Abstract
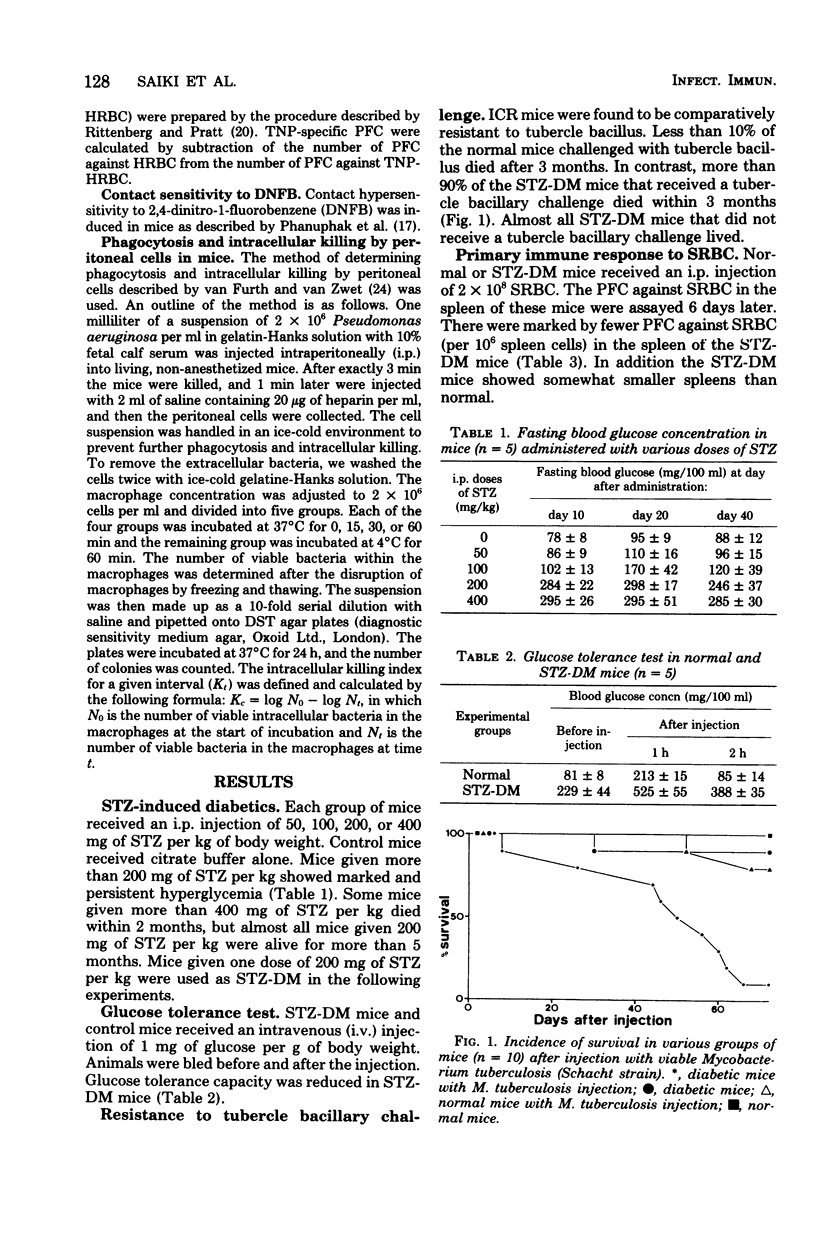
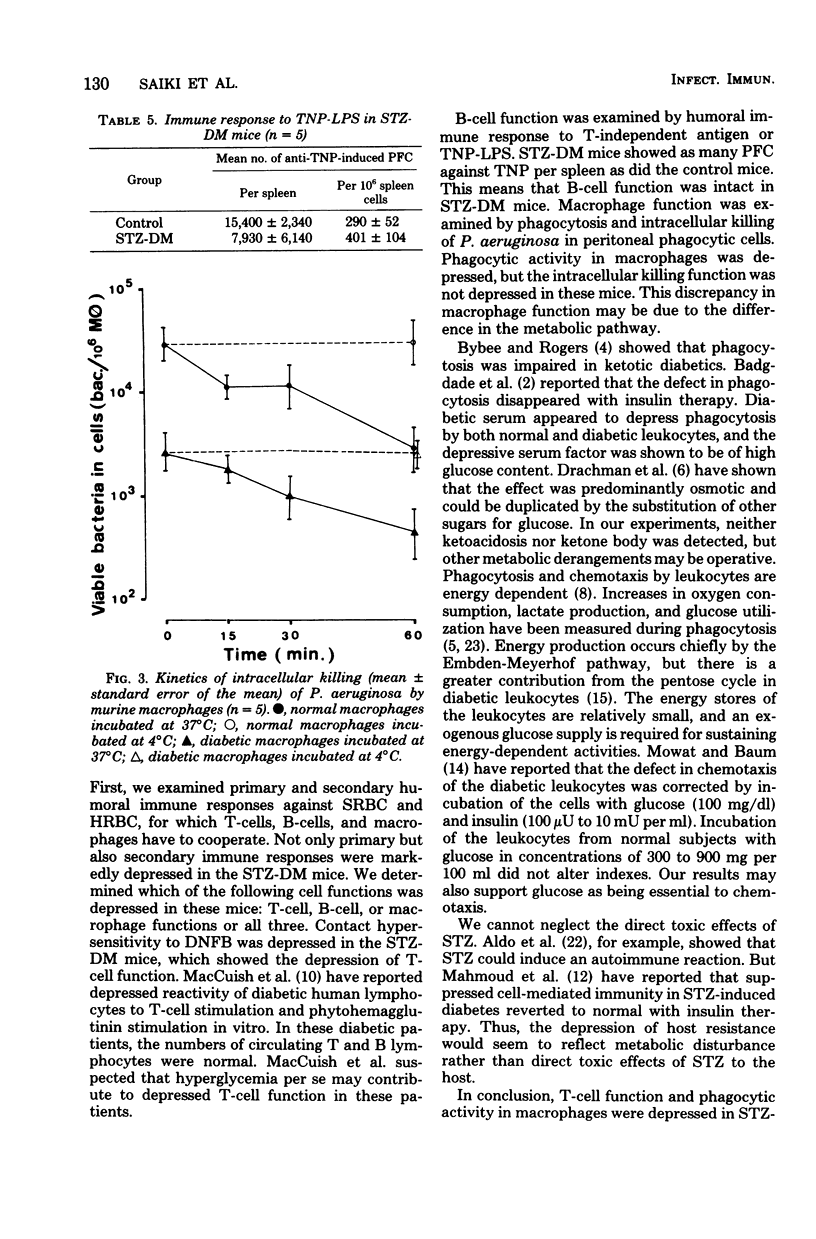
Persistent diabetes mellitus with marked hyperglycemia was induced in mice by the administration of streptozotocin. In these streptozotocin-induced diabetic mice, resistance to tubercle bacillus challenge and primary as well as secondardy humoral immune responses against foreign erythrocytes were markedly depressed. The T-cell function in delayed hypersensitivity to 2,4-dinitro-1-fluorobenzene and bacterial phagocytic activity or peritoneal macrophages were markedly depressed. In contrast, the B-cell function in antibody production against T-independent antigen and the intracellular killing of bacteria in peritoneal macrophages were intact. We concluded that depression of the T-cell function or the phagocytic activity of macrophages or both may be the main immunological defect in these mice.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson T., Schein P. S., McMenamin M. G., Cooney D. A. Streptozotocin diabetes. Correlation with extent of depression of pancreatic islet nicotinamide adenine dinucleotide. J Clin Invest. 1974 Sep;54(3):672–677. doi: 10.1172/JCI107805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BYBEE J. D., ROGERS D. E. THE PHAGOCYTIC ACTIVITY OF POLYMORPHONUCLEAR LEUKOCYTES OBTAINED FROM PATIENTS WITH DIABETES MELLITUS. J Lab Clin Med. 1964 Jul;64:1–13. [PubMed] [Google Scholar]
- Bagdade J. D., Root R. K., Bulger R. J. Impaired leukocyte function in patients with poorly controlled diabetes. Diabetes. 1974 Jan;23(1):9–15. doi: 10.2337/diab.23.1.9. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., MORSE S. I. Functional and metabolic properties of polymorphonuclear leucocytes. I. Observations on the requirements and consequences of particle ingestion. J Exp Med. 1960 May 1;111:667–687. doi: 10.1084/jem.111.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman R. H., Root R. K., Wood W. B., Jr Studies on the effect of experimental nonketotic diabetes mellitus on antibacterial defense. I. Demonstration of a defect in phagocytosis. J Exp Med. 1966 Aug 1;124(2):227–240. doi: 10.1084/jem.124.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINGSLEY G. R., GETCHELL G. Direct ultramicro glucose oxidase method for determination of glucose in biologic fluids. Clin Chem. 1960 Oct;6:466–475. [PubMed] [Google Scholar]
- Karnovsky M. L. The metabolism of leukocytes. Semin Hematol. 1968 Apr;5(2):156–165. [PubMed] [Google Scholar]
- MacCuish A. C., Urbaniak S. J., Campbell C. J., Duncan L. J., Irvine W. J. Phytohemagglutinin transformation and circulating lymphocyte subpopulations in insulin-dependent diabetic patients. Diabetes. 1974 Aug;23(8):708–712. doi: 10.2337/diab.23.8.708. [DOI] [PubMed] [Google Scholar]
- Mahmoud A. A., Cheever A. W., Warren K. S. Streptozotocin-induced diabetes mellitus and the host-parasite relation in murine schistosomiasis mansoni. J Infect Dis. 1975 Jun;131(6):634–642. doi: 10.1093/infdis/131.6.634. [DOI] [PubMed] [Google Scholar]
- Mahmoud A. A., Rodman H. M., Mandel M. A., Warren K. S. Induced and spontaneous diabetes mellitus and suppression of cell-mediated immunologic responses. Granuloma formation, delayed dermal reactivity and allograft rejection. J Clin Invest. 1976 Feb;57(2):362–367. doi: 10.1172/JCI108287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat A., Baum J. Chemotaxis of polymorphonuclear leukocytes from patients with diabetes mellitus. N Engl J Med. 1971 Mar 25;284(12):621–627. doi: 10.1056/NEJM197103252841201. [DOI] [PubMed] [Google Scholar]
- Munroe J. F., Shipp J. C. Glucose metabolism in leucocytes from patients with diabetes mellitus, with and without hypercholesteremia. Diabetes. 1965 Sep;14(9):584–590. doi: 10.2337/diab.14.9.584. [DOI] [PubMed] [Google Scholar]
- PERILLIE P. E., NOLAN J. P., FINCH S. C. Studies of the resistance to infection in diabetes mellitus: local exudative cellular response. J Lab Clin Med. 1962 Jun;59:1008–1015. [PubMed] [Google Scholar]
- Phanuphak P., Moorhead J. W., Claman H. N. Tolerance and contact sensitivity to DNFB in mice. I. In vivo detection by ear swelling and correlation with in vitro cell stimulation. J Immunol. 1974 Jan;112(1):115–123. [PubMed] [Google Scholar]
- Richardson R. IMMUNITY IN DIABETES: INFLUENCE OF DIABETES ON THE DEVELOPMENT OF ANTIBACTERIAL PROPERTIES IN THE BLOOD. J Clin Invest. 1933 Nov;12(6):1143–1149. doi: 10.1172/JCI100565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg M. B., Amkraut A. A. Immunogenicity of trinitrophenyl-hemocyanin: production of primary and secondary anti-hapten precipitins. J Immunol. 1966 Sep;97(3):421–430. [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Polk H. C., Jr The mechanism of infection in patients with diabetes mellitus: a review of leukocyte malfunction. Surgery. 1974 Jan;75(1):123–128. [PubMed] [Google Scholar]
- Rossini A. A., Like A. A., Chick W. L., Appel M. C., Cahill G. F., Jr Studies of streptozotocin-induced insulitis and diabetes. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2485–2489. doi: 10.1073/pnas.74.6.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj R. J., McRipley R. J., Sbarra A. J. The effect of phagocytosis and X-irradiation on human leukocyte metabolism. Cancer Res. 1967 Dec;27(12):2280–2286. [PubMed] [Google Scholar]



