Abstract
Buerger’s disease is a rare and severe disease affecting the blood vessels of the limbs. Adipose tissue-derived mesenchymal stem cells (ADSCs) have the potential to cure Buerger’s disease when developed as a stem cell drug. In the present study, we conducted a prospective, nonrandomized, no placebo-controlled, phase I/II clinical trial with a 2-year follow-up questionnaire survey. A total of 17 patients were intramuscularly administered autologous ADSCs at a dose of 5 million cells/kg. The incidence of adverse events (AEs), adverse drug reaction (ADR), and serious adverse events (SAEs) was monitored. No ADRs and SAEs related to stem cell treatment occurred during the 6-month follow-up. In terms of efficacy, the primary endpoint was increase in total walking distance (TWD). The secondary endpoint was improvement in rest pain, increase in pain-free walking distance (PFWD), toe–brachial pressure index (TBPI), transcutaneous oxygen pressure (TcPO2), and arterial brachial pressure index (ABPI). ADSCs demonstrated significant functional improvement results including increased TWD, PFWD, and rest pain reduction. No amputations were reported during the 6-month clinical trial period and in the follow-up questionnaire survey more than 2 years after the ADSC injection. In conclusion, intramuscular injection of ADSCs is very safe and is shown to prompt functional improvement in patients with severe Buerger’s disease at a dosage of 300 million cells per 60 kg of body weight. However, the confirmatory therapeutic efficacy and angiogenesis need further study.
Key words: Buerger’s disease, Mesenchymal stem cells (MSCs), Adipose tissue, Autologous
INTRODUCTION
Buerger’s disease (thromboangiitis obliterans) is an orphan disease with nonatherosclerotic, segmental inflammatory pathology that most commonly affects the small- and medium-sized arteries, veins, and nerves in the upper and lower extremities1. Typical patients with Buerger’s disease are young men (less than 40 to 45 years) with a history of tobacco use and present with progressive claudication, ischemic ulcers, or pain at rest1. In severe cases, patients with Buerger’s disease exhibit severe pain at rest and tissue death in the affected limbs, which eventually lead to amputation.
The most effective treatment for Buerger’s disease is smoking cessation. Patient education is important, but only 43%–70% of cases manage to give up smoking2. Drug treatment for Buerger’s disease is used to treat symptoms rather than the underlying cause and is palliative in nature. The most commonly used pharmacological agents are aspirin, cilostazol, and prostanoid. These pharmacological agents are used to improve pain-free walking distance (PFWD) through vasodilatatory and antiplatelet effects. However, the effects of these drugs are temporary and cannot prevent disease progression. To date, there is no pharmacological treatment to reverse or stop the disease progression. Therefore, new therapeutic development is essential and urgent for Buerger’s disease patients. The new therapeutic agent for Buerger’s disease should have 1) no adverse events (AEs), 2) an efficacy to reverse the disease condition through revascularization and tissue regeneration instead of temporary symptom relief, and 3) prevention or reduction of limb amputation.
Mesenchymal stem cells (MSCs) are potential new ideal therapeutic agents and hold great promise for use in tissue repair and regeneration3,4. Among the sources of MSCs, adipose tissue represents an abundant and practical source for obtaining autologous stem cell tissue through minimally invasive procedures. Furthermore, the safety and treatment efficacy of adipose tissue-derived stem cells (ADSCs) for various diseases have been reported in animal models and clinical trials5,6. We previously reported the safety and efficacy of intravenous (IV) infusion7 and intra-articular injection8 of autologous ADSCs. A number of studies have reported that ADSCs secrete multiple angiogenic growth factors such as vascular endothelial growth factor (VEGF) and hepatocyte growth factor at bioactive levels9, indicating that ADSCs can be used for therapeutic angiogenesis in a clinical setting. In this regard, the possibility of treatment for critical limb ischemia (CLI) with autologous ADSCs has been reported10–12. However, a clinical trial for the development of stem cell treatment using autologous ADSCs in patients with severe Buerger’s disease has not yet been reported.
Accordingly, we conducted phase I and II clinical trials to investigate the safety and efficacy of autologous ADSCs at a dosage of 300 million cells per 60 kg of body weight in Buerger’s disease patients who had no response to symptomatic or supportive treatment.
MATERIALS AND METHODS
Study Design and Patients
The phase I/II study of autologous ADSCs for Buerger’s disease was approved by the Ministry of Food and Drug Safety with Investigational New Drug Application No. 1206 (December 18, 2007), the Institutional Review Board (IRB) at the Catholic University of Korea Seoul St. Mary’s Hospital, Seoul, Republic of Korea (KCMC08MS041), and SMG-SNU Boramae Medical Center, Seoul, Republic of Korea (20120801/06-2012-179/122). The clinical trial (ClinicalTrials.gov identifier: NCT01302015) was started on July 31, 2008, and was completed on November 26, 2013. The subjects were given information regarding the expected efficacy and safety of the trial indicated by results from preclinical studies, that is, the Buerger’s model animals significantly improved limb ischemia with human ADSCs (hADSCs) and showed no adverse effects. The subjects were informed of the study and signed a consent form. The subjects were informed that this treatment was an experimental clinical trial and that the treatment of this study was performed without any payments. The ability to pay for treatment was not a prerequisite for inclusion in the study. The phase I and II clinical trials were designed as multisite, nonrandomized, uncontrolled, and open-label studies. The inclusion criteria included subjects between 20 and 80 years of age, whose onset of Buerger’s disease occurred at least 6 months prior, and who showed over 50% luminal stenosis in an angiogram. The patients classified as Rutherford class II-4, III-5, or III-6, who were experiencing rest pain or had ulcers or gangrenes, and who were not suitable subjects for revascularization or vascular bypass surgery were enrolled in the clinical trial13. Overall, the subjects enrolled in this clinical trial were individuals with severe cases of Buerger’s disease, who were classified as Rutherford class II-4 or higher, who experienced rest pain, who had no therapeutic effect with the existing pharmacological agents, and who could be treated surgically. Individuals whose estimated life expectancy was less than 6 months due to comorbidity, or who had end-stage renal disease, or who had uncontrolled diabetes were excluded from this study. Also individuals requiring immediate amputation due to life-threatening risks and individuals with acute cardiovascular diseases, such as acute myocardial infarction and angina pectoris, were excluded.
Prior to enrollment, all subjects were checked according to the inclusion and exclusion criteria. The subjects’ medical history, laboratory tests, physical examination results, and vitality signs were checked, and the efficacy endpoint examinations including the assessments of total walking distance (TWD), PFWD, and pain at rest were conducted.
Cell Isolation, Characterization, and Administration
The immunophenotype, karyotyping, stability, toxicity, and tumorigenicity of cultured hADSCs were confirmed as previously described7. The isolation and characterization of autologous ADSCs were performed by the previously established culture protocol7 under good manufacturing practice (GMP) conditions in the Stem Cell Research Institute of R Bio (Seoul, Republic of Korea). Adipose tissue samples were obtained through liposuction from the abdominal subcutaneous fats of the subjects a week after enrollment. Subcutaneous adipose tissues were digested with collagenase I (1 mg/ml; Gibco/Life Technologies, Grand Island, NY, USA) under gentle agitation for 60 min at 37°C and filtered through a 100-mm nylon sieve to remove cellular debris, followed by centrifugation at 470 × g for 5 min. After centrifugation, the pellet was resuspended in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA, USA)-based media containing 0.2 mM ascorbic acid and 10% fetal bovine serum (FBS; JR Scientific, Woodland, CA, USA) obtained from bovine spongiform encephalopathy-free herd. The cell fraction was cultured overnight at 37°C/5% CO2 in DMEM-based media containing 0.2 mM ascorbic acid and 10% FBS. After 24 h, the cell adhesion was checked under an inverted microscope, and nonadherent cells were removed by washing with phosphate-buffered saline (PBS). The cell medium was changed to keratinocyte serum-free medium (SFM; Invitrogen)-based media containing 0.2 mM ascorbic acid, 0.09 mM calcium, 5 ng/ml recombinant epidermal growth factor (rEGF; ProSpec, East Brunswick, NJ, USA ), and 5% FBS. The cells were maintained for 4 to 5 days until confluent (passage 0). When the cells reached 90% confluency, they were subculture expanded in keratinocyte SFM-based media containing 0.2 mM ascorbic acid, 0.09 mM calcium, 5 ng/ml rEGF, and 5% FBS until passage 3. FBS contaminants from cultured MSCs were completely removed by several washings with PBS and were verified through the test of albumin level below the measurement limit using a bovine albumin enzyme-linked immunosorbent assay (ELISA) quantitation kit (Bethyl Laboratories, Montgomery, TX, USA). Before transporting the cells for administration, aliquots of the ADSCs are tested for cell viability, fungal, bacterial, endotoxin, and mycoplasma contamination and immunophenotype for MSCs. Cell viability evaluated by trypan blue exclusion was >89%, and no evidence of bacterial, fungal, and mycoplasmal contamination was observed. The ADSCs showed a homogenous population of cells with high positive marker expression levels of CD73 and CD90 and very low negative marker expression levels of CD31, CD34, and CD45. CD73 and CD90 were expressed at a high level of >91% and >94%, respectively. Negative markers of CD31, CD34, and CD45 were expressed at a very low level of <2%. In the clinical trial, ADSCs (1 × 107 cells/500 μl/syringe) were injected intramuscularly with a volume of 5 × 106 cells/kg (based on the patient’s weight) about 3 weeks after the collection of adipose tissues. The ADSCs were injected into 30 points in the ischemic zone of the lower extremities (17 limbs from 17 patients). The subjects were instructed to visit the hospital at 1, 3, and 6 months after the injection to check various assessments for efficacy and safety.
Safety Measures
Safety endpoints were assessed for up to 6 months after the injection. Physical examinations, laboratory tests, and tumor marker tests were conducted on the subjects. Tolerability and AEs were also assessed. The AEs were classified based on the National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.0 Scale (NCI CTCAE ver4.0). The incidence of adverse drug reactions (ADRs) and serious adverse events (SAEs) was also monitored.
Efficacy Measures
The primary efficacy endpoint, TWD, was measured using the Bruce treadmill protocol14. The secondary efficacy endpoint, rest pain, was measured using the visual analog scale (VAS). No rest pain was indicated as VAS 0 and the severest pain as VAS 10. The PFWD was measured using the Bruce treadmill protocol14, with the distance measured until signs of claudication were apparent. Measurements were taken based on the toe–brachial pressure index (TBPI), transcutaneous oxygen pressure (TcPO2), arterial brachial pressure index (ABPI), and angiogram according to the previous study15. Computed tomography (CT) angiography was used to measure the formation of collateral vessels. New collateral vessel formation was assessed as +0 (no collateral development), +1 (slight collateral development), +2 (moderate collateral development), or +3 (rich collateral development).
Follow-Up Questionnaire Survey
A questionnaire was sent to the subjects enrolled in the clinical trial 2 years after the injection of ADSCs as a means to examine the progression of the disease. The survey was conducted following a review by the IRB of SMG-SNU Boramae Medical Center. The principal investigator delivered the questionnaire to the subjects after obtaining informed consent via phone or mail. The assessment items in the questionnaire included 1) smoking, 2) amputation in the area where ADSCs were injected, 3) amount of medication taken for pain associated with Buerger’s disease, 4) rest pain, 5) ulcer treatment in the area where ADSCs were injected, and 6) additional treatment at the administration site.
Statistical Analysis
A prior study of Ishida et al. was referenced to determine the number of subjects in the phase I and II clinical trials16. Baseline and week 4 TWD in meters from the clinical study16 were used as a basis to calculate the current sample size. Accordingly, a sample size of 18 was obtained including 10% dropouts. Safety was assessed based on the subjects included in the intention-to-treat (ITT) analysis group, while efficacy was assessed based on the patients in the per-protocol (PP) analysis group. The missing data were substituted using the last observation carried forward (LOCF) method. The significance level for statistical analysis was set at 0.05 and considered significant when p < 0.05. A paired t-test or Wilcoxon signed-rank test was run depending on the satisfaction of normality assumptions using statistical software SAS version 9.3 (SAS Institute Inc., Cary, NC, USA). Data were expressed as the mean ± standard deviation (SD), median, and range (min, max).
RESULTS
Disposition of Patients
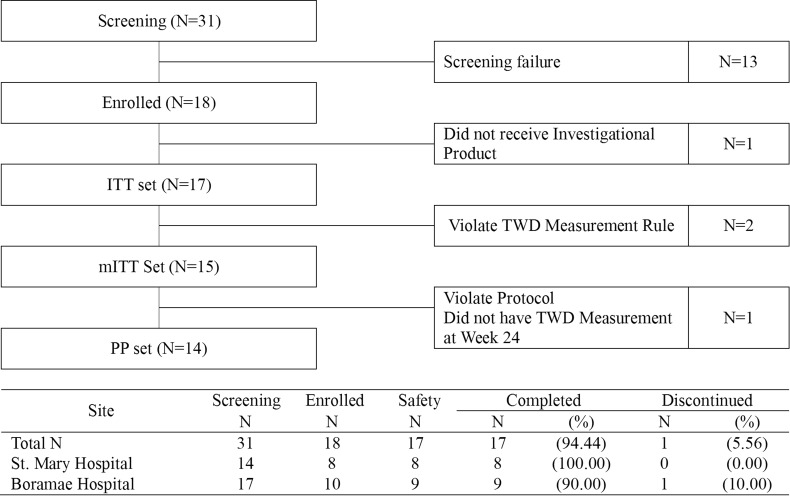
A total of 31 patients were screened and gave informed consent to enter the study. Of those, 18 (58%) patients were eligible for the study. Of the 18 patients, 17 patients received the investigational product. One patient decided not to receive the investigational product. Therefore, 17 patients were analyzed as safety and ITT sets. Of those, two patients violated protocol for the TWD measurement rule. Therefore, 15 patients were analyzed as the modified ITT (mITT) set. Of those, one patient did not have week 24 TWD measurement. Therefore, 14 patients were analyzed as the PP set (Fig. 1).
Figure 1.
Patient disposition. ITT, intention to treat; mITT, modified intention to treat; PP, per protocol; TWD, total walking distance; N, number.
Demographic and Other Baseline Characteristics
The average age of the patients was 43 years, 100% were male smokers, and 70.59% were with alcohol drinking histories (12 out of 17 patients) (see Table 1). The average age of the subjects was 42.59 ± 9.01, with individuals in their 30s and 40s accounting for more than 70% of the subjects.
Table 1.
Demographic and Other Baseline Characteristics
| Demographic Information | ITT Set (N = 17) |
|---|---|
| Age (years) | |
| 20–29 | 1 (5.88%) |
| 30–39 | 7 (41.18%) |
| 40–49 | 5 (29.41%) |
| 50–59 | 3 (17.65%) |
| ≥60 | 1 (5.88%) |
| Mean ± SD | 42.59 ± 9.01 |
| Median | 44 |
| Min/Max | 24/60 |
| Gender | |
| Male | 17 (100.00%) |
| Female | 0 (0.00%) |
| Cigarette smoking | |
| Yes | 12 (70.59%) |
| No | 5 (29.41%) |
| Ceased smoking | 7 (58.33%) |
| Presently smoking | 5 (41.67%) |
| Alcohol consumption (past) | |
| Yes | 12 (70.59%) |
| No | 5 (29.41%) |
| Alcohol consumption (present) | |
| Yes | 8 (47.06%) |
| No | 9 (52.94%) |
ITT, intention to treat.
Safety
Safety was assessed based on the 17 patients in the ITT set. Of 17 subjects, 11 (64.71%) (26 cases) experienced AEs during the clinical trial period, and treatment-emergent AEs were reported among 47.06% of the subjects (8/17 subjects, 22 cases). The details of the AEs are shown in Table 2. In the case of the 26 AEs reported, all involved pain of grade 2 or less, except for one case of grade 3 pain reported almost past the 6-month period. There were no AE-related dropouts. Moreover, the AEs were confirmed to be unassociated with the ADSC injections by the investigators who had administered the ADSCs. There were no subjects who experienced ADRs and SAEs in relation with ADSCs (Table 3). In addition, there were no statistically or clinically significant changes in the vitality signs or physical examination and laboratory test results.
Table 2.
Details of Adverse Events and Reactions
| No. | System Organ Class | Preferred Term | ADSCs Inj. Date | AE Start Date | AE End Date | Condition | Grade | Result | Association | Continuance of Clinical Trial | Treatment | SAE | Dropout |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 103 | Infections and infestations | Staphylococcal infection | 9/12/2008 | 9/5/2008 | 9/16/2008 | Continuous | Grade 2 | Recovered | Clearly unassociated | Continued | Yes | No | Continued participation |
| 112 | General disorders and administration site conditions | Pain | 5/27/2010 | 11/2010* | – | Continuous | Grade 3 | Continued | Clearly unassociated | Continued | No | No | Continued participation |
| 114 | Gastrointestinal disorders | Gastric ulcer | 7/28/2010 | 10/13/2010 | 10/28/2010 | Intermittent (1–2 times/week) | Grade 1 | Recovered | Clearly unassociated | Continued | Yes | No | Continued participation |
| 114 | Injury, poisoning, and procedural complications | Ligament rupture | 7/28/2010 | 11/1/2010 | – | Intermittent (ND) | Grade 1 | Continued | Clearly unassociated | Continued | Yes | No | Continued participation |
| 201 | Infections and infestations | Nasopharyngitis | 1/3/2013 | 1/26/2013 | 1/29/2013 | Continuous | Grade 2 | Recovered | Clearly unassociated | Continued | Yes | No | Continued participation |
| 204 | Gastrointestinal disorders | Large intestine polyp | 1/24/2013 | 4/12/2013 | 4/12/2013 | Continuous | Grade 2 | Recovered | Clearly unassociated | Continued | Yes | No | Continued participation |
| 204 | General disorders and administration site conditions | Ischemic ulcer | 1/24/2013 | 2/2013* | 8/8/2013 | Continuous | Grade 2 | Recovered | Clearly unassociated | Continued | Yes | No | Continued participation |
| 204 | General disorders and administration site conditions | Ischemic ulcer | 1/24/2013 | 2/2013* | 8/8/2013 | Continuous | Grade 2 | Recovered | Clearly unassociated | Continued | Yes | No | Continued participation |
| 204 | General disorders and administration site conditions | Ischemic ulcer | 1/24/2013 | 2/2013* | 8/8/2013 | Continuous | Grade 2 | Recovered | Clearly unassociated | Continued | Yes | No | Continued participation |
| 204 | Infections and infestations | Sinusitis | 1/24/2013 | 2/2013* | 2/2013* | Continuous | Grade 2 | Recovered | Clearly unassociated | Continued | Yes | No | Continued participation |
| 204 | Musculoskeletal and connective tissue disorders | Pain in extremity | 1/24/2013 | 3/2013* | – | Continuous | Grade 2 | Continued | Clearly unassociated | Continued | No | No | Continued participation |
| 205 | Injury, poisoning, and procedural complications | Ligament sprain | 2/1/2013 | 7/17/2013 | 7/22/2013 | Continuous | Grade 2 | Recovered | Clearly unassociated | Continued | Yes | No | Continued participation |
| 205 | Nervous system disorders | Nerve root lesion | 2/1/2013 | 7/17/2013 | 7/22/2013 | Continuous | Grade 2 | Recovered | Clearly unassociated | Continued | Yes | No | Continued participation |
| 208 | Gastrointestinal disorders | Diarrhea | 2/7/2013 | 2/21/2013 | 2/22/2013 | Continuous | Grade 2 | Recovered | Clearly unassociated | Continued | Yes | No | Continued participation |
| 208 | Gastrointestinal disorders | Dyspepsia | 2/7/2013 | 2/18/2013 | 2/19/2013 | Continuous | Grade 2 | Recovered | Clearly unassociated | Continued | Yes | No | Continued participation |
| 208 | Infections and infestations | Nasopharyngitis | 2/7/2013 | 4/18/2013 | 4/21/2013 | Continuous | Grade 2 | Recovered | Clearly unassociated | Continued | Yes | No | Continued participation |
| 208 | Musculoskeletal and connective tissue disorders | Back pain | 2/7/2013 | 5/31/2013 | 6/7/2013 | Continuous | Grade 2 | Recovered | Clearly unassociated | Continued | Yes | No | Continued participation |
| 208 | Musculoskeletal and connective tissue disorders | Musculoskeletal pain | 2/7/2013 | 5/3/2013 | 5/6/2013 | Continuous | Grade 2 | Recovered | Clearly unassociated | Continued | Yes | No | Continued participation |
| 208 | Musculoskeletal and connective tissue disorders | Musculoskeletal pain | 2/7/2013 | 5/31/2013 | 6/7/2013 | Continuous | Grade 2 | Recovered | Clearly unassociated | Continued | Yes | No | Continued participation |
| 208 | Musculoskeletal and connective tissue disorders | Myalgia | 2/7/2013 | 3/13/2013 | 3/16/2013 | Continuous | Grade 2 | Recovered | Clearly unassociated | Continued | Yes | No | Continued participation |
| 208 | Musculoskeletal and connective tissue disorders | Pain in extremity | 2/7/2013 | 5/3/2013 | 5/6/2013 | Continuous | Grade 2 | Recovered | Clearly unassociated | Continued | Yes | No | Continued participation |
| 208 | Respiratory, thoracic, and mediastinal disorders | Rhinitis allergic | 2/7/2013 | 1/12/2013 | 1/19/2013 | Continuous | Grade 2 | Recovered | Clearly unassociated | Continued | Yes | No | Continued participation |
| 211 | Nervous system disorders | Headache | 3/21/2013 | 4/2013* | 4/2013* | Continuous | Grade 2 | Recovered | Clearly unassociated | Continued | Yes | No | Continued participation |
| 215 | Skin and subcutaneous tissue disorders | Urticaria | 5/10/2013 | 4/10/2013 | 4/12/2013 | Continuous | Grade 2 | Recovered | Clearly unassociated | Continued | Yes | No | Continued participation |
| 216 | General disorders and administration site conditions | Injection site reaction | 5/23/2013 | 5/3/2013 | 5/6/2013 | Continuous | Grade 1 | Recovered | Clearly unassociated | Continued | No | No | Continued participation |
| 217 | Musculoskeletal and connective tissue disorders | Pain in extremity | 5/16/2013 | 7/2013* | – | Continuous | Grade 2 | Continued | Unassociated | Continued | Yes | No | Continued participation |
ADSCs, adipose tissue-derived mesenchymal stem cells; AE, adverse event; SAE, serious adverse event.
Day unknown.
Table 3.
Summary of Adverse Events and Reactions
| Adverse events and reactions | N = 17 | |
|---|---|---|
| N (%) | No. Cases | |
| Adverse events | 11 (64.71) | 26 |
| Treatment emergent adverse events | 8 (47.06) | 22 |
| Adverse drug reactions | 0 (0.00) | 0 |
| Serious adverse events | 0 (0.00) | 0 |
Clinical Improvement
The primary efficacy variable used to determine the efficacy of ADSCs was the change of TWD from screening to week 24. The results are presented in Table 4 for the ITT, mITT, and PP sets. The mean changes from screening to week 24 of TWD were significantly better in every set (97.39 m, p = 0.0406 for the ITT set; 124.21 m, p = 0.0149 for the mITT set; and 119.91 m, p = 0.0264 for the PP set using paired t-test at α = 0.05 level of significance). A similar result using the Wilcoxon signed-rank test was observed as a post hoc analysis.
Table 4.
Primary Efficacy Variable: Treadmill Walking Distance (TWD)
| TWD (Meters) | ITT (N = 17) | mITT (N = 15) | PP (N = 14) |
|---|---|---|---|
| Visit 1 (screening) | |||
| Mean (SD) | 300.18 (170.47) | 257.62 (117.04) | 250.06 (117.59) |
| Median | 291.20 | 270.60 | 250.00 |
| Min/Max | 35.30/764.00 | 35.30/446.00 | 35.30/446.00 |
| Visit 7 (week 24) | |||
| Mean (SD) | 397.57 (208.04) | 381.83 (214.85) | 369.97 (217.81) |
| Median | 425.00 | 375.50 | 339.70 |
| Min/Max | 81.80/722.30 | 81.80/722.30 | 81.80/722.30 |
| Change | |||
| Mean (SD) | 97.39 (180.25) | 124.21 (173.42) | 119.91 (179.13) |
| Median | 58.40 | 133.80 | 96.10 |
| Min/Max | −195.20/553.20 | −195.20/553.20 | −195.20/553.20 |
| 95% CI | (4.72, 190.10) | (28.17, 220.20) | (16.49, 223.30) |
| p Value* | 0.0406 | 0.0149 | 0.0264 |
| p Value† | 0.0569 | 0.0181 | 0.0295 |
ITT, intention to treat; mITT, modified intention to treat; PP, per protocol. Change = Visit 7 (week 24) − Visit 1 (screening).
*Paired t-test and †Wilcoxon Signed-Rank test.
The results for the secondary efficacy variable, change from screening to week 24 on the VAS, are presented in Table 5 for the ITT, mITT, and PP sets. The mean changes from screening to week 24 on the VAS were significantly better in every set (−1.76, p = 0.0289 for the ITT set; −2.47, p = 0.0013 for the mITT set; and −2.36, p = 0.0031 for the PP set using paired t-test at α = 0.05 level of significance). A similar result using the Wilcoxon signed-rank test was observed as a post hoc analysis. The results for the secondary efficacy variable, change from screening to week 24 on the PFWD, are presented in Table 6 for the ITT, mITT, and PP sets. The mean changes from screening to week 24 on the PFWD were significantly better in every set (119.62 m, p = 0.0118 for the ITT set; 145.57 m, p = 0.0042 for the mITT set; and 145.14 m, p = 0.0074 for the PP set using paired t-test at α = 0.05 level of significance). A similar result using the Wilcoxon signed-rank test was observed as a post hoc analysis. The results for the secondary efficacy variable, change from screening to week 24 on the ABPI are presented in Table 7 for the ITT, mITT, and PP sets. The mean changes from screening to week 24 on the ABPI for the right ankle were not significantly better in every set (0.01 mmHg, p = 0.8886 for the ITT set; 0.01 mmHg, p = 0.8892 for the mITT set; and −0.03 mmHg, p = 0.4080 for the PP set using paired t-test at α = 0.05 level of significance). The mean changes from screening to week 24 on the ABPI for the left ankle were significantly better in every set (0.05 mmHg, p = 0.0034 for the ITT set; 0.04 mmHg, p = 0.0105 for the mITT set; and 0.04 mmHg, p = 0.0225 for the PP set using paired t-test at α = 0.05 level of significance). A similar result using the Wilcoxon signed-rank test was observed as a post hoc analysis.
Table 5.
Secondary Efficacy Variable: Visual Analogue Scale (VAS)
| VAS | ITT (N = 17) | mITT (N = 15) | PP (N = 14) |
|---|---|---|---|
| Visit 1 (screening) | |||
| Mean (SD) | 5.59 (2.32) | 5.87 (2.33) | 5.86 (2.41) |
| Median | 6.00 | 6.00 | 6.5 |
| Min/Max | 2.00/10.00 | 2.00/10.00 | 2.00/10.00 |
| Visit 7 (week 24) | |||
| Mean (SD) | 3.82 (2.92) | 3.40 (2.75) | 3.50 (2.82) |
| Median | 2.00 | 2.00 | 2.00 |
| Min/Max | 1.00/10.00 | 1.00/10.00 | 1.00/10.00 |
| Change | |||
| Mean (SD) | −1.76 (3.03) | −2.47 (2.39) | −2.36 (2.44) |
| Median | −2.00 | −2.00 | −2.00 |
| Min/Max | −7.00/5.00 | −7.00/1.00 | −7.00/1.00 |
| 95% CI | (−3.32, −0.21) | (−3.79, −1.15) | (−3.76, −0.95) |
| p Value* | 0.0289 | 0.0013 | 0.0031 |
| p Value† | 0.0309 | 0.0020 | 0.0039 |
ITT, intention to treat; mITT, modified intention to treat; PP, per protocol. Change = Visit 7 (week 24) − Visit 1 (screening).
*Paired t-test and †Wilcoxon Signed-Rank test.
Table 6.
Secondary Efficacy Variable: Pain Free Walking Distance (PFWD)
| PFWD (Meters) | ITT (N = 17) | mITT (N = 15) | PP (N = 14) |
|---|---|---|---|
| Visit 1 (screening) | |||
| Mean (SD) | 217.79 (176.49) | 184.21 (114.53) | 171.40 (107.13) |
| Median | 177.30 | 143.90 | 142.80 |
| Min/Max | 35.30/762.00 | 35.30/369.50 | 35.30/369.50 |
| Visit 7 (week 24) | |||
| Mean (SD) | 337.00 (184.69) | 329.77(179.86) | 316.54(178.92) |
| Median | 323.00 | 323.90 | 313.90 |
| Min/Max | 64.50/662.70 | 64.50/662.70 | 64.50/662.70 |
| Change | |||
| Mean (SD) | 119.62 (173.61) | 145.57 (165.13) | 145.14 (171.35) |
| Median | 114.40 | 151.50 | 135.55 |
| Min/Max | −195.20/553.20 | −195.20/553.20 | −195.20/553.20 |
| 95% CI | (30.35, 208.90) | (54.12, 237.0) | (46.21, 244.10) |
| p Value* | 0.0118 | 0.0042 | 0.0074 |
| p Value† | 0.0079 | 0.0026 | 0.0040 |
ITT, intention to treat; mITT, modified intention to treat; PP, per protocol. Change = Visit 7 (week 24) − Visit 1 (screening).
*Paired t-test and †Wilcoxon Signed-Rank test.
Table 7.
Secondary Efficacy Variable: Ankle–Brachial Pressure Index (ABPI)
| ABPI (mmHg) | ITT (N = 17) | mITT (N = 15) | PP (N = 14) |
|---|---|---|---|
| Visit 1 (screening) | |||
| Right | |||
| Mean (SD) | 0.96 (0.24) | 0.97 (0.23) | 0.98 (0.23) |
| Median | 1.07 | 1.07 | 1.08 |
| Min/Max | 0.58/1.27 | 0.58/1.27 | 0.58/1.27 |
| Left | |||
| Mean (SD) | 0.76 (0.27) | 0.74 (0.27) | 0.75(0.28) |
| Median | 0.67 | 0.63 | 0.65 |
| Min/Max | 0.35/1.24 | 0.35/1.24 | 0.35/1.24 |
| Visit 7 (week 24) | |||
| Right | |||
| Mean (SD) | 0.97 (0.22) | 0.97 (0.20) | 0.95 (0.19) |
| Median | 1.00 | 1.00 | 0.96 |
| Min/Max | 0.61/1.24 | 0.63/1.23 | 0.63/1.20 |
| Left | |||
| Mean (SD) | 0.81 (0.26) | 0.78(0.26) | 0.78(0.26) |
| Median | 0.72 | 0.70 | 0.69 |
| Min/Max | 0.46/1.28 | 0.46/1.28 | 0.46/1.28 |
| Change | |||
| Right | |||
| Mean (SD) | 0.01 (0.17) | 0.01 (0.18) | −0.03 (0.13) |
| Median | −0.02 | −0.03 | −0.03 |
| Min/Max | −0.24/0.50 | −0.24/0.50 | −0.24/0.17 |
| 95% CI | (−0.08, 0.09) | (−0.09, 0.11) | (−0.10 ,0.04) |
| p Value* | 0.8886 | 0.8892 | 0.4080 |
| p Value† | 0.6193 | 0.6683 | 0.3490 |
| Left | |||
| Mean (SD) | 0.05 (0.05) | 0.04 (0.05) | 0.04 (0.05) |
| Median | 0.04 | 0.04 | 0.04 |
| Min/Max | −0.07/0.12 | −0.07/0.11 | −0.07/0.11 |
| 95% CI | (0.02, 0.07) | (0.01, 0.07) | (0.01, 0.07) |
| p Value* | 0.0034 | 0.0105 | 0.0225 |
| p Value† | 0.0040 | 0.0128 | 0.0226 |
ITT, intention to treat; mITT, modified intention to treat; PP, per protocol. Change = Visit 7 (week 24) − Visit 1 (screening).
*Paired t-test and †Wilcoxon Signed-Rank test.
The results for the secondary efficacy variable TBPI (Table 8) and TcPO2 (Table 9) were not significantly better in the ITT, mITT and PP sets using paired t-test and Wilcoxon signed-rank test. The results for the secondary efficacy variable, change of angiography from screening to week 24, are presented in Table 10. New collateral vessel formation was observed in only one patient in the PP set by CT angiography.
Table 8.
Secondary Efficacy Variable: Toe–Brachial Pressure Index (TBPI)
| TBPI (mmHg) | ITT (N = 17) | mITT (N = 15) | PP (N = 14) |
|---|---|---|---|
| Visit 1 (screening) | |||
| Right | |||
| n | 8 | 8 | 8 |
| Mean (SD) | 0.38 (0.20) | 0.38 (0.20) | 0.38 (0.20) |
| Median | 0.31 | 0.31 | 0.31 |
| Min/Max | 0.12/0.70 | 0.12/0.70 | 0.12/0.70 |
| Left | |||
| n | 5 | 5 | 5 |
| Mean (SD) | 0.26 (0.20) | 0.26 (0.20) | 0.26 (0.20) |
| Median | 0.23 | 0.23 | 0.23 |
| Min/Max | 0.08/0.56 | 0.08/0.56 | 0.08/0.56 |
| Visit 7 (week 24) | |||
| Right | |||
| n | 8 | 8 | 8 |
| Mean (SD) | 0.37 (0.27) | 0.37(0.27) | 0.37(0.27) |
| Median | 0.33 | 0.33 | 0.33 |
| Min/Max | 0.09/0.77 | 0.09/0.77 | 0.09/0.77 |
| Left | |||
| n | 6 | 6 | 6 |
| Mean (SD) | 0.23 (0.19) | 0.23 (0.19) | 0.23 (0.19) |
| Median | 0.17 | 0.17 | 0.17 |
| Min/Max | 0.11/0.61 | 0.11/0.61 | 0.11/0.61 |
| Change | |||
| Right | |||
| n | 8 | 8 | 8 |
| Mean (SD) | −0.02 (0.20) | −0.02 (0.20) | −0.02 (0.20) |
| Median | −0.01 | −0.01 | −0.01 |
| Min/Max | −0.44/0.18 | −0.44/0.18 | −0.44/0.18 |
| 95% CI | (−0.18, 0.15) | (−0.18, 0.15) | (−0.18, 0.15) |
| p Value* | 0.8353 | 0.8353 | 0.8353 |
| p Value† | 0.9453 | 0.9453 | 0.9453 |
| Left | |||
| n | 5 | 5 | 5 |
| Mean (SD) | −0.004 (0.06) | −0.004 (0.06) | −0.004 (0.06) |
| Median | 0.03 | 0.03 | 0.03 |
| Min/Max | −0.09/0.05 | −0.09/0.05 | −0.09/0.05 |
| 95% CI | (−0.08, 0.07) | (−0.08, 0.07) | (−0.08, 0.07) |
| p Value* | 0.8868 | 0.8868 | 0.8868 |
| p Value† | 1.0000 | 1.0000 | 1.0000 |
ITT, intention to treat; mITT, modified intention to treat; PP, per protocol. Change = Visit 7 (week 24) − Visit 1 (screening).
*Paired t-test and †Wilcoxon Signed-Rank test.
Table 9.
Secondary Efficacy Variable: Transcutaneous Oxygen Pressure (TcPO2)
| TcPO2 (mmHg) | ITT (N = 17) | mITT (N = 15) | PP (N = 14) |
|---|---|---|---|
| Visit 1 (screening) | |||
| Mean (SD) | 51.72 (25.34) | 55.82 (23.61) | 57.71 (23.30) |
| Median | 44.50 | 52.80 | 54.85 |
| Min/Max | 8.20/104.30 | 28.00/104.30 | 28.00/104.30 |
| Visit 7 (week 24) | |||
| Mean (SD) | 47.51 (14.57) | 48.60 (13.03) | 49.71 (12.76) |
| Median | 51.30 | 51.30 | 51.65 |
| Min/Max | 18.50/65.00 | 20.5/65.00 | 20.50/65.00 |
| Change | |||
| Mean (SD) | −4.21 (25.35) | −7.22 (25.35) | −7.99 (26.13) |
| Median | −3.00 | −5.50 | −7.05 |
| Min/Max | −52.30/36.60 | −52.30/36.60 | −52.30/36.60 |
| 95% CI | (−17.25, 8.82) | (−21.26, 6.82) | (−23.08, 7.09) |
| p Value* | 0.5032 | 0.2887 | 0.2730 |
| p Value† | 0.5791 | 0.2769 | 0.2412 |
ITT, intention to treat; mITT, modified intention to treat; PP, per protocol. Change = Visit 7 (week 24) − Visit 1 (screening).
*Paired t-test and †Wilcoxon Signed-Rank test.
Table 10.
Secondary Efficacy Variable: Angiography
| Visit 7 (Week 24) | +0 [n (%)] | +1 [n (%)] | +2 [n (%)] | +3 [n (%)] |
|---|---|---|---|---|
| ITT set (N = 15*) | ||||
| Visit 1 (screening) | ||||
| +0 | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| +1 | 0 (0.00) | 3 (20.00) | 0 (0.00) | 0 (0.00) |
| +2 | 0 (0.00) | 0 (0.00) | 5 (33.33) | 1 (6.67) |
| +3 | 0 (0.00) | 0 (0.00) | 0 (0.00) | 6 (40.00) |
| p Value | 0.8013 | |||
| mITT set (N = 15) | ||||
| Visit 1 (screening) | ||||
| +0 | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| +1 | 0 (0.00) | 3 (20.00) | 0 (0.00) | 0 (0.00) |
| +2 | 0 (0.00) | 0 (0.00) | 5 (33.33) | 1 (6.67) |
| +3 | 0 (0.00) | 0 (0.00) | 0 (0.00) | 6 (40.00) |
| p Value | 0.8013 | |||
| PP set (N = 14) | ||||
| Visit 1 (screening) | ||||
| +0 | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| +1 | 0 (0.00) | 2 (14.29) | 0 (0.00) | 0 (0.00) |
| +2 | 0 (0.00) | 0 (0.00) | 5 (35.71) | 1 (7.14) |
| +3 | 0 (0.00) | 0 (0.00) | 0 (0.00) | 6 (42.86) |
| p Value | 0.8013 | |||
ITT, intention to treat; mITT, modified intention to treat; PP, per protocol. +0: no collateral development; +1: slight collateral development; +2: moderate collateral development; +3: rich collateral development; McNemar Bowker’s test.
Two patients not included.
Follow-Up Questionnaire Survey
As for the follow-up questionnaire survey, eight out of nine subjects registered with the SMG-SNU Boramae Medical Center provided their informed consent. The longest follow-up was 2 years 8 months (Patient No. 201), and the shortest follow-up was 2 years 3 months (Patient No. 206) after ADSC injection. Despite the long lapse of time, none of the subjects (n = 8) had undergone amputation.
Although amputation was not included among the efficacy indicators for phase I and II clinical trials, it was possible to check for amputation in the Buerger’s disease patients by assessing the affected areas during the clinical trial period. While 4 of the 17 subjects had amputations before registering for the clinical trial, none of the subjects were found to have undergone any additional amputations based on the assessments of the affected areas during the clinical trial period and the follow-up questionnaire survey conducted 2 years after the ADSC injections.
Based on data regarding the symptomatic drugs taken during the clinical trial period and prior to the follow-up questionnaire survey, changes in the amount of medication used for Buerger’s disease were analyzed. As shown in Table 11, nine subjects (64.3%) stopped taking medications that they had taken prior to the clinical trial, and three subjects (21.4%) reduced the amount of medications, while two subjects (14.3%) reported no changes in the amount of medications taken.
Table 11.
Changes in the Intake of Pain Medications by the Clinical Trial Subjects
| Patient No. | Before ADSCs Administration (The Intake of Medications at Day of Screening) | 6 Months After ADSCs Administration | 2 Years After ADSCs Administration | Remarks |
|---|---|---|---|---|
| 103 | Oxycodone hydrochloride | Oxycodone hydrochloride | Unchecked | Reduced dose |
| Sarpogrelate hydrochloride | Cilostazol | |||
| Beraprost sodium | ||||
| Tramadol hydrochloride | ||||
| Aspirin (500 mg) | Aspirin (100 mg) | |||
| 106 | Cilostazol | Cilostazol | Unchecked | No change |
| 107 | Sarpogrelate hydrochloride | Unchecked | Ceased intake | |
| Limaprost alfadex | ||||
| 109 | Cilostazol | Cilostazol | Unchecked | No change |
| 110 | Beraprost sodium | Unchecked | Reduced dose | |
| Limaprost alfadex | ||||
| Aspirin | Aspirin | |||
| 201 | None* | Acetaminophen (325 mg) | Acetaminophen (162.5 mg) | Reduced dose |
| Tramadol hydrochloride (37.5 mg) | Tramadol hydrochloride (18.75 mg) | |||
| (Temporary intake before Visit 7) | ||||
| 202 | None† | Beraprost sodium | None | Ceased intake |
| Aspirin | ||||
| 204 | Cilostazol | Cilostazol | None | Ceased intake |
| 205 | Beraprost sodium | Beraprost sodium | None | Ceased intake |
| Aspirin | Aspirin | |||
| 208 | Beraprost sodium | Beraprost sodium | None | Ceased intake |
| 211 | None‡ | None | None | Ceased intake |
| 215 | Beraprost sodium | None | None | Ceased intake |
| 216 | Beraprost sodium | None | None | Ceased intake |
| 217 | Beraprost sodium | Beraprost sodium | None | Ceased intake |
| Kallidinogenase | Kallidinogenase |
ADSCs, adipose tissue-derived mesenchymal stem cells.
The patient took medications before the day of screening including Prena Tab (methylprednisolone), Retonase Tab (streptokinase–streptodornase), and Ibuprofen Tab 400 mg.
The patient took medications before the day of screening including Celebrex Cap 200 mg (celecoxib), Aspirin Protect Tab 100 mg (aspirin), Mevalotin Tab, and Berasil Tab (beraprost sodium).
The patient took Coumadin 5 mg before the day of screening.
The VAS score for pain at the end of the clinical trial was 3.5 ± 2.9 on average, and the average VAS score reported for a week in the questionnaire was 2.4 ± 3.1. This shows that there was an additional decrease of 1.1 ± 1.9 (32.1% decrease) in the VAS score for pain after more than 2 years of ADSC injections. However, this difference was not statistically significant (p = 0.068). Nonetheless, two (25.0%) of the subjects participating in the survey reported zero pain (VAS score: 0) at the end of the clinical trial, and four (50.0%) of the subjects participating in the survey reported zero pain (VAS score: 0) in the questionnaire (Table 12).
Table 12.
Results of the Follow-up Questionnaire Items on Rest Pain Measured by the Visual Analog Scale (N = 8)
| Category | Mean ± SD | Median | Min/Max |
|---|---|---|---|
| End of clinical trial | 3.5 ± 2.9 | 4 | 0/7 |
| In the past week | 2.4 ± 3.1 | 0.5 | 0/7 |
| Change | −1.1 ± 1.9 | 0 | −5/0 |
| p Value | 0.068 |
As shown in Table 13, at the time of the survey, three subjects (37.5%) fully recovered from the ulcers in the area where the therapeutic agent was administered, while one subject (12.5%) responded that the ulcer was healing. However, one subject (12.5%) answered that he had developed a new ulcer. After the termination of the clinical trial, the subjects were asked whether additional treatment was administered on the areas injected with the therapeutic agent under investigation. As shown in Table 14, seven of the eight subjects (87.5%) responded that they did not undergo any additional treatments, while only one subject (12.5%) received a vascular bypass and another treatment (leech therapy).
Table 13.
Results of the Follow-Up Questionnaire Items on Ulcer Treatment
| Questionnaire Results | n = 8 | Changes in Ulcer After Clinical Trial | n = 8 |
|---|---|---|---|
| No ulcers | 3 (37.5%) | No existing ulcers | 4 (50.0%) |
| Newly occurring ulcers | 1 (12.5%) | Existing ulcers | 4 (50.0%) |
| Amid recovery | 1 (12.5%) | Amid recovery | 1 (25.0%) |
| Full recovery from ulcers | 3 (37.5%) | Full recovery from ulcers | 3 (75.0%) |
| Total | 8 (100.0%) | Total | 8 (100.0%) |
Table 14.
Results of the Follow-Up Questionnaire Items on Additional Treatment on the Administration Site
| Category | n = 8 |
|---|---|
| Did not receive any other treatments | 7 (87.5%) |
| Sympathectomy | 0 (0.0%) |
| Vascular bypass | 1 (12.5%) |
| Amputation | 0 (0.0%) |
| Other treatments | 1 (12.5%) |
| Total | 8 (100.0%) |
DISCUSSION
Buerger’s disease is characterized by inflammatory occlusive vasculitis of the small- and medium-sized arteries and veins, and affects young adult smokers. Once the disease has become established, smoking cessation is the only effective way to prevent evolution of the disease and to reduce the risk of major amputations. Medical treatments with anticoagulants, vasodilators, or cilostazol may help improve the symptoms of the disease but cannot prevent disease progression17–20. Recognizing the need for a safe and effective alternative to discontinuation of tobacco use, phase I and II clinical trials of autologous ADSCs have been conducted for the treatment of patients with Buerger’s disease who were refractory to the traditional treatment including anticoagulants, vasodilators, and surgical regime and were experiencing rest pain. In other words, these patients had no therapeutic option with the current medications. Our results demonstrated that the autologous ADSC therapy was safe and prevented the risk of amputation after a 2-year follow-up. The current study also demonstrated that one-time intramuscular injection of autologous ADSCs was effective in reducing rest pain longer than 2 years in no-option Buerger’s disease patients. The ADSC treatment shown in this study suggested that stem cell therapy can be used safely for Buerger’s disease patients along with the use of the current medications.
Based on the conditions of Buerger’s disease, a nonrandomized, noncontrolled design was selected so that all enrolled patients were treated after screening. Because it would be unethical to instruct the subjects to stop taking the medications for clinical trial purposes, in this trial the use of existing symptomatic treatment methods such as cilostazol or beraprost sodium was allowed. Safety was assessed based on the subjects included in the ITT analysis group for 24 weeks. Changes in variables after the treatment compared to screening were examined for efficacy. Although treatment assignment blinding is important to lessen the potential for bias in study results, ensuring blinding is difficult for this treatment. For these correlated data, paired sample t-test between screening and week 24 was an appropriate statistical method for the analyses of primary and continuous secondary efficacy variables.
In terms of safety of autologous ADSCs, no SAEs were observed following the intramuscular injections of ADSCs in Buerger’s disease patients. Also no ADRs occurred, and none of the subjects dropped out from the study during the clinical trial period. These results confirm and expand the safety of ADSCs, which was previously confirmed in patients with spinal cord injury7 or osteoarthritis8. In line with our results, the administration of stem cells has been reported to be safe in numerous studies, confirming again the safety of stem cell drug21–25.
The efficacy results of this study demonstrated the clinical benefit of autologous ADSCs in the treatment of patients with Buerger’s disease. Treatment with ADSCs was superior on week 24 compared to screening in demonstrating a significantly better improvement on TWD and PFWD. In the phase I and II clinical trials for ADSCs, the walking distance was measured according to the Bruce treadmill protocol. The Bruce treadmill protocol involves increasing the speed and incline over time, that is, a gradual increase in the exercise load over time, and the walking distance inevitably decreases over time. In the case of clinical trials using cilostazol, the constant walking speed and gradual increase of incline were used, while in this study the speed and incline were increased gradually for measuring the maximal walking distance (MWD) (=TWD) and PFWD26–28. An increase in MWD by 95.7 m, from 236.9 to 332.6 m (40.40% increase), and an increase in PFWD by 95.5 m, from 211.4 to 306.9 m (45.18% increase), were reported26. An increase of 109 m, from 241 to 350 m (45.23%), for MWD and an increase of 94 m, from 124 to 218 m (75.81%), for PFWD were observed27. In addition, an increase of 73 m, from 262 to 335 m (27.86%), for MWD was reported28. In this study, the ADSCs resulted in 48% and 85% increases in MWD and PFWD on condition of gradually increasing walking speed and incline. Although direct comparison is not possible because of different test conditions of MWD and PFWD used in this and other studies26–28, the results of TWD and PFWD indicated that ADSCs can improve the walking distance in severe Buerger’s disease patients. In addition, since Buerger’s disease patients in this study had pains and walking disturbance despite taking medications such as cilostazol and beraprost sodium before ADSC injection, we believe that the differences of TWD and PFWD between the screening day and week 24 are purely the effect of stem cell treatment.
The other efficacy indicators in this clinical trial were rest pain assessed based on the VAS as well as the ABPI, TBPI, and TcPO2. Reduced rest pain resulting from ADSCs was observed during the clinical trial period, and further reduction in rest pain was reported in the follow-up questionnaire survey conducted 2 years later. To be more specific, 40.5% reduction in rest pain was observed during the clinical trial period, and an additional 33% reduction was reported 2 years afterward. These results clearly demonstrated that ADSCs indeed relieved the rest pain, which could not be ameliorated by the existing drugs, and thereby stopped or reduced the amount of drugs taken. Treatment with ADSCs was superior on week 24 compared to screening in demonstrating a significantly better improvement on the left ABPI. However, statistically significant results were not obtained in regard to TBPI. Since many patients did not have TBPI measurement at screening and week 24, it was difficult to make any statistical and clinical robust assessment for this efficacy variable. Taken together, the results of the VAS and left ABPI provided further support for the results of the primary efficacy variable.
Buerger’s disease is a progressive, irreversible disease accompanied by gradually aggravated symptoms that eventually results in amputation of the affected area regardless of treatment. In a previous study, major amputations were performed on 5 limbs (14.3%) of the 35 lower limbs among the 31 patients in the group that received artery bypass surgery29. A previous study reported that 14% of the 100 patients with Buerger’s disease received sympathectomy and amputation surgery simultaneously, and an additional 18% of the subjects received amputation surgery just a few months later due to recurrence of symptoms30. Additionally, in a clinical trial of iloprost conducted with Buerger’s disease patients, amputation surgery was performed on 6% of the subjects despite administration of iloprost31. The patients enrolled in this trial had high risk of amputation because of nonresponse to existing drugs and other therapeutic options. Therefore, evaluating the amputation rate is an important parameter for clinical benefits of ADSCs. In the current study, no amputations were reported during the 6-month clinical trial period and in the follow-up questionnaire survey more than 2 years after the ADSC injection. This result indicates that ADSCs present a better possible treatment option in preventing amputation for patients with severe Buerger’s disease.
The mechanism of the therapeutic effects of ADSCs is completely different from that of the conventional agents used for symptomatic treatment. The mechanism of actions of conventional agents such as cilostazol and argatroban involves vasodilation, thrombosis prevention, and platelet aggregation inhibition, with the purpose of alleviating or inhibiting disease symptoms resulting from arterial obstruction by preventing thrombosis or dilating blood vessels32–34. On the other hand, ADSCs are the type of therapeutic agent that works locally for angiogenesis and strengthening of blood vessels, which is also a known mechanism of other stem cells35,36. The mechanism of ADSCs used in this study was confirmed in our previous study through in vitro and in vivo animal studies7. In the previous study, we reported that ADSCs were differentiated into endothelial cells and prevented leg amputations by increasing the density of capillaries and the blood flow in ischemic limb model animals. The therapeutic angiogenic effect of ADSCs was through the secretion of diverse angiogenic factors including VEGF (data not shown). In line with our results, many studies have revealed the antigenic and therapeutic effect of VEGF through preclinical and clinical studies37–43. A study reported that an injection of recombinant VEGF protein (rVEGF) in laboratory animals resulted in the promotion of angiogenesis and that angiogenesis occurred at a higher rate when rVEGF was injected in combination with angiopoietin-2 (Ang-2)37. Furthermore, a number of studies reported that the injection of naked VEGF DNA effectively treated patients with peripheral arterial disease and Buerger’s disease in terms of improvement in clinical symptoms and ABI, generation of new blood vessels, and enhancement of the walking function38–43. In this study, we investigated the correlation in the results of therapeutic angiogenesis between our previous preclinical study and clinical study. Unfortunately, there were little to no direct angiogenesis effects observed in this clinical trial in contrast with the preclinical study. In the clinical trial, angiogenesis was observed in only one subject based on an angiogram, while the diverse symptoms of Buerger’s disease were ameliorated, and no amputation was observed. In line with our results, a study reported that amputation was prevented for all 37 subjects, yet angiogenesis was observed in only 23 subjects based on an angiogram after 24 months of administrating bone marrow-derived mesenchymal stem cells (BM-MSCs) and nucleated cells to patients with CLI44. In another study, angiogenesis was observed by angiography in only 3 patients (14%) out of 21 patients saved from amputation after 6 months of administrating the nucleated cells of the bone marrow to CLI patients45. Although functional improvements were shown in several parameters, the reasons for not observing angiogenesis in this trial in comparison with the previous study11 can be due to the technological limitations in assessing the formation of microvessels. Digital subtraction angiography (DSA) was used in the previous study11, and CT angiography was used in this study. The sensitivity of CT angiography is lower than that of DSA. In addition, even DSA cannot detect a microvessel less than 0.2 mm45. Therefore, the reasons for not observing angiogenesis in the current study can be due to the sensitivity and technical limitation of CT angiography. In addition, the short observation period (6 months) can be another reason. Further research is needed to explore the reasons for not observing angiogenesis.
CONCLUSION
Collectively, this study was conducted to investigate the safety and efficacy of intramuscular injection of autologous ADSCs in patients with severe Buerger’s disease. The results of the phase I and II clinical trials demonstrated that ADSCs are highly safe for administration in Buerger’s disease patients as no ADRs occurred, and none of the subjects dropped out from the study during the clinical trial period. In terms of efficacy, administration of ADSCs improves walking capacity, reduces rest pain, and potentially prevents the risk of amputation. However, further research is necessary for confirmation of the mechanism and efficacy of ADSCs.
ACKNOWLEDGMENTS
The authors thank the clinical staff at the Catholic University of Korea Seoul St. Mary’s Hospital, Seoul, including Professor S.H. Baek. This study was presented in part at the 12th Meeting of the International Federation for Adipose Therapeutics and Science, Amsterdam, Netherlands, December 13–16, 2014. J.C. Ra is a founder and a shareholder of R Bio. S.K. Kang and K.H. Choi are employees and shareholders of R Bio. S.J. Lee is an employee of R Bio. This study was sponsored by R Bio Co. Ltd.
Footnotes
E.C. Jeong declares no conflict of interest.
REFERENCES
- 1. Olin JW, Shih A. Thromboangiitis obliterans (Buerger’s disease). N Engl J Med. 2000;343:864–9. [DOI] [PubMed] [Google Scholar]
- 2. Fiessinger JN. Buerger’s disease or thromboangiitis obliterans. In: Tooke JE, Lowe GDO, editors. A textbook of vascular medicine. London (ENG): Arnold; 1996. p. 275–86. [Google Scholar]
- 3. Patel DM, Shah J, Srivastava AS. Therapeutic potential of mesenchymal stem cells in regenerative medicine. Stem Cells Int. 2013;2013:496218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xi J, Yan X, Zhou J, Yue W, Pei X. Mesenchymal stem cells in tissue repairing and regeneration: Progress and future. Burns Trauma 2013;1:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ishikawa T, Banas A, Hagiwara K, Iwaguro H, Ochiya T. Stem cells for hepatic regeneration: The role of adipose tissue derived mesenchymal stem cells. Curr Stem Cell Res Ther. 2010;5:182–9. [DOI] [PubMed] [Google Scholar]
- 6. Yim RL, Lee JT, Bow CH, Meij B, Leung V, Cheung KM, Vavken P, Samartzis D. A systematic review of the safety and efficacy of mesenchymal stem cell for disc degeneration: Insights and future directions for regenerative therapeutics. Stem Cells Dev. 2014;23:2553–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ra JC, Shin IS, Kim SH, Kang SK, Kang BC, Lee HY, Kim YJ, Jo JY, Yoon EJ, Choi HJ, Kwon E. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2001;20:1297–1308. [DOI] [PubMed] [Google Scholar]
- 8. Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, Kim JE, Shim H, Shin JS, Shin IS, Ra JC, Oh S, Yoon KS. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells 2014;32:1254–66. [DOI] [PubMed] [Google Scholar]
- 9. Konno M, Hamabe A, Hasegawa S, Ogawa H, Fukusumi T, Nishikawa S, Ohta K, Kano Y, Ozaki M, Noguchi Y, Sakai D, Kudoh T, Kawamoto K, Eguchi H, Satoh T, Tanemura M, Nagano H, Doki Y, Mori M, Ishii H. Adipose-derived mesenchymal stem cells and regenerative medicine. Dev Growth Differ. 2013;55:309–18. [DOI] [PubMed] [Google Scholar]
- 10. Nakagami H, Maeda K, Morishita R, Iguchi S, Nishikawa T, Takami Y, Kikuchi Y, Saito Y, Tamai K, Ogihara T, Kaneda Y. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2015;25:2542–7. [DOI] [PubMed] [Google Scholar]
- 11. Lee HC, An SG, Lee HW, Park JS, Cha KS, Hong TJ, Park JH, Lee SY, Kim SP, Kim YD, Chung SW, Bae YC, Shin YB, Kim JI, Jung JS. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: A pilot study. Circ J. 2012;76:1750–60. [DOI] [PubMed] [Google Scholar]
- 12. Bura A, Planat-Benard V, Bourin P, Silvestre JS, Gross F, Grolleau JL, Saint-Lebese B, Peyrafitte JA, Fleury S, Gadelorge M, Taurand M, Dupuis-Coronas S, Leobon B, Casteilla L. Phase I trial: The use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy 2014;16:245–57. [DOI] [PubMed] [Google Scholar]
- 13. Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, Jones DN. Recommended standards for reports dealing with lower extremity ischemia: Revised version. J Vasc Surg. 1997;26:517–38. [DOI] [PubMed] [Google Scholar]
- 14. Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85:546–62. [DOI] [PubMed] [Google Scholar]
- 15. McIntosh C, Green T. An overview of lower limb lymphoedema and diabetes. J Lymphoedema 2009;4:49–58. [Google Scholar]
- 16. Ishida A, Ohya Y, Sakuda H, Ohshiro K, Higashiuesato Y, Nakaema M, Matsubara S, Yakabi S, Kakihana A, Ueda M, Miyagi C, Yamane N, Koja K, Komori K, Takishita S. Autologous peripheral blood mononuclear cell implantation for patients with peripheral arterial disease improves limb ischemia. Circ J. 2005;69:1260–5. [DOI] [PubMed] [Google Scholar]
- 17. Seebald J, Gritters L. Thromboangiitis obliterans (Buerger disease). Radiol Case Rep. 2015;10:9–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vijayakumar A, Tiwari R, Kumar Prabhuswamy V. Thromboangiitis obliterans (Buerger’s disease)—Current practices. Int J Inflam. 2013;2013:156905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jorge VC, Araújo AC, Noronha C, Panarra A, Riso N, Vaz Riscado M. Buerger’s disease (thromboangiitis obliterans): A diagnostic challenge. BMJ Case Rep. 2011;2011 doi:10.1136/bcr.08.2011.4621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park UJ, Kim DI. Thromoboagiitis obliterans (TAO). Int J Stem Cells 2010;3:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garcia-Olmo D, Herreros D, Pascual I, Pascual JA, Del-Valle E, Zorrilla J, De-La-Quintana P, Garcia-Arranz M, Pascual M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: A phase II clinical trial. Dis Colon Rectum 2009;52:79–86. [DOI] [PubMed] [Google Scholar]
- 22. Fang B, Mai L, Li N, Song Y. Favorable response of chronic refractory immune thrombocytopenic purpura to mesenchymal stem cells. Stem Cells Dev. 2012;21:497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee PH, Kim JW, Bang OY, Ahn YH, Joo IS, Huh K. Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin Pharmacol Ther. 2008;83:723–30. [DOI] [PubMed] [Google Scholar]
- 25. Peng L, Xie DY, Lin BL, Liu J, Zhu HP, Xie C, Zheng YB, Gao ZL. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: Short-term and long-term outcomes. Hepatology 2011;54:820–8. [DOI] [PubMed] [Google Scholar]
- 26. Money SR, Herd JA, Isaacsohn JL, Davidson M, Cutler B, Heckman J, Forbes WP. Effect of cilostazol on walking distances in patients with intermittent claudication caused by peripheral vascular disease. J Vasc Surg. 1998;27:267–74. [DOI] [PubMed] [Google Scholar]
- 27. Dawson DL, Cutler BS, Hiatt WR, Hobson RW, Martin JD, Bortey EB, Forbes WP, Strandness DE Jr. A comparison of cilostazol and pentoxifylline for treating intermittent claudication. Am J Med. 2000;109:523–30. [DOI] [PubMed] [Google Scholar]
- 28. Elam MB, Heckman J, Crouse JR, Hunninghake DB, Herd JA, Davidson M, Gordon IL, Bortey EB, Forbes WP. Effect of the novel antiplatelet agent cilostazol on plasma lipoproteins in patients with intermittent claudication. Arterioscler Thromb Vasc Biol. 1998;18:1942–7. [DOI] [PubMed] [Google Scholar]
- 29. Ohta T, Ishioashi H, Hosaka M, Sugimoto I. Clinical and social consequences of Buerger disease. J Vasc Surg. 2004;39:176–80. [DOI] [PubMed] [Google Scholar]
- 30. Mohammadzadeh, Yadegari, Manzar, Akbar H. Clinical features of Buerger’s disease and therapeutic results of sympathectomy in Iranians. Internet J Thoracic Cardiovasc Surg. 2002;5:1. [Google Scholar]
- 31. The European TAO Study Group. Oral iloprost in the treatment of thromboangiitis obliterans (Buerger’s disease): A double-blind, randomised, placebo-controlled trial. Eur J Vasc Endovasc Surg. 1998;15:300–7. [DOI] [PubMed] [Google Scholar]
- 32. Beigi AA, Hoghoughi MA, Eshaghian A, Zade AH, Masoudpour H. The role of folic acid on the hyperhomocysteinemia in the Buerger’s disease (thromboangiitis obliterans). J Res Med Sci. 2014;19:1034–7. [PMC free article] [PubMed] [Google Scholar]
- 33. Goto S. Cilostazol: Potential mechanism of action for antithrombotic effects accompanied by a low rate of bleeding. Atheroscler Suppl. 2005;6:3–11. [DOI] [PubMed] [Google Scholar]
- 34. Jeske W, Walenga JM, Lewis BE, Fareed J. Pharmacology of argatroban. Expert Opin Investig Drugs 1999;8:625–54. [DOI] [PubMed] [Google Scholar]
- 35. Tao H, Han Z, Han ZC, Li Z. Proangiogenic features of mesenchymal stem cells and their therapeutic application. Stem Cells Int. 2016;2016:1314709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ichim TE, Solano F, Brenes R, Glenn E, Chang J, Chan K, Riordan NH. Placental mesenchymal and cord blood stem cell therapy for dilated cardiomyopathy. Reprod Biomed Online 2008;16:898–905. [DOI] [PubMed] [Google Scholar]
- 37. Brudno Y, Ennett-Shepard AB, Chen RR, Aizenberg M, Mooney DJ. Enhancing microvascular formation and vessel maturation through temporal control over multiple pro-angiogenic and pro-maturation factors. Biomaterials 2013;20:9201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baumgartner I, Pieczek A, Manor O, Blair R, Kearney M, Walsh K, Isner JM. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation 1998;97:1114–23. [DOI] [PubMed] [Google Scholar]
- 39. Iwaguro H, Yamaguchi J, Kalka C, Murasawa S, Masuda H, Hayashi S, Silver M, Li T, Isner JM, Asahara T. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation 2002;105:732–8. [DOI] [PubMed] [Google Scholar]
- 40. Isner JM, Baumgartner I, Rauh G, Schainfeld R, Blair R, Manor O, Razvi S, Symes JF. Treatment of thromboangiitis obliterans (Buerger’s disease) by intramuscular gene transfer of vascular endothelial growth factor: Preliminary clinical results. J Vasc Surg. 1998;28:964–73. [DOI] [PubMed] [Google Scholar]
- 41. Mack CA, Magovern CJ, Budenbender KT, Patel SR, Schwarz EA, Zanzonico P, Ferris B, Sanborn T, Isom P, Ferris B, Sanborn T, Isom OW, Crystal RG, Rosengart TK. Salvage angiogenesis induced by adenovirus-mediated gene transfer of vascular endothelial growth factor protects against ischemic vascular occlusion. J Vasc Surg. 1998;27:699–709. [DOI] [PubMed] [Google Scholar]
- 42. Kim HJ, Kim DK. Angiogenic gene therapy in patients with ischemic cardiovascular disease. Korean Circulation J. 2003;33:7–14. [Google Scholar]
- 43. Kim HJ, Jang SY, Kim JM, Kim SY, Kim BM, Kim WB, Kim DK. Case reports: Vascular endothelial growth factor (VEGF)-induced angiogenic gene therapy in a patient with critical limb ischemia. Korean J Med. 2003;64:85–91. [Google Scholar]
- 44. Lu D, Chen B, Liang Z, Deng W, Jiang Y, Li S, Xu J, Wu Q, Zhang Z, Xie B, Chen S. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: A double-blind, randomized, controlled trial. Diabetes Res Clin Pract. 2011;92:26–36. [DOI] [PubMed] [Google Scholar]
- 45. Amann B, Luedemann C, Ratei R, Schmidt-Lucke JA. Autologous bone marrow cell transplantation increases leg perfusion and reduces amputations in patients with advanced critical limb ischemia due to peripheral artery disease. Cell Transplant. 2009;18:371–80. [DOI] [PubMed] [Google Scholar]