuttleworth C. A., Melville T. H. A quantitative analysis of the cell wall amino acids of cariogenic and non-cariogenic streptococci. Arch Oral Biol. 1968 Aug;13(8):937–940. doi: 10.1016/0003-9969(68)90009-5. [DOI] [PubMed] [Google Scholar]
- Drysdale J. W., Righetti P., Bunn H. F. The separation of human and animal hemoglobins by isoelectric focusing in polyacrylamide gel. Biochim Biophys Acta. 1971 Jan 19;229(1):42–50. doi: 10.1016/0005-2795(71)90315-1. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F. Streptococcus mutans dextransucrase: mode of interaction with high-molecular-weight dextran and role in cellular aggregation. Infect Immun. 1976 Feb;13(2):365–372. doi: 10.1128/iai.13.2.365-372.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Glew R. H., Doyle R. J. Protection of sodium dodecyl sulfate-induced aggregation of concanalvalin A by saccharide ligands. Carbohydr Res. 1979 Aug;73:219–226. doi: 10.1016/s0008-6215(00)85491-9. [DOI] [PubMed] [Google Scholar]
- HORINISHI H., HACHIMORI Y., KURIHARA K., SHIBATA K. STATES OF AMINO ACID RESIDUES IN PROTEINS. 3. HISTIDINE RESIDUES IN INSULIN, LYSOZYME, ALBUMIN AND PROTEINASES AS DETERMINED WITH A NEW REAGENT OF DIAZO-I-H-TETRAZOLE. Biochim Biophys Acta. 1964 Jun 8;86:477–489. [PubMed] [Google Scholar]
- Habeeb A. F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966 Mar;14(3):328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- Hamada S., Torii M., Kotani S., Masuda N., Ooshima T., Yokogawa K., Kawata S. Lysis of Streptococcus mutans cells with mutanolysin, a lytic enzyme prepared from a culture liquor of Streptomyces globisporus 1829. Arch Oral Biol. 1978;23(7):543–549. doi: 10.1016/0003-9969(78)90268-6. [DOI] [PubMed] [Google Scholar]
- Inoue M., Hamada S., Ooshima T., Kotani S., Kato K. Chemical composition of Streptococcus mutans cell walls and their susceptibility to Flavobacterium L-11 enzyme. Microbiol Immunol. 1979;23(5):319–328. doi: 10.1111/j.1348-0421.1979.tb00469.x. [DOI] [PubMed] [Google Scholar]
- Kelstrup J., Funder-Nielsen T. D. Adhesion of dextran to Streptococcus mutans. J Gen Microbiol. 1974 Apr;81(2):485–489. doi: 10.1099/00221287-81-2-485. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H. K., Ingersoll L. Interaction of glucosyltransferase with the cell surface of Streptococcus mutans. Infect Immun. 1978 Jun;20(3):652–659. doi: 10.1128/iai.20.3.652-659.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Schauer S. V. Studies on the bacterial components which bind Streptococcus sanguis and Streptococcus mutans to hydroxyapatite. Arch Oral Biol. 1975 Sep;20(9):609–615. doi: 10.1016/0003-9969(75)90082-5. [DOI] [PubMed] [Google Scholar]
- Longworth J. W. Conformations and interactions of excited states. II. Polystyrene, polypeptides, and proteins. Biopolymers. 1966 Dec;4(10):1131–1148. doi: 10.1002/bip.1966.360041009. [DOI] [PubMed] [Google Scholar]
- McCabe M. M., Hamelik R. M. Multiple forms of dextran-binding proteins from Streptococcus mutans. Adv Exp Med Biol. 1978;107:749–759. doi: 10.1007/978-1-4684-3369-2_84. [DOI] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E. Relationship between cell-bound dextransucrase and the agglutination of Streptococcus mutans. Infect Immun. 1975 Sep;12(3):512–520. doi: 10.1128/iai.12.3.512-520.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. I. Roles of insoluble dextran-levan synthetase enzymes and cell wall polysaccharide antigen in plaque formation. Infect Immun. 1973 Oct;8(4):555–562. doi: 10.1128/iai.8.4.555-562.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. II. Nature of the binding site and the adsorption of dextran-levan synthetase enzymes on the cell-wall surface of the streptococcus. Infect Immun. 1974 Feb;9(2):419–429. doi: 10.1128/iai.9.2.419-429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Structure and immunological specificity of the Streptococcus mutans group b cell wall antigen. Infect Immun. 1973 Apr;7(4):578–585. doi: 10.1128/iai.7.4.578-585.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan J. F., Sokolovsky M., Vallee B. L. The functional tyrosyl residues of carboxypeptidase A. Nitration with tetranitromethane. Biochemistry. 1967 Nov;6(11):3609–3617. doi: 10.1021/bi00863a036. [DOI] [PubMed] [Google Scholar]
- Rosan B. Determination of muramic acid in automated amino acid analysis. Anal Biochem. 1972 Aug;48(2):624–628. doi: 10.1016/0003-2697(72)90119-4. [DOI] [PubMed] [Google Scholar]
- Rosan B. Relationship of the cell wall composition of group H streptococci and Streptococcus sanguis to their serological properties. Infect Immun. 1976 Apr;13(4):1144–1153. doi: 10.1128/iai.13.4.1144-1153.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. R. Wall-associated protein antigens of Streptococcus mutans. J Gen Microbiol. 1979 Sep;114(1):109–115. doi: 10.1099/00221287-114-1-109. [DOI] [PubMed] [Google Scholar]
- Sjöquist J., Meloun B., Hjelm H. Protein A isolated from Staphylococcus aureus after digestion with lysostaphin. Eur J Biochem. 1972 Sep 25;29(3):572–578. doi: 10.1111/j.1432-1033.1972.tb02023.x. [DOI] [PubMed] [Google Scholar]
- Sjöquist J., Movitz J., Johansson I. B., Hjelm H. Localization of protein A in the bacteria. Eur J Biochem. 1972 Oct 17;30(1):190–194. doi: 10.1111/j.1432-1033.1972.tb02086.x. [DOI] [PubMed] [Google Scholar]
- Sokolovsky M., Vallee B. L. Azocarboxypeptidase: functional consequences of tyrosyl and histidyl modification. Biochemistry. 1967 Mar;6(3):700–708. doi: 10.1021/bi00855a008. [DOI] [PubMed] [Google Scholar]
- Staat R. H., Doyle R. J., Langley S. D., Suddick R. P. Modification of in vitro adherence of Streptococcus mutans by plant lectins. Adv Exp Med Biol. 1978;107:639–647. doi: 10.1007/978-1-4684-3369-2_72. [DOI] [PubMed] [Google Scholar]
- Staat R. H., Langley S. D., Doyle R. J. Streptococcus mutans adherence: presumptive evidence for protein-mediated attachment followed by glucan-dependent cellular accumulation. Infect Immun. 1980 Feb;27(2):675–681. doi: 10.1128/iai.27.2.675-681.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallinder I. B., Neujahr H. Y. CELL WALL AND PEPTIDOGLYCAN FROM Lactobacillus fermenti. J Bacteriol. 1971 Mar;105(3):918–926. doi: 10.1128/jb.105.3.918-926.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wietzerbin-Falszpan J., Das B. C., Gros C., Petit J. F., Lederer E. The amino acids of the cell wall of Mycobacterium tuberculosis var. bovis, strain BCG. Presence of a poly(L-glutamic acid). Eur J Biochem. 1973 Feb 1;32(3):525–532. doi: 10.1111/j.1432-1033.1973.tb02637.x. [DOI] [PubMed] [Google Scholar]
- Wolff J., Covelli I. Factors in the iodination of histidine in proteins. Eur J Biochem. 1969 Jun;9(3):371–377. doi: 10.1111/j.1432-1033.1969.tb00618.x. [DOI] [PubMed] [Google Scholar]
- Yokogawa K., Kawata S., Nishimura S., Ikeda Y., Yoshimura Y. Mutanolysin, bacteriolytic agent for cariogenic Streptococci: partial purification and properties. Antimicrob Agents Chemother. 1974 Aug;6(2):156–165. doi: 10.1128/aac.6.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Ichihara S., Mizushima S. A major outer membrane protein (O-8) of Escherichia coli K-12 exists as a trimer in sodium dodecyl sulfate solution. FEBS Lett. 1979 Apr 1;100(1):71–74. doi: 10.1016/0014-5793(79)81133-3. [DOI] [PubMed] [Google Scholar]


