Abstract
Atopic dermatitis (AD) is a common inflammatory skin disease affecting up to 20% of children and 3% of adults worldwide and is associated with dysregulation of the skin barrier. While type 2 responses are implicated in AD, emerging evidence indicates potential role for the IL-17A signalling axis in AD pathogenesis. In this study we show that in the filaggrin mutant mouse model of spontaneous AD, IL-17RA deficiency (Il17ra-/-) resulted in severe exacerbation of skin inflammation. Interestingly, Il17ra-/- mice without the filaggrin mutation also developed spontaneous progressive skin inflammation with eosinophilia, increased levels of thymic stromal lymphopoietin (TSLP) and IL-5 in the skin. Il17ra-/- mice have a defective skin barrier with altered filaggrin expression. The barrier dysregulation and spontaneous skin inflammation in Il17ra-/- mice was dependent on TSLP, but not the other alarmins IL-25 and IL-33. The associated skin inflammation was mediated by IL-5 expressing pathogenic effector (pe) Th2 cells and was independent of TCRγδ T cells and IL-22. An absence of IL-17RA in non-hematopoietic cells, but not in the hematopoietic cells, was required for the development of spontaneous skin inflammation. Skin microbiome dysbiosis developed in the absence of IL-17RA, with antibiotic intervention resulting in significant amelioration of skin inflammation and reductions in skin infiltrating peTh2 cells and TSLP. This study describes a previously unappreciated protective role for IL-17RA signalling in regulation of the skin barrier and maintenance of skin immune homeostasis.
Introduction
Atopic dermatitis (AD) is frequently recognized as the first manifestation of allergic disease in early life, with 15-30% of children affected in the developed world (1). The development of AD is dependent on interplay between genetic and environmental factors involving a disruption in skin homeostasis altering the functional integrity of the skin and leading to aberrant inflammation. In AD patients, inflammation in the acute lesions is associated with infiltration of IL-4, IL-5 and IL-13 expressing CD4+ T helper (h) 2 cells, eosinophils (2) and type 2 innate lymphoid cells. While this is indicative of a type 2 atopic inflammation, there is a recognition that other cytokines, including IL-17A and IL-22, may also contribute to the pathogenesis of AD (2). Dysregulation of the IL-17A/IL-17RA axis is implicated in inflammatory skin diseases, with antibodies against IL-17A and IL-17RA showing significant efficacy for the treatment of psoriasis (3). In AD patients, the increased expression of IL-17A in skin lesions raises the potential to target IL-17RA and its ligands IL-17A and IL-17F as a possible therapeutic strategy (4, 5).
In a subgroup of AD patients, defects in the integrity of the skin barrier arise due to mutations in the filaggrin (FLG) encoding gene, with the filaggrin protein having a central role in normal skin barrier function and barrier integrity maintenance (6). The Flaky tail mouse strain, which has natural mutations in Flg and also Tmem79, develops AD-like skin inflammation analogous to the inflammation that occurs in AD patients with FLG mutations (7–9). Recently, we generated a mouse strain with only the Flg mutation, that has a compromised skin barrier and develops spontaneous AD-like inflammation when maintained on the Th2/17 prone BALB/c strain background, with skin inflammation characterized by elevated Th2 cytokines, as well as elevated IL-17A (10). In this study, it was noted that when Flg mutant mice were crossed to IL-17RA deficient mice, they developed severely exacerbated allergic skin inflammation. Unexpectedly, Il17ra-/- mice, in the absence of the Flg mutation, also developed spontaneous and progressive skin inflammation. IL-17RA deficient mice had aberrant filaggrin expression in the skin, a defective skin barrier and cutaneous inflammation associated with skin eosinophilia and elevated IL-5 and TSLP, with no contribution from IL-22 or TCRγδ+ T cells. The development of allergic skin inflammation due to IL-17RA deficiency was dependent on CD4+ T cells with a significant role for IL-5 producing pathogenic effector Th2 (peTh2) cells. Interestingly, absence of IL-17RA in the non-hematopoietic, but not in the hematopoietic compartment, was required for the induction of skin inflammation. Deficiency in TSLP mediated signals, but not IL-25 or IL-33, restored barrier homeostasis and protected from the development of spontaneous allergic skin inflammation. IL-17RA deficiency also resulted in skin microbiome dysbiosis, while antibiotic treatment ameliorated skin inflammation and led to a significant reduction in the frequency of peTh2 cells. These results uncover a novel role of IL-17RA signalling in maintaining barrier integrity and immune system homeostasis in the skin.
Materials and Methods
Mice
All mice were on a congenic BALB/c strain with BALB/c mice used as wild-type (WT) controls. Il17ra-/- mice on a C57BL/6 background were provided by Amgen (Amgen Thousand Oaks, California, USA) and backcrossed in-house to BALB/c. Rag1-deficient (Rag1-/-), Il22-deficient (Il22-/-), TCRδ-deficient (TCRδ-/-) mice were obtained from Jackson Laboratories (Jackson Laboratory Bar Harbor, ME, US). Il5-cerulean florescent protein (Il5cfp/cfp (10)), filaggrin mutant (Flgft/ft (10)), IL-17BR deficient (Il17br-/-), IL-33 deficient (Il33cit/cit(11)), IL-33 receptor deficient (Il1rl1-/-), filaggrin deficient (Flg-/- (12)) and mice deficient in Il4, Il5, Il9 and Il13 (Il4/5/9/13-/-(13)) were described previously. Il17ra-/- mice were crossed to Flgft/ft, Rag1-/-, Tslpr-/-, Il17br-/-, Il1rl1-/-, Il33cit/cit, Il17br-/-, Il22-/-, TCRδ-/- mice to generate dual deficient mice. Mice were housed in SPF conditions, with irradiated diet, bedding, and water ad libitum. All animal experiments were performed in compliance with the Irish Department of Health and Children and approved by the Trinity College Dublin’s BioResources ethical review board.
Clinical scoring of skin
Clinical skin severity of inflammation was scored blindly using macroscopic criteria as previously described (10). Briefly, scores of 0, none; 1, mild; 2, moderate; and 3, severe was applied to the signs of edema, erythema, scaling, and erosion. The total score for each mouse, maximum score of 12, was calculated from the sum of individual scores for each parameter.
Histology
Skin tissue samples were placed in 4% neutral buffered formalin. Following fixation samples were processed in paraffin wax, sectioned and stained by eosin and haematoxylin. Epidermal thickness and cell infiltration were analysed as described (10). Images were acquired using a Leica microsystems DM3000 LED microscope with an attached DFC495 camera; image analysis was performed on LAS v4.0 software (Leica microsystems).
MC903 treatment
MC903 (Calcipotriol) was dissolved in ethanol; mice were treated daily for 4 consecutive days as previously described (14). Briefly, 25μl with a total of 4nmol of MC903 were applied on one ear; the second ear was used as an internal control and was treated with ethanol only. Mice were scored daily and were sacrificed 24 hours post the final treatment with MC903.
ELISA
The detection of cytokines (IL-4, IL-5, IL-13, IL-17A, IL-22, IL-1β, IL-25, IL-33 and TSLP) from culture supernatant or tissue lysates was performed via ELISA according to manufacturers (R&D Systems) instructions.
RNA isolation and real-time PCR
RNA was isolated from skin of 5-week non-inflamed and 12-week inflamed Il17ra-/- mice and sex- and age-matched controls. RNA isolation was performed by using the RNeasy kit (Qiagen, UK) and was followed by reverse transcription with the Quantitect reverse transcription kit incorporating a genomic DNA elimination step (Qiagen) as per the manufacturer’s instructions. Real-time quantitative PCR was performed on an AB StepOnePLus Real-time PCR system (Life Technologies, UK), using predesigned TaqMan gene expression assays specific for murine Tslp (Mm00498739_m1), Il25 (Mm00499822_m1), Il33 (Mm01195784_m1), S100a7 (Mm01218201_m1), S100a8 (Mm00496696_m1), S100a9 (Mm00656925_m1) Defb2 (Mm00657074_m1) and Cramp (Mm00438285_m1). Specific gene expression was normalized to murine glyceraldehyde-3-phosphate dehydrogenase (VIC probe, 4352339E; Life Technologies, UK). Fold expression was calculated by using the comparative cycle threshold method of analysis and is presented as relative quantification.
Trans-epidermal water loss (TEWL)
The measurement of TEWL was performed on ear skin of mice using a Courage and Khazaka Tewameter TM210 (Enviroderm, Evesham, UK), as described previously (7). TEWL was recorded at ambient temperature 19-21°C and humidity 50% ± 5.
Western blotting
Western blot analysis of skin for filaggrin detection was performed as previously described (10). In brief, epidermis was separated from dermis following incubation in PBS with 5 mM EDTA at 56°C. Epidermis was homogenized in protein extraction buffer (50 mM Tris, 8 M Urea, pH 7.6 with HALT protease inhibitor cocktail (Thermo Scientific)) using an Ultra-Turrax. Lysates were clarified by centrifugation (13,000 g, 4°C for 15 min). Proteins were resolved by SDS-PAGE and transferred onto a PVDF membrane (Thermo Scientific). Membrane-bound proteins were incubated with primary rabbit anti-mouse filaggrin antibody (Covance) and secondary HRP-conjugated goat anti-rabbit immunoglobulins (Dako). Enhanced chemiluminescence substrate (Thermo Scientific) was used to visualise membrane bound filaggrin.
Primary keratinocyte culture
Culture of primary murine keratinocytes was performed as previously described (15). Briefly, the epidermis was separated from the dermis of neonatal mouse skin after overnight incubation at 4°C in 0.25% trypsin (Lonza). Cells were resuspended in KGM-2 BulletKit media (Lonza), adjusted to 0.05 mmol/L Ca2+, supplemented with penicillin-streptomycin (50 U/mL, 50 μg/mL; Gibco). Cells were plated on fibronectin/collagen-coated 6-well plates and cultured at 36°C at 5% CO2 for 4 days, or to 90% confluence. Keratinocytes were differentiated in KGM-2 BulletKit media adjusted to 0.5 mmol/L Ca2+. Cells were harvested for analysis at 72 hours following the initiation of differentiation, and were analysed by western blot for filaggrin expression.
Cell transfer
CD4+ T cells were isolated from BALB/c mice or from the indicated donor mice by negative selection using magnetic separation on an AutoMacs pro-cell separator (Miltenyi Biotec) according to manufacturer’s instructions. A total of 1 x 107 CD4+ cells, >92% purity, were transferred intravenously to Il17ra-/-Rag1-/- recipient mice.
Flow cytometry
Single cell suspension was prepared and Fc receptors were blocked with anti-CD16/anti-CD32 (24G2, eBioscience) in a 2% FBS in PBS solution. Cells were incubated with combinations of the following anti-mouse antibodies against: CD3 (145-2C11), CD4 (GK1.5), CD45.2 (104), CD193 (J073E5) (Biolegend), SiglecF (E50-2440; BD Biosciences). Samples were stained with a fixable live dead stain (Aqua, Thermo Scientific) or Propidium Iodide (Sigma-Aldrich). For intracellular staining, cells were fixed and permeabilised using a commercial intracellular staining kit Cytofix/Cytoperm (BD Bioscience) according to manufacturer’s instructions. Cells were stained with the following anti-mouse cytokine antibodies for IL-4 (11B11), IL-13 (eBio13A) (eBiosciences), IL-5 (TRFK5) (BD Biosciences). All samples were acquired using a Beckman Coulter Cyan ADP using Summit (v4.3) while analysis was performed with FlowJo (v8.87).
Cell stimulation and intracellular cytokine staining
All cell cultures were performed in the presence of RPMI-1640 (Invitrogen) supplemented with 10% heat-inactivated FBS (Sigma Aldrich), 2 mmol L-glutamine (Invitrogen) 50 IU/ml penicillin and 50 μg/ml streptomycin (Invitrogen). Cells were stimulated in vitro with plate-bound anti-CD3 (10 μg/ml; 2C11, BD) and anti-CD28 mAb (2 μg/ml; 37.51, BD) for 72 hours. Followed by 50 ng/ml PMA and 1 μg/ml ionomycin for 1 hour prior to a 4 hour incubation with 2 μM monensin (eBiosciences) supplemented media. Cell culture supernatants were collected and cells were stained for flow cytometry analysis as described above.
Bone marrow chimeras
Bone marrow chimeric mice were generated as previously described (16). In brief, donor bone marrow cells were prepared from Il17ra-/- mice, or WT mice. 2 x 107 bone marrow cells were reconstituted in 100 μl PBS and intravenously injected into sub-lethally irradiated (900 rad in two doses of 500 and 400 rad) WT or Il17ra-/- mice. Bone marrow chimeric mice were scored weekly for skin inflammation and weight changes.
Skin microbiome analysis
Skin microbiome samples were collected by directly exposing sterile swabs to ear skin of Il17ra-/- and sex- and age-matched control mice. The same surface area was sampled for all mice and in order to avoid sample cross contamination fresh sterile gloves were used for each collection. Samples were instantly frozen in liquid nitrogen and 16S ribosomal RNA gene sequencing and microbiome community analysis was performed by Second Genome (San Francisco USA), as described previously (17).
Antibiotic treatment and co-housing studies
Mice were treated with a broad-spectrum antibiotic treatment protocol, as described previously (18). Briefly, 12-week old female WT and Il17ra-/- mice were treated daily by oral gavage for 10 consecutive days with vancomycin (0.5mg/ml), neomycin (1mg/ml), metronidazole (1mg/ml), ampicillin (1mg/ml) and gentamicin (1mg/ml) (Sigma-Aldrich) prepared in 0.2ml of autoclaved water. Control mice received 0.2ml of autoclaved water daily by oral gavage. Microbiome transfer was achieved by co-housing WT mice that had been treated for 10 days with the antibiotic regime, as above, with untreated Il17ra-/- mice as microbiome donors for eight weeks. As a control group, WT mice treated with antibiotics were co-housed with untreated WT mice as microbiome donor mice. Mice were scored weekly for skin inflammation, as above.
Statistical analyses
Data are expressed as mean ± SEM and were analyzed by two-way analysis of variance (ANOVA) test or unpaired Student's t-tests (Prism 6; GraphPad Software). Significance for all statistical tests was shown in figures as P < 0.05 (*), P < 0.01 (**), P < 0.001 (***) and P < 0.0001 (****).
Results
IL-17RA deficiency leads to spontaneous progressive allergic skin inflammation
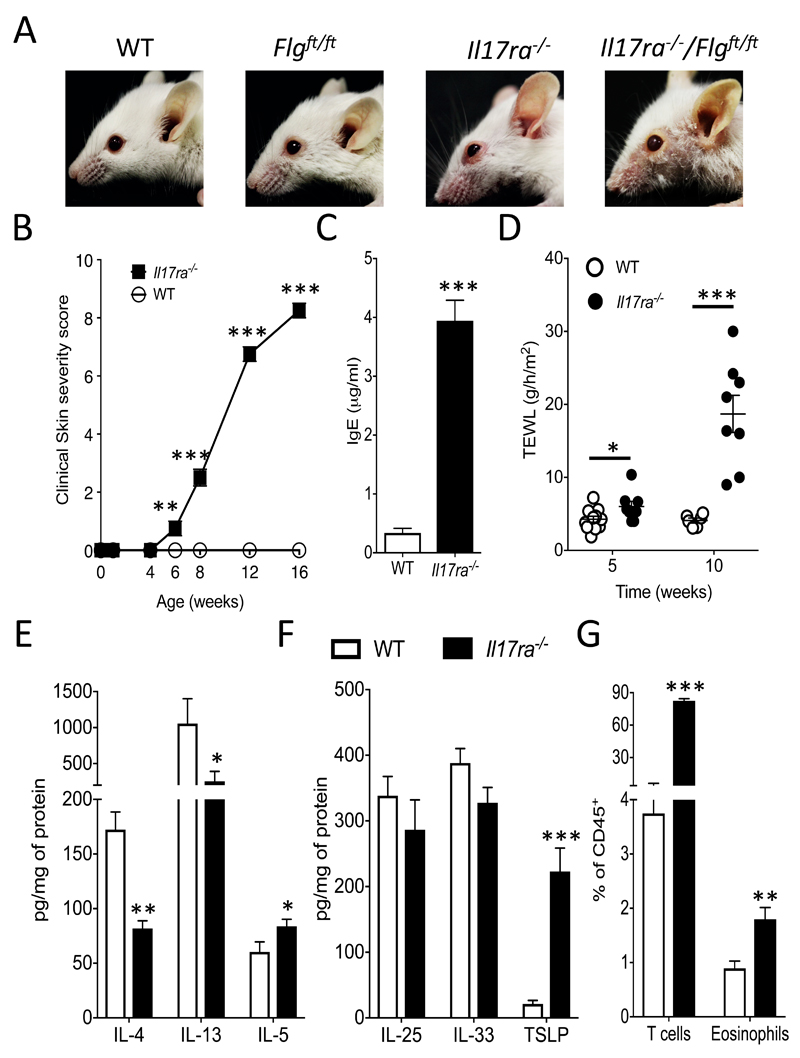
We have previously described that filaggrin mutant mice (Flgft/ft) have a defective skin barrier and are predisposed to developing AD-like skin inflammation that was associated with elevated levels of IL-17A (10). To investigate the role of IL-17A in spontaneous generation of AD-like inflammation in Flg mutant mice, dual Il17ra-/- and Flgft/ft (Il17ra-/-Flgft/ft) mice were generated on a BALB/c background. Unexpectedly, rather than ameliorating disease, in the absence of IL-17RA signalling adult filaggrin mutant mice developed exacerbated skin inflammation (Fig. 1A). Strikingly, the IL-17RA deficient mice developed spontaneous skin inflammation, irrespective of the presence of the Flg mutation (Fig. 1A). The spontaneous skin inflammation in Il17ra-/- mice developed after 6 weeks of age, was progressive (Fig 1B) and associated with high levels of serum IgE (Fig. 1C). Il17ra-/- mice have a defective skin barrier that predates the development of overt skin inflammation, with significantly (P<0.05) elevated trans-epidermal water loss (TEWL) in 5-week old IL-17RA deficient mice that have no overt skin inflammation (Fig 1B), relative to age-matched WT mice (Fig. 1D). After the onset of skin inflammation in Il17ra-/- mice there was pronounced barrier disruption, with significantly (P<0.001) increased TEWL in older (10-week old) mice (Fig. 1D). Our initial interest in IL-17RA arose from studies on filaggrin mutant mice, which also have a defective skin barrier (10). The filaggrin protein has a pivotal role in maintaining skin barrier integrity, it is synthesised as a precursor protein (pro-filaggrin) in keratohyalin granules within the stratum corneum and is subsequently proteolytically processed into filaggrin monomers (19). Alterations in filaggrin processing, including loss of filaggrin expression and altered pre-mature filaggrin processing have been linked with impaired skin barrier function (20, 21). A previous study has shown that IL-17A treatment of human keratinocytes directly altered filaggrin production and processing leading to reduced expression of filaggrin monomers (22). Using in vitro differentiated primary murine keratinocytes from WT mice, IL-17A treatment also reduced filaggrin monomer formation (Fig. S1 A). Similarly, primary keratinocytes from Il17ra-/- mice constitutively express increased levels of filaggrin monomer when compared to keratinocytes from WT mice (Fig. S1 B). Importantly western blot analysis of epidermal skin from 5-week old Il17ra-/- mice, with barrier disruption but no frank skin inflammation (Fig. 1), recapitulates the results from the in vitro differentiated primary keratinocytes, with increased filaggrin monomer expression in the epidermis of Il17ra-/- mice when compared to filaggrin expression in the epidermis of age-matched WT mice (Fig. S1 C), and increased filaggrin expression in epidermis of skin sections of IL-17RA deficient mice (Fig. S1 D). These data demonstrate aberrant filaggrin processing in the absence of IL-17RA mediated signalling, which can have detrimental effects on the homeostasis of the skin barrier.
Figure 1. IL-17RA deficiency leads to spontaneous progressive allergic skin inflammation.
A. Representative pictures of 12-week old Il17ra-/-/Flgft/ft and Il17ra-/- mice compared to sex- and age-matched Flgft/ft and WT control mice. B. Clinical skin severity score. C. Total serum levels of IgE at 12-weeks of age. D. Measurement of trans epidermal water loss (TEWL) of mice as indicated (5-6 mice per group) at the specified time points. E. Skin cytokine protein levels for IL-4, IL-13 and IL-5 prior to clinical skin inflammation, and F levels for TSLP, IL-33 and IL-25, determined by ELISA and expressed as pg of cytokine per mg of total skin protein. G. Frequency of T cells and eosinophils in the skin expressed as a percentage of CD45+ cells. Data are presented as mean and SEM, from 4-9 mice per group, and statistical differences between groups were determined using Student's t test.
The IL-17RA deficient mice used in this study were originally generated on a C57BL/6 background and backcrossed congenic to BALB/c background in-house. C57BL/6 Il17ra-/- mice do not develop spontaneous skin inflammation (Fig. S2 A and B) or elevated IgE (Fig. S2 C). It is relevant that these C57BL/6 Il17ra-/- mice were bred and housed in the same animal facility, thus with identical environmental conditions, as the BALB/c IL-17RA deficient mice that do develop skin inflammation (Fig. 1). However, in the MC903-induced model of AD-like skin inflammation C57BL/6 IL-17RA deficient mice had significantly (P<0.001) accelerated skin inflammation when compared to C57BL/6 controls (Fig. S2 D), with a significant (P<0.05) increase in Th2 (GATA3+ CD4+) cells (Fig. S2 E). Therefore, while Il17ra-/- mice on a C57BL/6 strain background do not spontaneously develop AD-like inflammation, they do have a lower threshold to skin inflammatory insult compared to WT C57BL/6 strain mice.
The spontaneous AD-like skin inflammation and elevated IgE in Il17ra-/- mice indicates potential roles for the cardinal type 2 associated cytokines (IL-4, IL-5 and IL-13). We therefore examined the expression of these cytokines in non-lesional skin. In the skin of 5-week old Il17ra-/- mice, prior to the development of overt clinical skin inflammation, there were significantly (P<0.05) increased levels of IL-5 protein but decreased levels of both IL-4 (P<0.001) and IL-13 (P<0.05; Fig. 1E). Upstream of these Th2 cytokines, the alarmin cytokines IL-25, IL-33 and TSLP are key initiators of type-2 associated inflammation in epithelial tissues, including the skin (23, 24). Prior to the appearance of overt skin inflammation in the skin of 5 week-old Il17ra-/- mice, TSLP, but not IL-33 or IL-25, expression was significantly (P<0.001) elevated (Fig. 1F). Furthermore, the mRNA levels of TSLP, but not IL-25 or IL-33, were over thirty-fold increased when compared to wild type mice at 5- (no inflammation) and 12-weeks of age, when clinical signs of inflammation were evident (Fig. S3 A). As with 5-week old non-inflamed Il17ra-/- mice, 12-week old inflamed Il17ra-/- mice have significantly reduced levels of IL-4 (P<0.05) and IL-13 (P<0.05), but no alteration in IL-5, in the skin when compared to WT controls (Fig. S3 B). These results are consistent with a previous study showing reduced skin IL-4 expression in a mouse model of AD-like skin inflammation in the absence of IL-17A mediated signals (25). It is noteworthy that in the skin of Il17ra-/- mice protein levels of IL-17A were elevated, as reported previously (26, 27), while other cytokines including IL-22 and IL-1β were not significantly different when compared to controls (Fig. S3 C). There was significant (P<0.0001) infiltration of T cells and, consistent with elevated expression of IL-5, eosinophils (P<0.01) in the skin of Il17ra-/- mice when compared to control mice (Fig. 1G). In contrast, ILC2 and basophils populations, both implicated in AD, were not expanded in the skin of Il17ra-/- mice (data not shown). These results highlight potential roles for IL-5 and TSLP in the early genesis of spontaneous allergic skin inflammation in IL-17RA deficient mice.
Non-hematopoietic IL-17RA expression protects from spontaneous AD-like skin inflammation
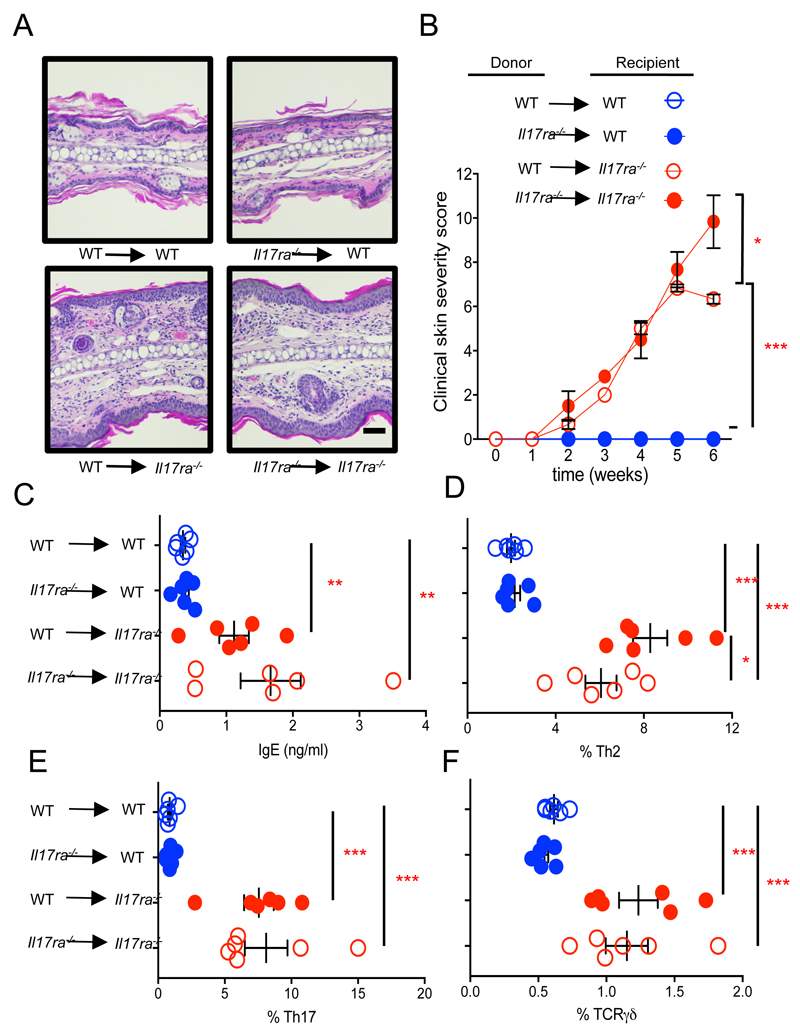
In order to assess whether IL-17RA deficiency in the hematopoietic or the non-hematopoietic cell compartment is required for the development of spontaneous skin inflammation, we generated bone marrow chimeric mice. Irradiated WT mice that received bone marrow from Il17ra-/- or WT donors did not develop skin inflammation, while Il17ra-/- mice recipients of WT or Il17ra-/- derived bone marrow developed severe progressive skin inflammation (Fig. 2A and B). Furthermore, spontaneous serum IgE, increased Th2, Th17 and TCRγδ T cells in the skin draining lymph-nodes developed when IL-17RA deficient mice were used as recipients irrespective of whether bone marrow cells were derived from WT or Il17ra-/- donors (Fig. 2C-F). These data demonstrate that IL-17RA signalling on radio-resistant skin resident cells is required for the spontaneous development of allergic skin inflammation.
Figure 2. Absence of IL-17RA in the non-hematopoietic and not the hematopoietic compartment is required for spontaneous skin inflammation.
A. Representative images of H&E-stained ear tissue biopsies from sex- and age-matched bone marrow chimeric mice, (bone marrow donor → recipient, magnification x20, bar equals 100 μm) and B clinical skin severity score, x axis indicates time post bone marrow transfer (5 mice per group). C. Total serum IgE levels for the indicated bone marrow chimeric mice. D-F. Flow cytometric analysis of Th2 (Gata3high), T17 (Rorγt+) and TCRγδ 17 (Rorγt+) T cells respectively for skin draining lymph-nodes of the indicated bone marrow chimeric mice, each symbol represents individual mice (5 mice per group). Data are presented as mean and SEM and statistical differences between groups was determined using Student's t test.
Skin inflammation in IL-17RA deficient mice is independent of TCRγδ T cells and IL-22 but is dependent on CD4+ T cells and mediated by IL-5
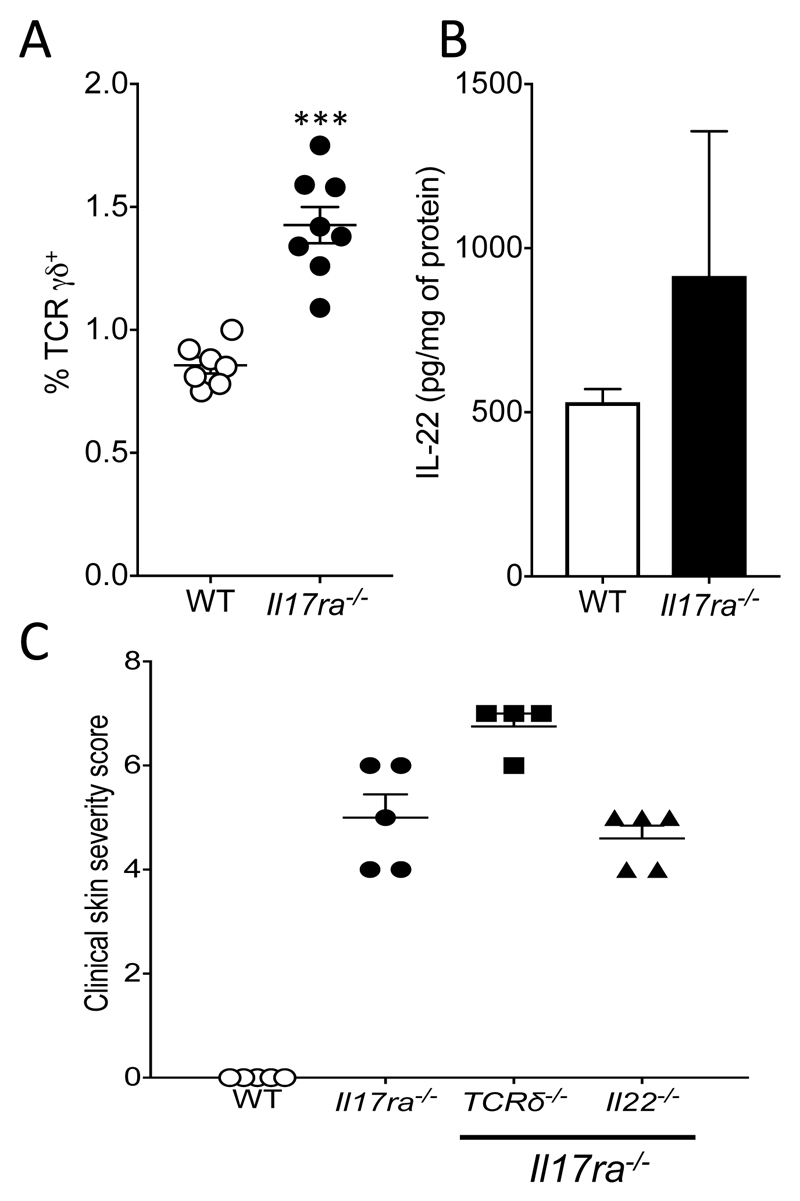
The increased numbers of TCRγδ T cells in Il17ra-/- bone marrow chimeric mice (Fig. 2F) indicates a potential role for these cells in the spontaneous skin inflammation observed. Therefore we further characterized TCRγδ T cells in Il17ra-/- mice. TCRγδ T cells are significantly increased (P<0.001) in Il17ra-/- mice when compared to controls (Fig. 3A), an increase primarily due to infiltration of TCRγδ T cells with an activated tissue memory phenotype (data not shown). To comprehensively assess the role of TCRγδ T cells in the spontaneous skin inflammation developed by Il17ra-/- mice, mice deficient in both TCRγδ T cells and IL-17RA (Tcrδ/ Il17ra-/-) were generated. TCRγδ T cell deficiency did not prevent Il17ra-/- mice from developing spontaneous skin inflammation (Fig. 3C).
Figure 3. Skin inflammation in IL-17RA deficient mice is independent of TCRγδ T cells and IL-22.
A. Flow cytometric analysis of TCRγδ T cells in WT and Il17ra-/- mice, 8 mice per group. B. ELISA for the detection of IL-22 in the skin of WT and Il17ra-/- mice, 3 mice per group. C. Clinical skin severity score for the indicated sex- and age-matched mice, 3-5 mice per group. Data are presented as mean and SEM and statistical differences between groups was determined using Student's t test.
While IL-22 was not upregulated in the skin of Il17ra-/- mice (Fig. 3B), it is widely implicated in mouse models of skin damage and inflammation (28), including skin inflammation downstream of IL17RA signaling via Act1 (29). We therefore formally examined the contribution of IL-22 to the Il17ra-/- skin phenotype by generating mice deficient in both IL-17RA and IL-22 (Il17ra/Il22-/-). IL-22 deficient Il17ra-/- mice developed spontaneous skin inflammation (Fig. 3C)
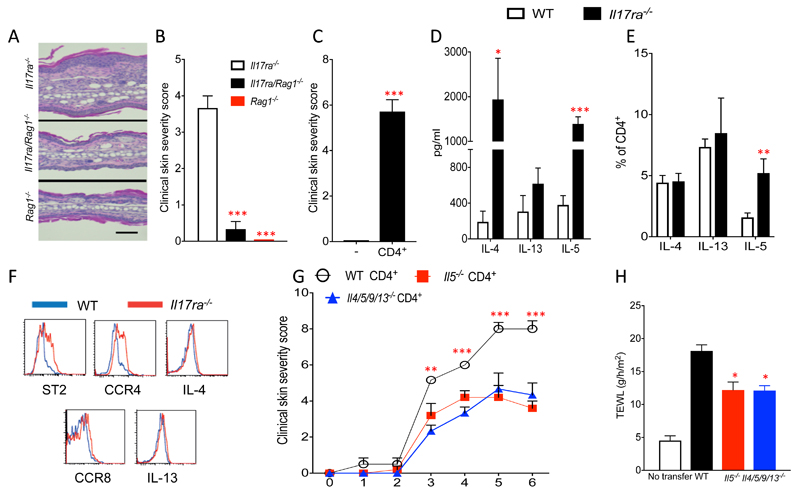
While there is no role for TCRγδ T cells in the spontaneous skin phenotype (Fig. 3C), the marked T cell skin infiltration (Fig. 1G) suggests a role for adaptive immunity in the genesis of the skin inflammation. To formally address the contribution of adaptive immunity to the development of skin inflammation in the absence of IL-17RA signaling we generated dual Il17ra-/- and Rag1-/- mice (Il17ra/Rag1-/-). In the absence of T and B cells with ILCs present, Il17ra-/- mice did not develop overt skin inflammation (Fig. 4A and B). However, reconstitution of Il17ra/Rag1-/- mice with WT CD4+ T cells restored the development of spontaneous skin inflammation (Fig. 4C). As these data identify a central role for CD4+ cells in the development of type 2 skin inflammation due to IL-17RA deficiency, the frequency of IL-4, IL-13 and IL-5 expressing Th2 cells was examined in skin draining lymph nodes from Il17ra-/- and WT controls. The protein levels of IL-4 and IL-5 were significantly, P<0.05 and P<0.01, respectively, higher in the supernatants of in vitro cultured lymph node cells derived from Il17ra-/- mice compared to cells from WT mice (Fig. 4D). The frequency of IL-5 expressing CD4+ T cells was significantly (P<0.001) increased in Il17ra-/- skin when compared to controls, with no significant differences in the frequency of IL-4 or IL-13 expressing Th2 cells (Fig. 4E). We further characterized the IL-5 producing CD4+ T cells that were expanded in the skin of Il17ra-/- mice. These skin-infiltrating IL-5+ CD4+ T cells did not have increased IL-4 or IL-13 expression or CCR8 expression but had increased surface expression of T1/ST2 and CCR4 when compared to controls (Fig. 4F). The phenotype of these cells in IL-17RA deficient mice is consistent with previously described IL-5+ pathogenic effector Th2 (peTh2) cells (30). Such peTh2 cell populations have been implicated in the pathogenesis of type 2 allergic diseases, including skin eosinophilia in patients with AD (31).
Figure 4. Skin inflammation in IL-17RA deficient mice is CD4+ T cell dependent and mediated by IL-5.
A. H&E-stained ear tissue biopsies from sex- and age-matched Il17ra-/-, Il17ra/Rag1-/- and Rag1-/-, mice (magnification x63, bar equals 50 μm) and B clinical skin severity score (4-5 mice per group). C. Clinical skin severity score of Il17ra/Rag1-/- mice following reconstitution with CD4+ T cells compared to non-reconstituted Il17ra/Rag1-/- control mice. D. ELISA for the detection of IL-4, IL-5 and IL-13 in the supernatant of in vitro stimulated skin draining lymph-node cells of Il17ra-/- and WT control mice (5 mice per group). E. Flow cytometric analysis for frequency of IL-4, IL-13 and IL-5 expressing CD4+ T cells in skin draining lymph nodes (3-10 mice per group). F. Flow cytometric analysis of IL-5 expressing CD4+ T cells from WT or Il17ra-/- mice and expression of T1/ST2, CCR4, CCR8, IL-4 and IL-13. G. Clinical skin severity score of Il17ra/Rag1-/- mice reconstituted with CD4+ T cells from donor mice (3-6 mice per group). H. Measurement of trans epidermal water loss (TEWL) of 3-6 mice per group. Data are presented as mean and SEM and statistical differences between groups was determined using Student's t test.
The increased levels of IL-5 in the skin of Il17ra-/- mice and the infiltration of IL-5 expressing peTh2 cells in mice warranted further investigation of the role of CD4+ T cell derived IL-5 in the development of spontaneous skin inflammation. CD4+ T cells from WT or IL-5 deficient (Il5cfp/cfp) mice were transferred into Il17ra/Rag1-/- mice, that do not develop skin inflammation (Fig. 4A and B), and the development of spontaneous skin inflammation in CD4+ T cell recipients was evaluated. Recipients of Il5cfp/cfp CD4+ T cells had significantly ameliorated skin inflammation when compared to recipients of CD4+ T cells from WT donors (Fig. 4G). Interestingly, recipients of CD4+ T cells from mice deficient in all four cardinal Th2 cytokines (IL-4, IL-5, IL-9 and IL-13) developed the same degree of protection as did recipients of IL-5 deficient CD4+ T cells; indicating a specific role for pathogenic IL-5 expressing CD4+ T cells in the spontaneous skin inflammation of the Il17ra-/- mice (Fig. 4G). Furthermore, while transfer of WT CD4+ cells into Il17ra/Rag1-/- mice induced a defective skin barrier, reflected in elevated TEWL, IL-5 deficient or IL-4/IL-5/IL-9/IL-13 deficient CD4+ T cells evoked significantly reduced skin barrier disruption (Fig. 4H). These studies identify a role for IL-5+ peTh2 cells in the generation of spontaneous skin inflammation in IL-17RA deficient mice.
TSLP is required for skin inflammation in IL-17RA deficient mice
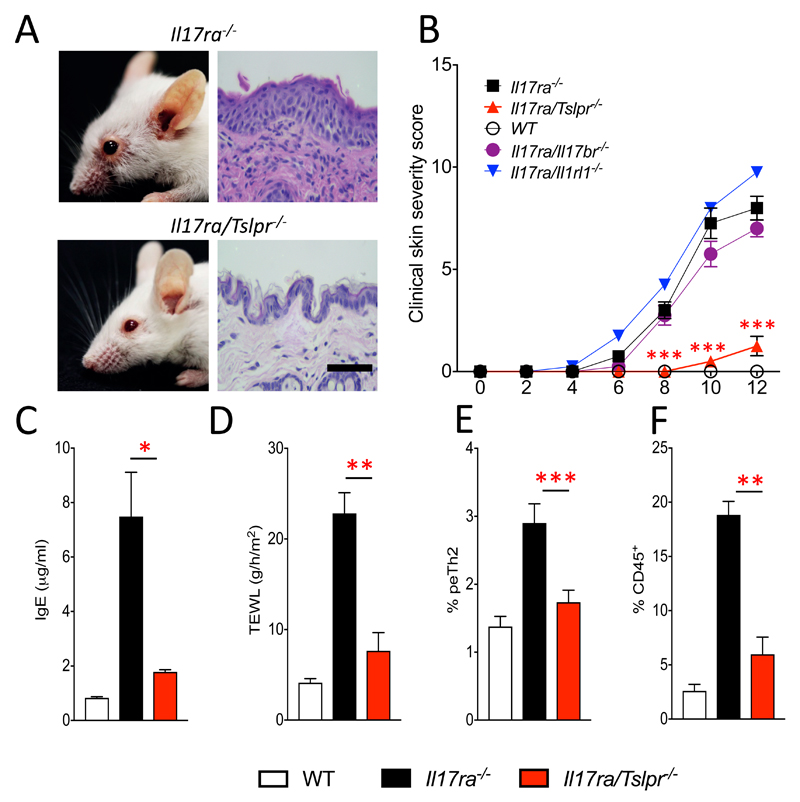
Due to the increased levels of TSLP in the skin of IL-17RA deficient mice, we further examined the contribution of TSLP, as well as other skin alarmins, IL-25 and IL-33, in the development of spontaneous skin inflammation by generating mice deficient in both IL-17RA and TSLPR (Il17ra/Tslpr-/-), or IL-25 receptor (Il17ra/Il17br-/-) or IL-33 receptor (Il17ra/Il1rl1-/-). TSLPR deficiency profoundly protected Il17ra-/- mice from developing spontaneous skin inflammation (Fig. 5A and B). In contrast, Il17ra/Il17br-/- and Il17ra/Il1rl1-/- mice developed skin inflammation comparable to Il17ra-/- mice (Fig. 5B), thus reinforcing the specific pathogenic role for TSLP in this model. In the absence of TSLP receptor signaling in IL-17RA deficient mice there was significantly reduced (P<0.05) serum levels of IgE (Fig. 5C), ameliorated (P<0.01) skin barrier disruption (Fig. 5D) with cell infiltration of the skin (Fig. 5E) were significantly reduced (P<0.001). In addition, the frequency of IL-5+ peTh2 cells in skin draining lymph nodes was significantly (P<0.005) reduced compared to Il17ra-/- mice (Fig. 5F).
Figure 5. TSLP is required for skin inflammation in IL-17RA deficient mice.
A. H&E-stained skin tissue biopsies from sex- and age-matched Il17ra-/- and Il17ra/Tslpr-/- mice (magnification x63, bar equals 50 μm). B. Clinical skin severity score of Il17ra-/-, Il17ra/Tslpr-/-, Il17ra/Il17br-/-, Il17ra/Il1rl1-/- and WT control mice (4-6 mice per group). C. Total serum levels of IgE. D. Measurement of trans epidermal water loss (TEWL) of mice as indicated, compared to sex- and age-matched controls (3-5 mice per group). E. Frequency of infiltrating cells to the skin (7-9 mice per group). F. Flow cytometry analysis of IL-5 expressing CD4+ peTh2 in skin draining lymph nodes (7-9 mice per group). Data are presented as mean and SEM and statistical differences between groups was determined using Student's t test.
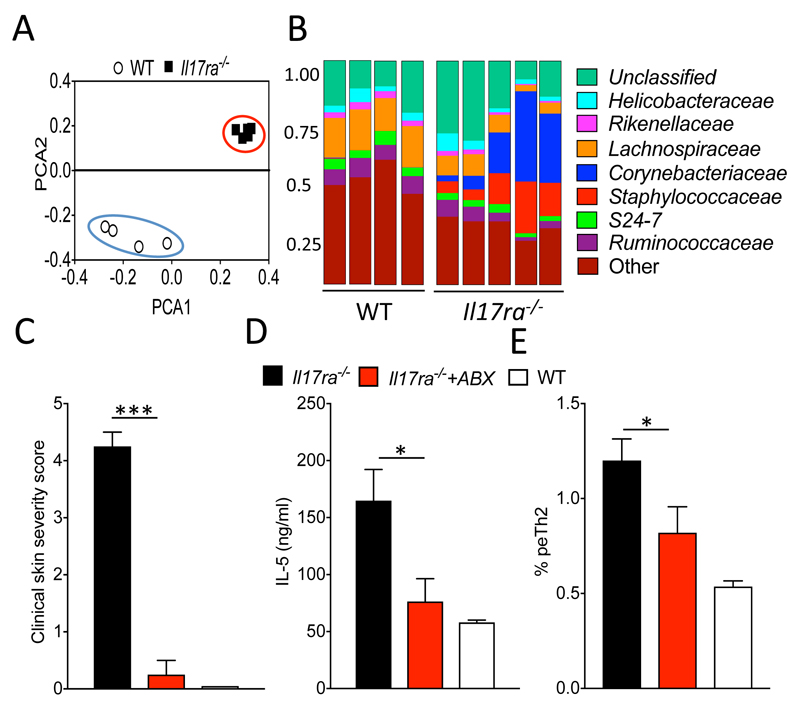
Skin microbiome dysbiosis in the absence of IL-17RA
Alterations in the skin microbiome are associated with AD and also a defective skin barrier (32, 33). 16S ribosomal sequencing analysis of skin from sex- and age-matched IL-17RA deficient and WT mice revealed bacterial microbiome dysbiosis in the skin of Il17ra-/- mice, with principal component analysis illustrating a dramatic segregation between the skin bacterial genera and also their relative abundances between the two groups (Fig. 6A). Bacterial taxonomic classification of the skin microbiome revealed an increase in bacteria of the Staphylococcaceae and Corynebacteriaceae families, which are associated with skin inflammation in AD in man and mouse models (33), in the skin of IL-17RA deficient mice when compared to WT controls (Fig. 6B). Microbiome composition differences were also evident on the genus level with a significant (P<0.05) increase in the abundance of the AD-associated Corynebacterium, Staphylococcus and Gemella (Fig. S4 A). Further confirmation of microbiome dysbiosis in in Il17ra-/- mice was demonstrated by the significant amelioration (P<0.0001) of skin inflammation in Il17ra-/- mice following treatment of mice with a broad-spectrum antibiotic regime (Fig. 6C). Consistent with pathogenic functions for IL-5 in the genesis of skin inflammation, antibiotic-treated Il17ra-/- mice had significantly (P<0.05) reduced circulating levels of IL-5 and, furthermore, also a significant reduction of peTh2 cells (P<0.05; Fig. 6D).
Figure 6. Skin microbiome dysbiosis in the absence of IL-17RA and antibiotic treatment attenuated skin inflammation.
A. Principal component analysis of skin microbiome samples isolated from Il17ra-/- and sex- and age-matched WT control mice. B. Bacterial families identified on the skin of Il17ra-/- and WT mice, mean relative abundance is shown. C. Clinical skin severity score of indicated groups of mice following treatment with an antibiotic (ABX) regime or treatment with autoclaved water (5 mice per group). D. Serum levels of IL-5 and frequency of IL-5 expressing CD4+ peTh2 cells in skin draining lymph nodes (5 mice per group). Data are presented as mean and SEM and statistical differences between groups was determined using Student's t test.
Due to the microbiome dysbiosis and aberrant inflammation in the skin of Il17ra-/- mice, the expression levels of a panel of anti-microbial peptides (AMP) implicated in skin homeostasis was analysed. The expression of S100 proteins (S100a7, S100a8 and S100a9), β-defensin 2 (Defb2) and cathelin-related antimicrobial peptide (Cramp) was assessed in the skin of Il17ra-/- mice at 5-weeks of age (no overt inflammation) and at 12-weeks of age (inflamed skin) and compared to age- and sex- matched WT controls (Fig. S4 B). There were no differences between expression of selected AMP genes in the skin of 5-week old Il17ra-/- mice and WT animals, whereas in the skin of 12-week old IL-17RA deficient mice there was statistically reduced (P<0.05) expression of S100a8 and S100a9 (Fig. S4 B). These data indicate changes in certain AMP in the skin of Il17ra-/- mice only when the mice have developed skin inflammation. To address if the dysbiotic microbiome of the IL-17RA deficient mice is able to elicit spontaneous skin inflammation in WT mice, co-housing studies were undertaken. Antibiotic-treated WT mice that were co-housed with Il17ra-/- mice with a dysbiotic microbiome did not develop spontaneous skin inflammation (Fig. S4 C). Furthermore, consistent with no skin inflammation, the levels of L-5 and TSLP in the skin of WT antibiotic-treated mice cohoused with Il17ra-/- or WT mice were comparable (data not shown). Collectively, these results show requirements for a dysbiotic microbiome, compromised skin barrier and aberrant immunity for the development of spontaneous allergic like-skin inflammation due to IL-17RA deficiency.
Discussion
Dysregulation of the IL-17A/IL-17RA axis is implicated in inflammatory skin diseases, with antibodies against IL-17A and IL-17RA showing significant efficacy for the treatment of psoriasis (3, 34). In AD patients, the increased expression of IL-17A in skin lesions raises the potential to target IL-17A or IL-17RA as a therapeutic strategy (4, 5). Additionally, absence of IL-17A has been shown to attenuate the development of skin inflammation in mouse models of AD (25). While the functions of IL-17A signalling in skin inflammation is well investigated, a better understanding of the immunological function of IL-17RA in the skin is required. Our results highlight a previously unappreciated protective role for IL-17RA signaling in the maintenance of the integrity of the skin barrier and skin homeostasis. Interestingly, in the intestine IL-17RA signaling is implicated in the maintenance of the intestinal barrier, via IL-17A regulated expression of the tight junction protein occludin in the intestinal epithelial cells (35, 36). We now demonstrate that in mouse skin, keratinocyte IL-17RA signalling regulates the expression and processing of the skin barrier protein filaggrin, with absence of IL-17RA signalling creating an imbalance of monomeric filaggrin to profilaggrin composition of the skin leading to the subsequent skin barrier dysregulation. Therefore in both the skin and gut IL-17RA signaling may be required for the preservation of barrier integrity and local immune homeostasis. However, while we report a role for IL-17RA signaling in maintenance of skin homeostasis, it remains to be determined in what degree IL-17A, IL-17F, IL17A/F or IL-17E contribute to the generation of skin inflammation. As antibody based biologics against IL-17 family members are being developed it will be interesting to determine if alterations in barrier function, regulation of the microbiome or immunity in the skin, as addressed herein, and also in the gut, develop in patients with sustained blocking of IL-17RA or other IL-17 receptors and their ligands.
IL-17RA signaling may have regulatory roles in skin inflammation as mice deficient in Act1, also on a BALB/c background as used herein, which is downstream of IL-17RA and mediates the subsequent activation of NFkB, develop spontaneous skin inflammation (29, 37). Hyper-active Th17 cells and primarily IL-22 were shown to be responsible for the spontaneous skin phenotype observed in these mice (29). In contrast to deficiency in Act1, in this study IL-17RA deficiency does not require IL-22 for the induction of spontaneous skin inflammation. This indicates that the genesis of skin inflammation due to Act1-deficiency and IL-17RA-deficiency are mechanistically different. The use of bone marrow chimeras indicated that IL-17RA expression on skin radio-resistant cells, such as epidermal langerhans cells and keratinocytes, was responsible for the development of the AD-like skin inflammation. In a mouse model of IL-17A and IL-17C psoriatic skin inflammation it was also recently reported that disease was mediated by non-hematopoietic cells (27), with the same IL-17A expressing cellular compartment responsible for synovitis in a model of joint inflammation (38). These studies highlight a specific role for IL-17RA expression on non-hematopoietic cells in the pathogenesis of inflammation in different tissues.
In this study IL-17RA deficient mice on the BALB/c mouse strain, commonly used for the study of allergic diseases (39), developed marked spontaneous skin inflammation, i.e. without the need for skin insult and barrier disruption. The majority of available studies in the public domain using IL-17RA signalling deficient mice involve the C57BL/6 background with no reports of a spontaneous skin inflammatory phenotype. However, IL-17RA deficient mice on a C57BL/6 background also have a dramatically reduced threshold for the development of skin inflammation following insult with the chemical agent MC903, and develop severely accelerated skin inflammation. It is relevant that in mice with a defective skin barrier due to a filaggrin mutation, there is also more exacerbated disease when on a BALB/c background but not on a C57BL/6 background (10). Similar to human AD, the development of skin inflammation due to IL-17RA deficiency in mice may also be a polygenic condition, with mouse strain genetic differences, as well as impact of animal breeding environment, influencing the magnitude of skin dysregulation and resulting inflammation.
In IL-17RA deficient mice skin inflammation was characterized by elevated IL-5 in this skin, with CD4+ T cell transfer showing inflammation was IL-5 dependent. In patients with AD, polymorphisms in the IL-5 encoding gene have previously been associated with disease (40), while changes in IL-5 serum levels have been shown to reflect clinical improvement of AD in patients (41). Recently emerging evidence implicates IL-5+ producing pathogenic effector Th2 cells in the initiation of chronic inflammatory diseases including AD (42). IL-5 producing peTh2 cells drive eosinophilic airway inflammation and skin eosinophilia leading to tissue damage and chronicity of the immune response, and they have been shown in both human and mouse models (30, 31). Little is however known regarding the regulation of these cells and the mechanisms that influence their expansion. Here we reported that IL-17RA-deficiency induced an increase in peTh2 T cells that was associated with spontaneous skin inflammation and skin eosinophilia and importantly we demonstrated a specific role for IL-5. While AD patients treated with a humanized IL-5 neutralizing antibody showed only modest improvement, further study into the contribution of IL-5 in AD is warranted (43). While our data show a role for IL-5 in the development of skin inflammation, it is clear that other IL-5 independent mechanisms may also be involved. However, while IL-5 deficiency reduced the severity of skin inflammation, TSLP was mandatory for the development of AD-like inflammation in IL-17RA deficient mice. These data raise the potential of exploring combined biologic targeting of the alarmin initiator and cytokine effector, in this case TSLP and IL-5 for AD, respectively, as a novel bispecific therapeutic strategy for inflammatory diseases.
Several studies have implicated TSLP in atopic diseases including atopic dermatitis (44). TSLP is primarily expressed by epithelial cells at barrier surfaces, including skin keratinocytes (45). Keratinocyte specific overexpression of TSLP in mice has previously been shown to induce spontaneous skin inflammation with several characteristics of AD (46). Interestingly IL-17A inhibits secretion of TSLP by human and mouse keratinocytes ((47) data not shown), therefore absence of IL-17A mediated inhibitory signals due to an IL-17RA deficiency could be a major confounding factor for the high levels of TSLP expression observed in IL-17RA deficient mice. While TSLP can induce type 2 responses (48) and can elicit allergic skin inflammation via ILC2 and/or basophils (49, 50), both ILC2 and basophils were not altered in the skin of IL-17RA deficient mice (data not shown). Another mechanism for TSLP initiated skin inflammation involves TSLP activating dendritic cells to drive naïve T cell polarization to IL-4 expressing Th2 cells (48). Indeed, keratinocyte derived TSLP has been shown to act on skin langerhans cells, that respond to TSLP to elicit strong Th2 responses in a model of allergic skin dermatitis (51). It remains to be elucidated how TSLP is contributing to the spontaneous skin inflammation observed in IL-17RA deficient mice.
An important role of IL-17RA in skin microbiome maintenance was show by mice deficient in IL-17RA signaling developing microbiome dysbiosis. Furthermore, the skin inflammation in IL-17RA deficient mice was ameliorated by antibiotic intervention supporting the premise that the aberrant microbiome directly contributes to the skin inflammation. However, microbiome transfer co-housing studies indicated that the dysbiotic microbiome of IL-17RA deficient mice is, in isolation, not adequate to lead to spontaneous skin inflammation when transferred to wild type mice that have an intact skin barrier. In various inflammatory skin diseases, including AD, there are alterations in the expression of AMP in the skin, that may function in cutaneous defense as well as contribute to pathogenesis (52, 53). In 5 week-old IL-17RA deficient animals, when the skin has no overt inflammation, the expression S100a7, S100a8, S100a9, Defb2 and Cramp was comparable to wild type mice. In older Il17ra-/- mice with inflamed skin, while there was no change in expression of S100a7, Defb2 and Cramp there was reduced expression of S100a8 and S100a9, two AMP implicated in AD and activated by IL-17A (54). While it remains to be determined what the initiator of the spontaneous skin inflammation in the absence of IL-17RA signaling is, this study highlights the intricate interplay of the triad of a defective skin barrier, aberrant inflammation and microbial dysbiosis in skin function in health and disease.
Skin microbiome dysbiosis has previously been associated with AD in humans and mice (32, 33). Such is the contribution of the skin microbiome in AD that age-related differences in disease prevalence could be a result of composition differences of the microbiome in pediatric and adult AD (55). Disease severity and disease flares show strong association with changes in microbiome diversity in patients with AD with particularly strong correlation between severity and bacteria of the Staphylococcus, Corynebacterium and Gemella genera (33, 56). Interestingly the genera Staphylococcus and Corynebacterium were both increased in the skin of IL-17RA deficient mice in this study. While this is a mouse study, intriguingly humans carrying mutations leading to IL-17RA signaling deficiency develop chronic mucocutaneous candidiasis and, as seen herein, also may develop additional staphylococcal infections of the skin as well as eczema (57).
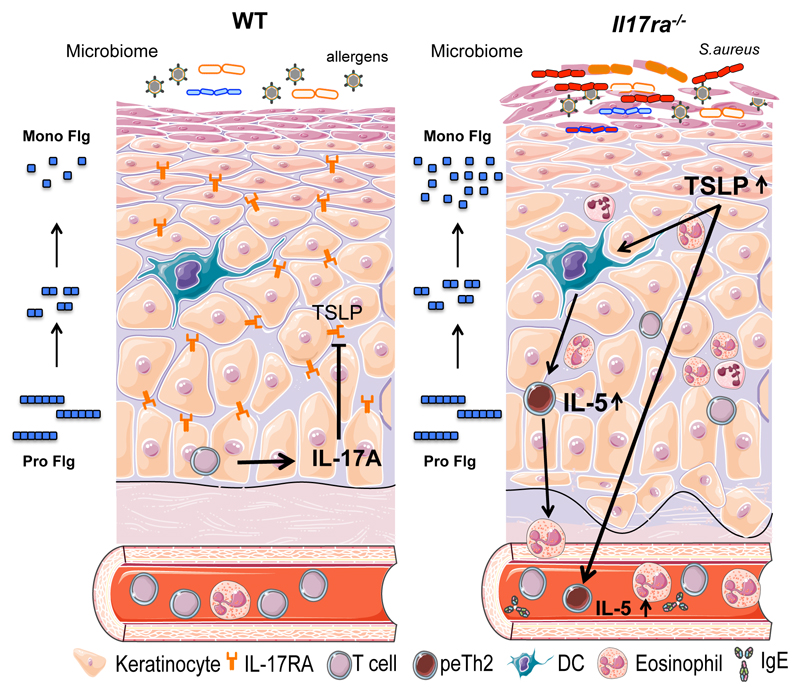
Collectively these results reveal that dsyregulation of the skin in Il17ra-/- mice involves skin microbiome dysbiosis, a defective skin barrier with alteration in filaggrin expression, and aberrant skin inflammation mediated by IL-5 and peTh2 cells and dependent on TSLP (Fig. 7). This study advances our current understanding of the regulatory role of IL-17RA in maintaining the skin barrier and immune system homeostasis to prevent the spontaneous development of allergic dermatitis.
Figure 7. Schematic of the aberrant skin barrier and development of allergic cutaneous inflammation in the absence of IL-17RA signaling.
Supplementary Material
Acknowledgments
We are grateful to Masayuki Amagai for kindly providing filaggrin deficient mice.
1This work was supported by the Wellcome Trust (092530/Z/10/Z), National Children’s Research Centre, Science Foundation Ireland (10/IN.1/B3004) and the Irish Research Council.
Abbreviations used in this article:
- AD
atopic dermatitis
- AMP
antimicrobial peptides
- TSLP
thymic stromal lymphopoietin
- Flg
filaggrin
- peTh2
pathogenic effector Thelper 2
- TEWL
Trans-epidermal water loss
- WT
wild type
References
- 1.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 2.Werfel T, Allam JP, Biedermann T, Eyerich K, Gilles S, Guttman-Yassky E, Hoetzenecker W, Knol E, Simon HU, Wollenberg A, Bieber T, et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol. 2016;138:336–349. doi: 10.1016/j.jaci.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, Aras G, Li J, Russell CB, Thompson EH, Baumgartner S. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181–1189. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- 4.Noda S, Krueger JG, Guttman-Yassky E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J Allergy Clin Immunol. 2015;135:324–336. doi: 10.1016/j.jaci.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Gittler JK, Shemer A, Suarez-Farinas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, Mitsui H, Cardinale I, de Guzman Strong C, Krueger JG, Guttman-Yassky E. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130:1344–1354. doi: 10.1016/j.jaci.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irvine AD, McLean WHI, Leung DYM. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365:1315–1327. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- 7.Fallon PG, Sasaki T, Sandilands A, Campbell LE, Saunders SP, Mangan NE, Callanan JJ, Kawasaki H, Shiohama A, Kubo A, Sundberg JP, et al. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet. 2009;41:602–608. doi: 10.1038/ng.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saunders SP, Goh CS, Brown SJ, Palmer CN, Porter RM, Cole C, Campbell LE, Gierlinski M, Barton GJ, Schneider G, Balmain A, et al. Tmem79/Matt is the matted mouse gene and is a predisposing gene for atopic dermatitis in human subjects. J Allergy Clin Immunol. 2013;132:1121–1129. doi: 10.1016/j.jaci.2013.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasaki T, Shiohama A, Kubo A, Kawasaki H, Ishida-Yamamoto A, Yamada T, Hachiya T, Shimizu A, Okano H, Kudoh J, Amagai M. A homozygous nonsense mutation in the gene for Tmem79, a component for the lamellar granule secretory system, produces spontaneous eczema in an experimental model of atopic dermatitis. J Allergy Clin Immunol. 2013;132:1111–1120 e1114. doi: 10.1016/j.jaci.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 10.Saunders SP, Moran T, Floudas A, Wurlod F, Kaszlikowska A, Salimi M, Quinn EM, Oliphant CJ, Nunez G, McManus R, Hams E, et al. Spontaneous atopic dermatitis is mediated by innate immunity, with the secondary lung inflammation of the atopic march requiring adaptive immunity. J Allergy Clin Immunol. 2016;137:482–491. doi: 10.1016/j.jaci.2015.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardman CS, Panova V, McKenzie AN. IL-33 citrine reporter mice reveal the temporal and spatial expression of IL-33 during allergic lung inflammation. Eur J Immunol. 2013;43:488–498. doi: 10.1002/eji.201242863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawasaki H, Nagao K, Kubo A, Hata T, Shimizu A, Mizuno H, Yamada T, Amagai M. Altered stratum corneum barrier and enhanced percutaneous immune responses in filaggrin-null mice. J Allergy Clin Immunol. 2012;129:1538–1546. doi: 10.1016/j.jaci.2012.01.068. [DOI] [PubMed] [Google Scholar]
- 13.Fallon PG, Jolin HE, Smith P, Emson CL, Townsend MJ, Fallon R, Smith P, McKenzie ANJ. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity. 2002;17:7–17. doi: 10.1016/s1074-7613(02)00332-1. [DOI] [PubMed] [Google Scholar]
- 14.Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, Huang L-C, Johnson D, Scanlon ST, McKenzie ANJ, Fallon PG, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kezic S, O'Regan GM, Lutter R, Jakasa I, Koster ES, Saunders S, Caspers P, Kemperman PM, Puppels GJ, Sandilands A, Chen H, et al. Filaggrin loss-of-function mutations are associated with enhanced expression of IL-1 cytokines in the stratum corneum of patients with atopic dermatitis and in a murine model of filaggrin deficiency. J Allergy Clin Immunol. 2012;129:1031–1039. doi: 10.1016/j.jaci.2011.12.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan AR, Hams E, Floudas A, Sparwasser T, Weaver CT, Fallon PG. PD-L1hi B cells are critical regulators of humoral immunity. Nat Commun. 2015;6:5997. doi: 10.1038/ncomms6997. [DOI] [PubMed] [Google Scholar]
- 17.Miezeiewski M, Schnaufer T, Muravsky M, Wang S, Caro-Aguilar I, Secore S, Thiriot DS, Hsu C, Rogers I, DeSantis T, Kuczynski J, et al. An in vitro culture model to study the dynamics of colonic microbiota in Syrian golden hamsters and their susceptibility to infection with Clostridium difficile. ISME J. 2015;9:321–332. doi: 10.1038/ismej.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill DA, Hoffmann C, Abt MC, Du Y, Kobuley D, Kirn TJ, Bushman FD, Artis D. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol. 2010;3:148–158. doi: 10.1038/mi.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egawa G, Kabashima K. Multifactorial skin barrier deficiency and atopic dermatitis: Essential topics to prevent the atopic march. J Allergy Clin Immunol. 2016;138:350–358. doi: 10.1016/j.jaci.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Mocsai G, Gaspar K, Nagy G, Irinyi B, Kapitany A, Biro T, Gyimesi E, Toth B, Marodi L, Szegedi A. Severe skin inflammation and filaggrin mutation similarly alter the skin barrier in patients with atopic dermatitis. Br J Dermatol. 2014;170:617–624. doi: 10.1111/bjd.12743. [DOI] [PubMed] [Google Scholar]
- 21.Gruber R, Elias PM, Crumrine D, Lin TK, Brandner JM, Hachem JP, Presland RB, Fleckman P, Janecke AR, Sandilands A, McLean WH, et al. Filaggrin genotype in ichthyosis vulgaris predicts abnormalities in epidermal structure and function. Am J Pathol. 2011;178:2252–2263. doi: 10.1016/j.ajpath.2011.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutowska-Owsiak D, Schaupp AL, Salimi M, Selvakumar TA, McPherson T, Taylor S, Ogg GS. IL-17 downregulates filaggrin and affects keratinocyte expression of genes associated with cellular adhesion. Exp Dermatol. 2012;21:104–110. doi: 10.1111/j.1600-0625.2011.01412.x. [DOI] [PubMed] [Google Scholar]
- 23.Vannella KM, Ramalingam TR, Borthwick LA, Barron L, Hart KM, Thompson RW, Kindrachuk KN, Cheever AW, White S, Budelsky AL, Comeau MR, et al. Combinatorial targeting of TSLP, IL-25, and IL-33 in type 2 cytokine-driven inflammation and fibrosis. Sci Transl Med. 2016;8:337ra365. doi: 10.1126/scitranslmed.aaf1938. [DOI] [PubMed] [Google Scholar]
- 24.Hammad H, Lambrecht BN. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima S, Kitoh A, Egawa G, Natsuaki Y, Nakamizo S, Moniaga CS, Otsuka A, Honda T, Hanakawa S, Amano W, Iwakura Y, et al. IL-17A as an inducer for Th2 immune responses in murine atopic dermatitis models. J Invest Dermatol. 2014;134:2122–2130. doi: 10.1038/jid.2014.51. [DOI] [PubMed] [Google Scholar]
- 26.El Malki K, Karbach SH, Huppert J, Zayoud M, Reissig S, Schuler R, Nikolaev A, Karram K, Munzel T, Kuhlmann CR, Luhmann HJ, et al. An alternative pathway of imiquimod-induced psoriasis-like skin inflammation in the absence of interleukin-17 receptor a signaling. J Invest Dermatol. 2013;133:441–451. doi: 10.1038/jid.2012.318. [DOI] [PubMed] [Google Scholar]
- 27.Monin L, Gudjonsson JE, Childs EE, Amatya VN, Xing X, Verma AH, Coleman BM, Garg AV, Killeen M, Mathers A, Ward NL, et al. MCPIP1/Regnase-1 Restricts IL-17A- and IL-17C-Dependent Skin Inflammation. J Immunol. 2017;198:767–775. doi: 10.4049/jimmunol.1601551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon J, Leyva-Castillo JM, Wang G, Galand C, Oyoshi MK, Kumar L, Hoff S, He R, Chervonsky A, Oppenheim JJ, Kuchroo VK, et al. IL-23 induced in keratinocytes by endogenous TLR4 ligands polarizes dendritic cells to drive IL-22 responses to skin immunization. J Exp Med. 2016;213:2147–66. doi: 10.1084/jem.20150376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang C, Wu L, Bulek K, Martin BN, Zepp JA, Kang Z, Liu C, Herjan T, Misra S, Carman JA, Gao J, et al. The psoriasis-associated D10N variant of the adaptor Act1 with impaired regulation by the molecular chaperone hsp90. Nat Immunol. 2013;14:72–81. doi: 10.1038/ni.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Endo Y, Hirahara K, Yagi R, Tumes DJ, Nakayama T. Pathogenic memory type Th2 cells in allergic inflammation. Trends Immunol. 2014;35:69–78. doi: 10.1016/j.it.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Mitson-Salazar A, Yin Y, Wansley DL, Young M, Bolan H, Arceo S, Ho N, Koh C, Milner JD, Stone KD, Wank SA, et al. Hematopoietic prostaglandin D synthase defines a proeosinophilic pathogenic effector human T(H)2 cell subpopulation with enhanced function. J Allergy Clin Immunol. 2016;137:907–918 e909. doi: 10.1016/j.jaci.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, Nomicos E, Polley EC, Komarow HD, Murray PR, Turner ML, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850–859. doi: 10.1101/gr.131029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi T, Glatz M, Horiuchi K, Kawasaki H, Akiyama H, Kaplan DH, Kong HH, Amagai M, Nagao K. Dysbiosis and Staphylococcus aureus Colonization Drives Inflammation in Atopic Dermatitis. Immunity. 2015;42:756–766. doi: 10.1016/j.immuni.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, Puig L, Nakagawa H, Spelman L, Sigurgeirsson B, Rivas E, et al. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014;371:326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 35.Maxwell JR, Zhang Y, Brown WA, Smith CL, Byrne FR, Fiorino M, Stevens E, Bigler J, Davis JA, Rottman JB, Budelsky AL, et al. Differential Roles for Interleukin-23 and Interleukin-17 in Intestinal Immunoregulation. Immunity. 2015;43:739–750. doi: 10.1016/j.immuni.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 36.Lee JS, Tato CM, Joyce-Shaikh B, Gulen MF, Cayatte C, Chen Y, Blumenschein WM, Judo M, Ayanoglu G, McClanahan TK, Li X, et al. Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity. 2015;43:727–738. doi: 10.1016/j.immuni.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, Kordula T, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8:247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 38.Lubberts E, Schwarzenberger P, Huang W, Schurr JR, Peschon JJ, van den Berg WB, Kolls JK. Requirement of IL-17 receptor signaling in radiation-resistant cells in the joint for full progression of destructive synovitis. J Immunol. 2005;175:3360–3368. doi: 10.4049/jimmunol.175.5.3360. [DOI] [PubMed] [Google Scholar]
- 39.Shin YS, Takeda K, Gelfand EW. Understanding asthma using animal models. Allergy Asthma Immunol Res. 2009;1:10–18. doi: 10.4168/aair.2009.1.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Namkung JH, Lee JE, Kim E, Cho HJ, Kim S, Shin ES, Cho EY, Yang JM. IL-5 and IL-5 receptor alpha polymorphisms are associated with atopic dermatitis in Koreans. Allergy. 2007;62:934–942. doi: 10.1111/j.1398-9995.2007.01445.x. [DOI] [PubMed] [Google Scholar]
- 41.Kondo S, Yazawa H, Jimbow K. Reduction of serum interleukin-5 levels reflect clinical improvement in patients with atopic dermatitis. J Dermatol. 2001;28:237–243. doi: 10.1111/j.1346-8138.2001.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 42.Islam SA, Chang DS, Colvin RA, Byrne MH, McCully ML, Moser B, Lira SA, Charo IF, Luster AD. Mouse CCL8, a CCR8 agonist, promotes atopic dermatitis by recruiting IL-5(+) T(H)2 cells. Nat Immunol. 2011;12:167–U186. doi: 10.1038/ni.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corren J. Inhibition of Interleukin-5 for the Treatment of Eosinophilic Diseases. Discovery Med. 2012;13:305–312. [PubMed] [Google Scholar]
- 44.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11:289–293. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cianferoni A, Spergel J. The importance of TSLP in allergic disease and its role as a potential therapeutic target. Expert Rev Clin Immunol. 2014;10:1463–1474. doi: 10.1586/1744666X.2014.967684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoo J, Omori M, Gyarmati D, Zhou BH, Aye T, Brewer A, Comeau MR, Campbell DJ, Ziegler SF. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bogiatzi SI, Guillot-Delost M, Cappuccio A, Bichet JC, Chouchane-Mlik O, Donnadieu MH, Barillot E, Hupe P, Chlichlia K, Efremidou EI, Aractingi S, et al. Multiple-checkpoint inhibition of thymic stromal lymphopoietin-induced T(H)2 response by T(H)17-related cytokines. J Allergy Clin Immunol. 2012;130:233. doi: 10.1016/j.jaci.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 48.Leyva-Castillo JM, Hener P, Michea P, Karasuyama H, Chan S, Soumelis V, Li M. Skin thymic stromal lymphopoietin initiates Th2 responses through an orchestrated immune cascade. Nat Commun. 2013;4:2847. doi: 10.1038/ncomms3847. [DOI] [PubMed] [Google Scholar]
- 49.Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, Hepworth MR, Van Voorhees AS, Comeau MR, Artis D. TSLP Elicits IL-33-Independent Innate Lymphoid Cell Responses to Promote Skin Inflammation. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noti M, Kim BS, Siracusa MC, Rak GD, Kubo M, Moghaddam AE, Sattentau QA, Comeau MR, Spergel JM, Artis D. Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin-basophil axis. J Allergy Clin Immunol. 2014;133:1390–U1643. doi: 10.1016/j.jaci.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakajima S, Igyarto BZ, Honda T, Egawa G, Otsuka A, Hara-Chikuma M, Watanabe N, Ziegler SF, Tomura M, Inaba K, Miyachi Y, et al. Langerhans cells are critical in epicutaneous sensitization with protein antigen via thymic stromal lymphopoietin receptor signaling. J Allergy Clin Immunol. 2012;129:1048–1055 e1046. doi: 10.1016/j.jaci.2012.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Niyonsaba F, Kiatsurayanon C, Chieosilapatham P, Ogawa H. Friends or Foes? Host defense (antimicrobial) peptides and proteins in human skin diseases. Exp Dermatol. 2017 Feb 13; doi: 10.1111/exd.13314. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi T, Gallo RL. The Critical and Multifunctional Roles of Antimicrobial Peptides in Dermatology. Dermatologic clinics. 2017;35:39–50. doi: 10.1016/j.det.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 54.Jin S, Park CO, Shin JU, Noh JY, Lee YS, Lee NR, Kim HR, Noh S, Lee Y, Lee JH, Lee KH. DAMP molecules S100A9 and S100A8 activated by IL-17A and house-dust mites are increased in atopic dermatitis. Exp Dermatol. 2014;23:938–941. doi: 10.1111/exd.12563. [DOI] [PubMed] [Google Scholar]
- 55.Shi BC, Bangayan NJ, Curd E, Taylor PA, Gallo RL, Leung DYM, Li HY. The Skin Microbiome Differs with Age in Atopic Dermatitis. J Allergy Clin Immunol. 2016;137:Ab407–Ab407. doi: 10.1016/j.jaci.2016.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chng KR, Tay ASL, Li CH, Ng AHQ, Wang JJ, Suri BK, Matta A, McGovern N, Janela B, Wong XFCC, Sio YY, et al. Whole metagenome profiling reveals skin microbiome-dependent susceptibility to atopic dermatitis flare. Nat Microbiol. 2016;11:16106. doi: 10.1038/nmicrobiol.2016.106. [DOI] [PubMed] [Google Scholar]
- 57.Levy R, Okada S, Beziat V, Moriya K, Liu C, Chai LY, Migaud M, Hauck F, Al Ali A, Cyrus C, Vatte C, et al. Genetic, immunological, and clinical features of patients with bacterial and fungal infections due to inherited IL-17RA deficiency. Proc Natl Acad Sci U S A. 2016;113:E8277–E8285. doi: 10.1073/pnas.1618300114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.