Abstract
Adipose-derived mesenchymal stem cells (ASCs) release factors beneficial for islets in vitro and protect against hyperglycemia in rodent models of diabetes. Oxygen tension has been shown to induce metabolic changes and alter ASCs’ release of soluble factors. The effects of hypoxia on the antidiabetic properties of ASCs have not been explored. To investigate this, we incubated human ASCs for 48 h in 21% (normoxia) or 1% O2 (hypoxia) and compared viability, cell growth, surface markers, differentiation capability, and soluble factors in the conditioned media (CM). Human islets were exposed to CM from ASCs incubated in either normoxia or hypoxia, and islet function and apoptosis after culture with or without proinflammatory cytokines were measured. To test hypoxic preconditioned ASCs’ islet protective effects in vivo, ASCs were incubated for 48 h in normoxia or hypoxia before being injected into Balb/c Rag 1–/– immunodeficient mice with streptozotocin-induced insulitis. Progression of diabetes and insulin content of pancreas were measured. We found that incubation in hypoxia was well tolerated by ASCs and that levels of VEGF-A, FGF-2, and bNGF were elevated in CM from ASCs incubated in hypoxia compared to normoxia, while levels of HGF, IL-8, and CXCL1 were reduced. CM from ASCs incubated in hypoxia significantly improved human islet function and reduced apoptosis after culture, and reduced cytokine-induced apoptosis. In our mouse model, pancreas insulin content was higher in both groups receiving ASCs compared to control, but the mice receiving preconditioned ASCs had lower random and fasting blood glucose, as well as improved oral glucose tolerance compared to untreated mice. In conclusion, our in vitro results indicate that the islet protective potential of ASCs improves in hypoxia, and we give insight into factors involved in this. Finally we show that hypoxic preconditioning potentiates ASCs’ antidiabetic effect in vivo.
Key words: Adipose-derived stem cells (ASCs), Hypoxia, Islet transplantation, Diabetes
INTRODUCTION
Type 1 diabetes (T1D) is a disease where the destruction of insulin-producing β-cells normally found in the islets of Langerhans results in a chronic insulin deficiency. Islet transplantation is a treatment option offering to restore endogenous insulin production to patients suffering from T1D, but long-term results show deteriorating islet function over time1,2. It has been demonstrated that human bone marrow-derived mesenchymal stem cells (BM-MSCs) can protect islets from proinflammatory cytokines and prolong graft survival in a mouse model with rebuilt human immunity3,4. Mesenchymal stem cells (MSCs) have been isolated from various tissues in the body, including adipose tissue5. Adipose-derived mesenchymal stem cells (ASCs) can be harvested more safely and less painfully compared to BM-MSCs and are also relatively more abundant6. Cotransplantation of syngeneic ASCs with islets in mice show enhanced graft function, and human ASCs have been shown to protect against streptozotocin (STZ)-induced hyperglycemia in mice through paracrine and systemic effects7,8. The exact mechanism of action on islets is not fully understood, but secretion of soluble factors appears to be a critical component in other disease models9,10.
Oxygen concentration is an important environmental factor that affects ASCs. While ASCs are commonly cultured in 21% O2 during expansion and in vitro experiments, the oxygen tension in adipose tissue in vivo varies between 3% and 11%11. Incubating ASCs in 1% O2 has been shown to reduce apoptosis and increase secretion of soluble factors such as vascular endothelial growth factor A (VEGF-A) and fibroblast growth factor 2 (FGF-2) while retaining ASCs’ immunosuppressive abilities12,13. Preconditioning mouse BM-MSCs in 1% O2 has been shown to increase BM-MSC glycogen storage in vitro and improve survival of injected BM-MSC in vivo in a mouse limb ischemia model14. Here we investigate how human ASCs respond to incubation in 1% O2 (hypoxia) compared to 21% O2 (normoxia). We investigate the levels of soluble factors in conditioned media (CM) from ASCs incubated in normoxia (21% O2 CM) compared to CM from ASCs incubated in hypoxia (1% O2 CM), and we investigate the effect of these different CM on human islets. We find that 1% O2 CM contains increased levels of VEGF-A, β-nerve growth factor (bNGF), and FGF-2, and decreased levels of hepatocyte growth factor (HGF), interleukin-8 (IL-8), and chemokine C-X-C motif ligand 1 (CXCL1) compared to 21% O2 CM, and we show that 1% O2 CM improves function, reduces apoptosis, and protects against cytokine-induced apoptosis in human islets in vitro. Finally, we investigate the effect of preconditioning human ASCs in hypoxia prior to administration in a mouse model of STZ-induced insulitis and hyperglycemia and find that hypoxic preconditioning potentiates ASCs’ ability to protect against hyperglycemia in vivo.
MATERIALS AND METHODS
Isolation and Culture of ASCs
Lipoaspirates were obtained from the flank or thigh of six females aged 60.4 ± 4.2 years undergoing elective plastic surgical operations at the Department for Plastic Surgery, Radiumhospitalet, Oslo University Hospital. All donors signed informed consent, and the use of ASCs was approved by the Regional Committee for Medical and Health Research Ethics (2014/838). The stromal vascular fraction (SVF) was extracted using the Celution® system (Cytori Therapeutics Inc., San Diego, CA, USA) according to the manufacturer’s instructions. Briefly, the tissue was washed with Ringer’s solution, enzymatically digested by a proprietary enzyme solution (Celase®) during constant agitation, and then washed and concentrated by centrifugation15. Directly after processing, the SVF was washed once with 5% human serum albumin (Octapharma, Jessheim, Norway) and centrifuged at 600 × g for 10 min at 21°C. Mononuclear cells were counted using a hemocytometer (Kova, Garden Grove, CA, USA) and seeded at 3,000 cells/cm2 in a T75 flask (Nunc; Thermo Fisher Scientific, Waltham, MA, USA) with supplemented minimum essential medium (MEM) α containing 10% fetal bovine serum (FBS) (both from Gibco, Thermo Fisher Scientific) and 50 μg/ml gentamicin (Braun, Esbjerg, Denmark). Cells were cultured at standard condition of 37°C in a humidified atmosphere with 21% O2 and 5% CO2. The primary cells were allowed to attach for 2 days before being washed three times with 37°C phosphate-buffered saline (PBS; Lonza, Basel Switzerland) to remove nonadherent cells. The culture medium was changed every 2–3 days until the cells reached 70%–80% confluence, then harvested using TrypLE Express (Gibco), and subsequently seeded at a density of 3,000 cells/cm2 for the next passage. ASCs of passages 1 to 2 were cryopreserved in medium containing 10% dimethyl sulfoxide (DMSO; Cryo-Sure; Wak-chemie, Steinbach, Germany) and 20% human serum albumin, initially cooled to −80°C at a rate close to −1°C/min using a freezing container (Mr. Frosty; Thermo Fisher Scientific) and subsequently transferred to a −196°C liquid nitrogen vapor tank for storage. Cells were thawed in room temperature (RT), and DMSO was rapidly diluted in supplemented ASC medium before centrifugation and use in experiments.
Hypoxic Incubation of ASCs
ASCs were thawed and cultured in supplemented MEMα for 1–2 passages before experiments. ASCs were plated at 3,000 cells/cm2 and incubated in normoxia for 4 h, allowing the cells to attach. Flasks were then divided into two groups and incubated under either normoxic or hypoxic conditions for 48 h. Hypoxic conditions were obtained using an IN VIVO2 200 Hypoxic workstation supplied with a Gas Mixer Q advanced gas mixing system (Ruskinn, Bridgend, South Wales, UK) or a New Brunswick Galaxy 48 R (Hamburg-Eppendorf, Hamburg, Germany) with a mixture of N2-adjusted O2 1%, CO2 5%, and 37°C. After 48 h, CM from ASCs incubated in either normoxia or hypoxia was collected in 50-ml tubes (Corning, Corning, NY, USA). The respective CM was centrifuged at 5,000 × g at 4°C for 10 min, and the supernatant was stored at −80°C until use.
In Vitro Analysis of ASCs
Experiments were performed immediately after incubating ASCs in normoxia or hypoxia. Viability was assessed by dye exclusion test using trypan blue solution 0.4% (Gibco) and counting cells in a hemocytometer. Analysis of surface antigen expression was done by flow cytometry using a BD FACSCanto II (Becton Dickinson, San Diego, CA, USA) and BD Stemflow Human MSC Analysis Kit (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instructions. Briefly, 5 × 105 cells/100 μl were incubated with the conjugated monoclonal or isotype-matched IgG control antibodies, then analyzed by fluorescence-activated cell sorting (FACS) to measure the levels of positive [cluster of differentiation 105 peridinin chlorophyll protein-cyanine 5.5 (CD105 PerCP-Cy™5.5)/CD73 allophycocyanin (APC)/CD90 fluorescein isothiocyanate (FITC)] and negative [CD45/CD34/CD11b/CD19/human leukocyte antigen-DR phycoerythrin (HLA-DR PE)] markers of ASCs16. Differentiation capacity was evaluated using StemPro Adipogenesis and Osteogenesis differentiation kits (Gibco) following the manufacturer’s instruction. Briefly, 1 × 104 cells/cm2 cells were seeded into 12-well tissue culture plates (Costar, Corning, NY, USA) and incubated for 14 days in species-specific medium changed every 3–4 days. At the end of the differentiation period, all cultures were fixed with 4% formaldehyde (Chemi-teknik, Oslo, Norway) before differentiated cells were stained with 0.3% Oil red O solution (Sigma-Aldrich, St. Louis, MO, USA) for 10 min or 2% Alizarin red S solution (pH 4.2; Sigma-Aldrich) for 3 min. Plastic adherence, correct surface markers, and differentiation potential were established before ASCs were used for further experiments. Hypoxia-inducible factor 1α (HIF-1α) expression was measured by Western immunoblotting. Cells (1 × 106) were plated out in 10-cm dishes and 48 h later lysed in 60 μl of 5× Lane Marker Reducing Sample Buffer (Thermo Fisher Scientific) and 7 μl of 10% sodium dodecyl sulfate (SDS; Sigma-Aldrich) while still in 1% O2. Protein lysates were boiled 5 min at 95°C before 12 μl was loaded onto 15-well 4–20% Mini-PROTEAN TGX Precast Gel (Bio-Rad, Hercules, CA, USA) and transferred to nitrocellulose membranes (TransBlot Turbo Transfer System; Bio-Rad). The membrane was blocked [5% skim milk powder (Sigma-Aldrich) in PBS-Tween (PBS-T; Sigma-Aldrich)] and incubated with anti-HIF-1α (1:800; ab51608; Abcam; Cambridge, UK) and anti-γ tubulin (1:2,000; T6557; Sigma-Aldrich) at 4°C overnight, washed 3 × 5 min in PBS-T, followed by horseradish peroxidase (HRP)-conjugated secondary antibody with a dilution of 1:10,000 (Jackson Laboratories, West Grove, PA, USA) for 1 h at RT. SuperSignal West Substrate (Thermo Fisher Scientific) was used for the detection of signal with a ChemiDoc instrument (Bio-Rad). ASC clonogenicity was evaluated by the colony-forming unit fibroblast assay (CFU-F) (MesenCult™ Proliferation Kit; Stem Cell Technologies, Vancouver, Canada). Briefly, 500 ASCs at P1–2 were incubated for 48 h in normoxic or hypoxic conditions before being seeded in triplicate into a six-well plate (Nunc; Thermo Fisher Scientific) in assay medium for 14 days at 37°C (5% CO2) with medium changes every 3 days. Thereafter, colonies were rinsed with PBS, fixed and stained with crystal violet dye (Sigma-Aldrich) in methanol (Merck KGaA, Damstadt, Germany) for 30 min, and the numbers of colonies were manually counted.
Analysis of Soluble Factors in CM
Frozen CM was thawed for measurement of soluble factors. The levels of IL-10, IL-8, IL-6, VEGF-A, FGF-2, monocyte chemoattractant protein 1 (MCP-1), eosinophil chemotactic protein (eotaxin), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) in CM from six different donors were measured using a Bio-Plex human cytokine panel (Bio-Rad). In CM produced from two donors for in vitro islet experiments, 45 different factors were measured by multiplex analysis using a Human Cytokine/Chemokine/Growth Factor Panel 1 (Product No. EPX45012171-901), and three additional factors, transforming growth factor-β1 (TGF-β1; EPX01A-10249-901), indoleamine 2,3-dioxygenase (IDO; EXP01A-12213-901), and programmed death ligand 1 (PD-L1; EXP01A-12212-901) (ProcartaPlex; eBioscience, San Diego, CA, USA), were determined by simplex analysis. After incubating the sample with antibody-coated, color-coded beads, the levels of analytes were determined by a Bio-Plex 200 dual laser flow-based analysis system (Bio-Rad).
Human Islet Experiments
Human islets were isolated as described previously17 from the pancreata of six female and nine male deceased donors, with a mean age of 56 years (18–75 years) and body mass index (BMI) of 25 (22–33), at the islet isolation facility of Nordic Network in Uppsala, Sweden, or Oslo University Hospital, Oslo, Norway, after appropriate consent was given. Average purity judged by dithizone (Sigma-Aldrich) staining was 68% (38%–90%), and the islets were disqualified for clinical transplantation due to quantitative insufficiency. Islet preparations were maintained in supplemented Connaught Medical Research Laboratories (CMRL) 1066 (Corning) containing 10% human serum (Milan Analytica, Rheinfelden, Switzerland), 10 mM nicotinamide (Swedish Pharmacy, Gothenburg, Sweden), 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES), 2 mM l-glutamine, 50 μg/ml gentamicin (Gibco, Life Technologies AS, Oslo, Norway), and 20 μg/ml ciprofloxacin (Bayer Healthcare, Leverkusen, Germany) at 37°C (5% CO2) for the first 12–24 h after isolation and then stored at 25°C (5% CO2) with medium change every 2–3 days until use in experiments. For incubation experiments, 500 handpicked islets were incubated at 37°C (5% CO2) in 90-mm uncoated Petri dishes (Sterilin, Newport, UK) containing a 1:1 mix of supplemented CMRL islet media with either supplemented MEMα (control), 21% O2 CM, or 1% O2 CM. The islets in combined media were then incubated for 48 h in normoxia (37°C, 5% CO2). For cytokine experiments, 150 handpicked islets were incubated for 24 h in normoxia (37°C, 5% CO2) in 60-mm uncoated Petri dishes (Sterilin) containing either supplemented MEMα without or with a mixture of cytokines (TNF-α, 10 ng/ml; Il-1β, 10 ng/ml; IFN-γ, 50 ng/ml; all from R&D Systems, Oxon, UK), 21% O2 CM with the mixture of cytokines, or 1% O2 CM with the mixture of cytokines.
In Vitro Analysis of Islet Functional Viability
Glucose-stimulated insulin secretion (GSIS) was performed by handpicking 20 islets and transferring them into Transwell cell culture inserts with 12-μm pore size (Millipore, Billerica, MA, USA) placed in 24-well plates (Costar). The islets were preincubated in Krebs–Ringer bicarbonate buffer (Merck KGaA) containing 1.67 mmol/L glucose (B. Braun, Meslungen, Germany) at 37°C for 15 min, and then transferred into a new well and incubated for 30 min for equilibration. The islets were then incubated for 1 h in fresh Krebs–Ringer bicarbonate buffer containing 1.67 mmol/L glucose (basal), and then finally incubated for 1 h in fresh Krebs–Ringer bicarbonate buffer containing 20.0 mmol/L glucose (stimulated). The supernatants were subsequently collected, and insulin secretion was measured using human insulin ELISA (Mercodia AB, Uppsala, Sweden). The capacity for insulin release was expressed as the stimulation index (SI), calculated as the ratio of stimulated over basal insulin secretion. Induced cell death in islets was determined by the detection of DNA–histone complexes present in the cytoplasmic fraction in 30 handpicked islets using Cell Death Detection ELISAPLUS (Roche, Basel, Switzerland) according to the manufacturer’s descriptions. Remaining islets were lysed by sonication using Microson Ultrasonic Cell Disruptor XL 2007 (Misonix, Farmingdale, NY, USA) and two freeze–thaw cycles. After centrifugation for 10 min at 10,000 × g, caspase 3/7 activity in the supernatant was measured using Caspase-Glo 3/7 assay (Promega, Madison, WI, USA) according to the manufacturer’s instructions and normalized to total protein content measured by bicinchoninic acid (BCA) protein assay kit (Pierce, Thermo Fisher Scientific).
Mice and Induction of Experimental Diabetes
The research protocol was approved by the Norwegian National Animal Research Authority (Project License No. FOTS 72/13). The animal experiments were performed in accordance with the European Directive 2010/63/EU and the Guide for the Care and Use of Laboratory Animals, 8th edition (NRC 2011, National Academic Press). The animals were housed no more than five mice per cage, maintained in a 12-h light/dark cycle, and given free access to food and water. The mice were handled by an experienced animal technician at all times, and all efforts were made to minimize suffering. The same technician monitored animal welfare in accordance to standardized requirements for the animal unit at Oslo University Hospital, administered the treatment, and performed the blood sampling. Diabetes was induced in 12- to 17-week-old male Balb/c recombination-activating gene 1-deficient (Rag 1–/–) immunodeficient mice [C.129S7(B6)-Rag1tm1Mom/J; stock 003145; Jackson Laboratory, Sacramento, CA, USA] by intravenous STZ (Sigma-Aldrich) injections (50 mg/kg body weight) repeated daily for 4 days to induce insulitis and mimic onset of T1D. STZ effect was confirmed by measured blood glucose above 10 mmol/L (180 mg/dl) using a glucometer (Accu-Chek Avia Nano; Roche Diagnostics) the day after the last STZ injection.
In Vivo Experiment With Preconditioned ASCs (PC-ASCs)
The experiment was run as two separate experiments at two different time points, and results were then summarized. Diabetic mice were randomized into three groups to receive 1 day after the last dose of STZ an injection into the tail vein of either 0.2 ml of PBS (PBS) (n = 10) or 0.2 ml of PBS containing either 0.8 × 106 ASCs incubated in normoxia prior to injection (ASCs) (n = 8) or 0.8 × 106 ASCs preconditioned by incubation for 48 h in hypoxia prior to injection (PC-ASCs) (n = 7). Nonfasting blood glucose was measured once weekly at 9:00 a.m. using a glucometer. Fasting blood glucose was measured after 4 h of fasting starting at 9:00 a.m. before the oral glucose tolerance test (OGTT). OGTT was performed on day 13 or 16 after ASC injection by administering oral gavage of 1.5 g/kg d-glucose (Fresenius Kabi, Oslo, Norway) to fasting animals. Blood glucose was measured at 0, 15, 30, 60, and 120 min after glucose administration using a glucometer. At days 21–23, the mice were anesthetized with an intraperitoneal injection of 0.1 ml/10 g body weight tiletamine and zolazepam 3.32 mg/ml (Zoletil Forte; Virbac, Carros, France), 0.45 mg/ml xylazine (Rompun; Bayer), and 2.65 μg/ml fentanyl (Actavis, Oslo, Norway) before being sacrificed by dislocation of the neck. The lungs and pancreas were harvested and snap frozen on liquid nitrogen and stored at −80°C until analysis of human-specific DNA. Frozen tissue was minced by mortar before extracting DNA using micro columns (GenElute; Sigma-Aldrich). Human DNA was amplified using a TaqMan PCR master Mix (Applied Biosystems, Waltham, MA, USA) with a human-specific VEGF primer (Hs03929046_s1; Thermo Fisher Scientific). Samples were titrated to contain 100 ng of dsDNA/reaction using NanoDrop 2000 (Thermo Fisher Scientific), and a standard was prepared containing 100, 10, 1, 0.1, 0.01, or 0.001 ng of human DNA supplemented with mouse DNA to reach a total DNA concentration of 100 ng/reaction. The samples were analyzed on a 7900HT Real-Time PCR System (Applied Biosystems). Pancreas insulin content was measured by sonicating minced tissue (Microson XL-2000; Misonix) in Milli-q purified water (Millipore), incubating the homogenate overnight in 70% (final concentration) acid ethanol (0.3 M HCL), and measuring mouse insulin by Ultra Sensitive Mouse Insulin Elisa (Mercodia AB). Insulin content was normalized to total protein of the samples, measured by Pierce’s BCA Protein Assay Kit.
Statistical Analysis
Differences between groups were examined for significance with a two-tailed Student’s test, Wilcoxon signed rank test was used in paired analysis, and Mann–Whitney was used for unpaired analysis. Calculations and graphs were made by the GraphPad Prism statistics software (GraphPad Prism 6.07; GraphPad Software Inc., San Diego, CA, USA). A value of p < 0.05 was taken to indicate the presence of a significant difference.
RESULTS
Short-Term Hypoxia Does Not Alter ASC Morphology, Viability, Surface Marker Expression, or Differentiation Potential
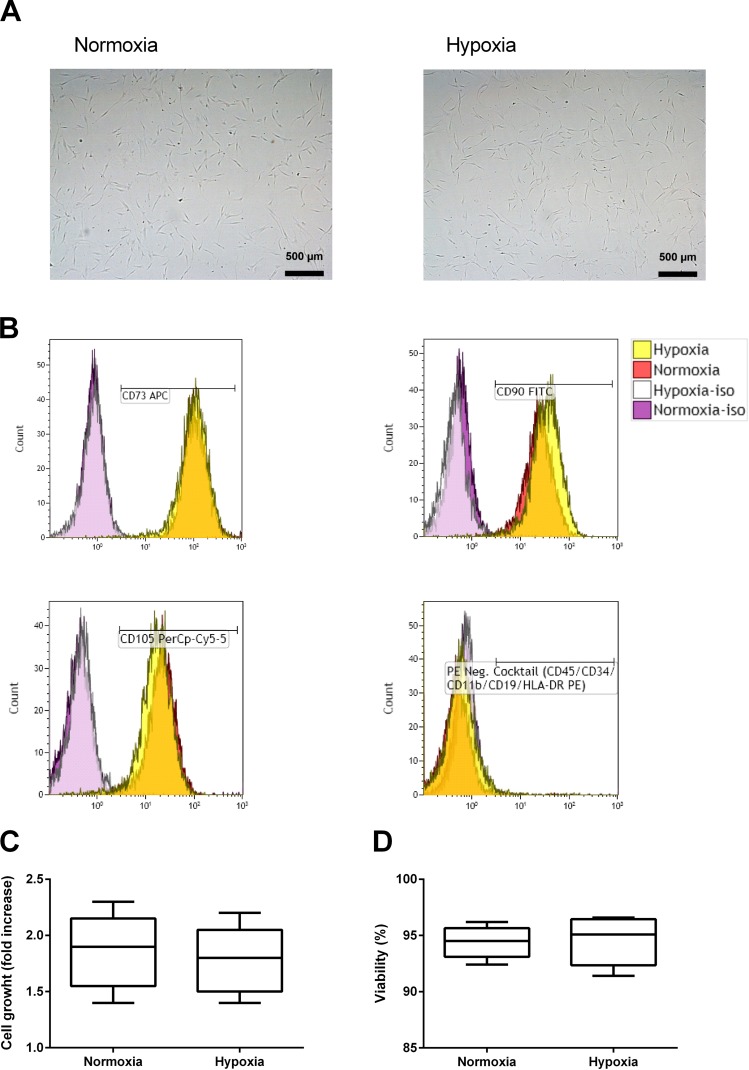
In order to investigate the effects of short-term incubation in hypoxia, we incubated ASCs in either normoxia or hypoxia. After 48 h of incubation, we saw no difference in macroscopic cellular morphology (Fig. 1A), and ASCs incubated in hypoxia maintain International Society for Cellular Therapy (ISCT) surface marker expression fulfilling the criteria for MSCs16, with positive expression (>97%+) of CD105, CD73, and CD90 and negative expression (<2%+) of HLA-DR and CD19, as well as CD34, CD11b, and CD45 (Fig. 1B). Donor variability was low, with no more than 2.5 percentage point difference between donors. Cell growth (Fig. 1C) and ASC viability (Fig. 1D), as assessed by the trypan blue exclusion method, were similar in the two groups (n = 5). Differentiation potential into adipocytes and osteoblasts was investigated by first incubating ASCs in normoxia or hypoxia for 48 h, followed by culture according to mesenchymal cell differentiation kits over 14 days in standard normoxic culture conditions. ASCs from all donors were able to differentiate into adipocytes and osteoblasts irrespective of whether they were incubated in normoxia or hypoxia prior to the experiment.
Figure 1.
Adipose-derived mesenchymal stem cells (ASCs) tolerate 48 h of incubation in hypoxia. Representative light microscopy images of ASC morphology after incubation in normoxia (21% O2) or hypoxia (1% O2) (A). Representative flow cytometry histogram for negative and positive surface markers, with isotype controls (iso), before and after incubation in hypoxia (B). Cell growth (C) and viability (D) determined by trypan blue exclusion test comparing ASCs incubated in normoxia or hypoxia. Results are presented as a box plot, with whiskers min. to max. (n = 5). CD73 APC, cluster of differentiation 73 allophycocyanin; CD90 FITC, CD90 fluorescein isothiocyanate; CD105 PerCP-Cy5.5, CD105 peridinin chlorophyll protein-cyanine 5.5; PE, phycoerythrin; HLA-DR, human leukocyte antigen-DR.
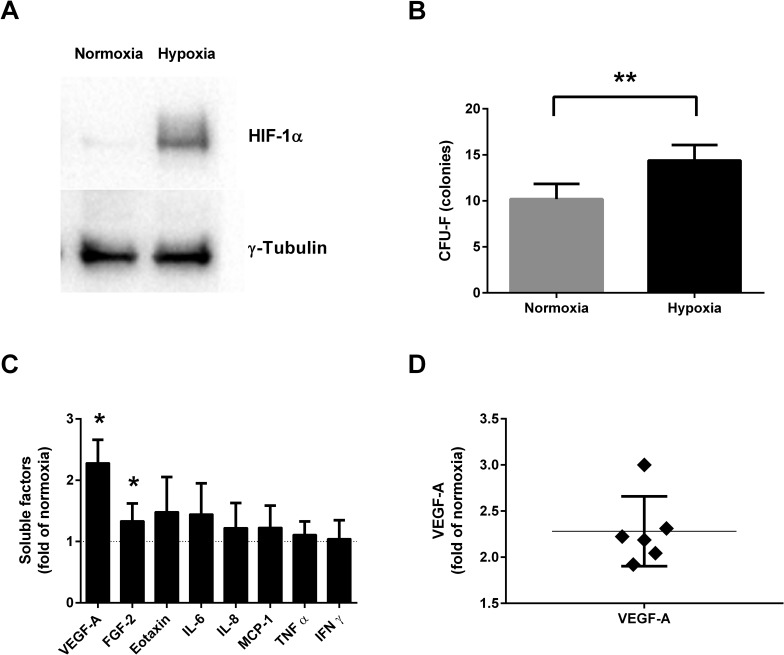
Short-Term Incubation in Hypoxia Induces HIF-1α, Increases CFU-F Colony Count, and Increases Secretion of VEGF-A and FGF-2
To confirm that ASCs respond to hypoxia, we measured the levels of HIF-1α by Western immunoblotting. HIF-1α was detected in ASC lysate after 48 h of incubation in hypoxia, while being barely detectable in ASCs incubated in normoxia (Fig. 2A). Short-term exposure to hypoxia before the CFU-F assay resulted in a significantly increased colony number (1.7-fold increase, n = 5, p = 0.0079) compared to normoxia (Fig. 2B). Cytokine release measurement in CM from cells of six different donors was performed by an eight-plex cytokine assay as described above. In the CM from ASCs incubated in hypoxia, we found significantly increased levels of VEGF-A (2.28-fold of normoxia, n = 6, p = 0.031) and FGF-2 (1.34-fold of normoxia, n = 6, p = 0.031) compared to ASCs incubated in normoxia (Fig. 2C). Changes in secretion of eotaxin, IL-6, IL-8, MCP-1, TNF-α, and IFN-γ did not reach significance in this assay. The increase in VEGF-A ranged from 1.9- to 3.0-fold of normoxia between donors, and the increase in five out of six donors was within 16% of the mean, showing a consistent response between donors for this factor (mean: 2.28-fold increase, SD: 0.38) (Fig. 2D).
Figure 2.
ASC response to hypoxia and altered levels of soluble factors in conditioned media (CM) after incubation in hypoxia (1% O2) compared to normoxia (21% O2). Representative Western blot of hypoxia-inducible factor 1α (HIF-1α) in ASC lysate after 48 h of incubation in normoxia or hypoxia (A). Colony number comparing cells incubated for 48 h in normoxia or hypoxia prior to colony-forming unit fibroblast assay (CFU-F) (n = 5) (B). Levels of soluble factors in 1% O2 CM compared to 21% O2 CM in ASCs from six different donors (n = 6) (C). Distribution of vascular endothelial growth factor A (VEGF-A) increase in 1% O2 CM compared to 21% O2 CM (D). Results are presented as mean ± SD. *p < 0.05, **p < 0.01. FGF-2, fibroblast growth factor 2; IL-6, interleukin-6; MCP-1, monocyte chemoattractant protein 1; TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ.
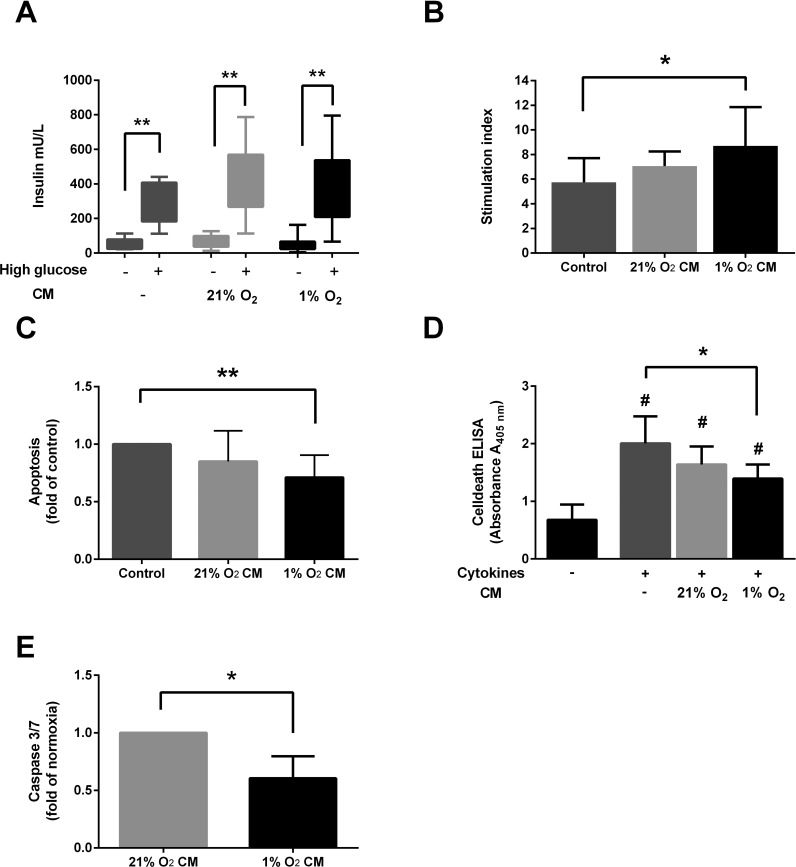
1% O2 CM Improves Human Islet Function, Reduces Apoptosis, and Protects Against Inflammatory Cytokines
To evaluate the effect of soluble factors in 1% O2 CM on human islets, we cultured human islets for 48 h in a 1:1 mix of supplemented CMRL islet media with either supplemented MEMα (control), 21% O2 CM, or 1% O2 CM. In order to reduce ASC donor variability, multiple batches of CM from two randomly selected ASC donors were prepared for these experiments. When looking at the insulin secretion in low and high glucose, we see a trend of elevated insulin secretion from islets exposed to both 21% O2 CM and 1% O2 CM in high glucose, while islets incubated in 1% O2 CM have a tendency toward lower insulin secretion in a low-glucose setting (Fig. 3A). When calculating a SI for these results, we find that islets cultured in media containing 1% O2 CM performed significantly better than the control group after 48 h of culture (mean SI: 7.9 ± 3.9 vs. 5.2 ± 2.4, n = 9, p = 0.039) (Fig. 3B). To evaluate apoptosis, we measured DNA fragmentation by ELISA. The islets exposed to 1% O2 CM showed a significantly lower apoptosis compared to the control group (0.71-fold of control, n = 10, p = 0.0039) (Fig. 3C). In order to evaluate the anti-inflammatory potential of CM, we cultured human islets in either unconditioned, supplemented MEMα, 21% O2 CM, or 1% O2 CM, and added a cytokine mix of TNF-α (10 ng/ml), IL-1β (10 ng/ml), and IFN-γ (50 ng/ml). After 24 h of culture, Cell Death ELISA revealed a significantly reduced apoptosis in the islets incubated in 1% O2 CM compared to unconditioned media (0.73-fold of unconditioned media, n = 6, p = 0.031) (Fig. 3D). To support this finding, we measured total protein-adjusted activated caspase 3/7 in islet lysate and found a significant reduction in the islets incubated in 1% O2 CM compared to 21% O2 CM (0.60-fold of 21% O2 CM, n = 6, p = 0.031) (Fig. 3E).
Figure 3.
Exposing human islets to CM from ASCs incubated in hypoxia (1% O2 CM) significantly improves function and reduces apoptosis after culture and protects against cytokine-induced apoptosis. Human islets were cultured for 48 h in a 1:1 mixture of supplemented Connaught Medical Research Laboratories (CMRL) media and either unconditioned minimum essential media (MEM) α (Control), 21% O2 CM, or 1% O2 CM. Insulin release in low (–) and high (+) glucose (A) and calculated stimulation index (n = 9) (B). Apoptosis measured by Cell Death ELISA after 48 h of culture (n = 10) (C). Cell Death ELISA after 24 h of culture in control media, 21% O2 CM, or 1% O2 CM with or without a mixture of cytokines (TNF-α, IL-1β, and IFN-γ) (n = 6) (D). Caspase 3/7 activation in lysates of islets incubated for 24 h with a mixture of cytokines (n = 6) (E). Results are presented as mean ± SD. *p < 0.05, **p < 0.01, #p < 0.05 versus no cytokines.
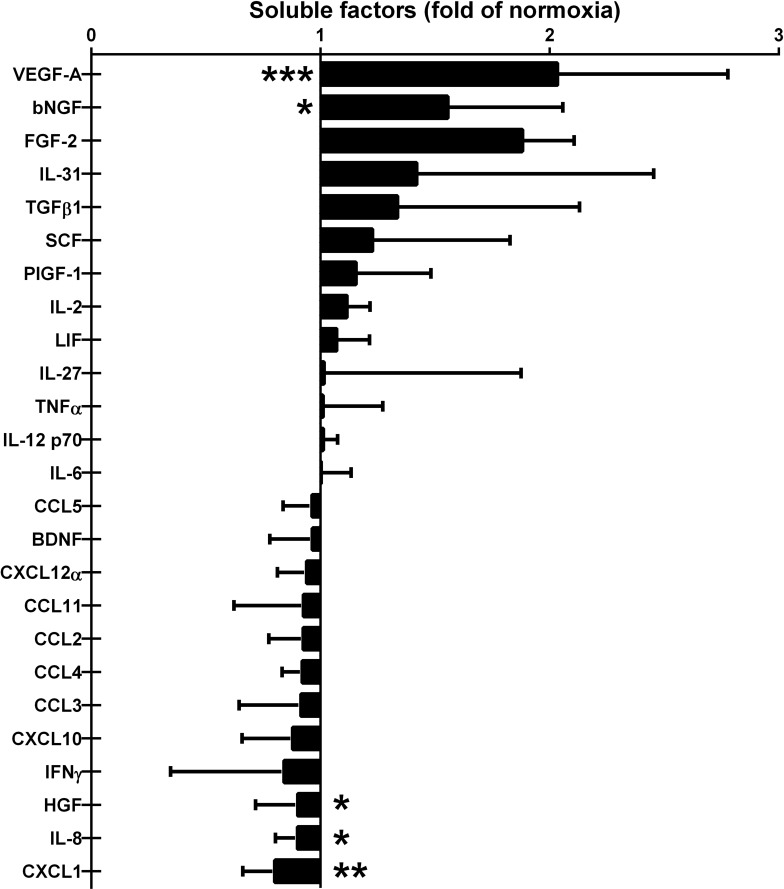
Altered Levels of Soluble Factors in 1% O2 CM
In order to describe the altered composition of secreted factors by ASCs in more detail, we analyzed eight batches of the CM used in the islet experiments described above for 45 different factors using multiplex technology, and additionally levels of TGF-β1, IDO, and PD-L1 were determined by simplex analysis. Of the factors measured, we found a significant increase in the levels of VEGF-A (2.02-fold of normoxia, p = 0.0005) and bNGF (1.46-fold of normoxia, p = 0.031) in the 1% O2 CM compared to the 21% O2 CM (Fig. 4A). The levels of HGF (0.87-fold of normoxia, p = 0.043), IL-8 (0.86-fold of normoxia, p = 0.021), and CXCL1 (0.78-fold of normoxia, p = 0.0078) were significantly reduced (Fig. 4B). In this assay, the increase in FGF-2 in 1% O2 CM failed to reach statistical significance (p = 0.125). The increase in TGF-β1 also did not reach statistical significance (p = 0.19), and levels of IDO and PD-L1 were undetectable.
Figure 4.
Detectable soluble factors in CM from ASCs incubated for 48 h in hypoxia (1% O2 CM) compared to normoxia (21% O2 CM). Multiple batches of 1% O2 CM and 21% O2 CM were prepared using ASCs from two different donors before use in islet experiments described above. This figure shows the relative levels of detectable factors out of a total of 48 factors analyzed. Results are presented as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001 (n = 8).
ASCs Preconditioned in Hypoxia Protect Against STZ Damage in Immunodeficient Mice
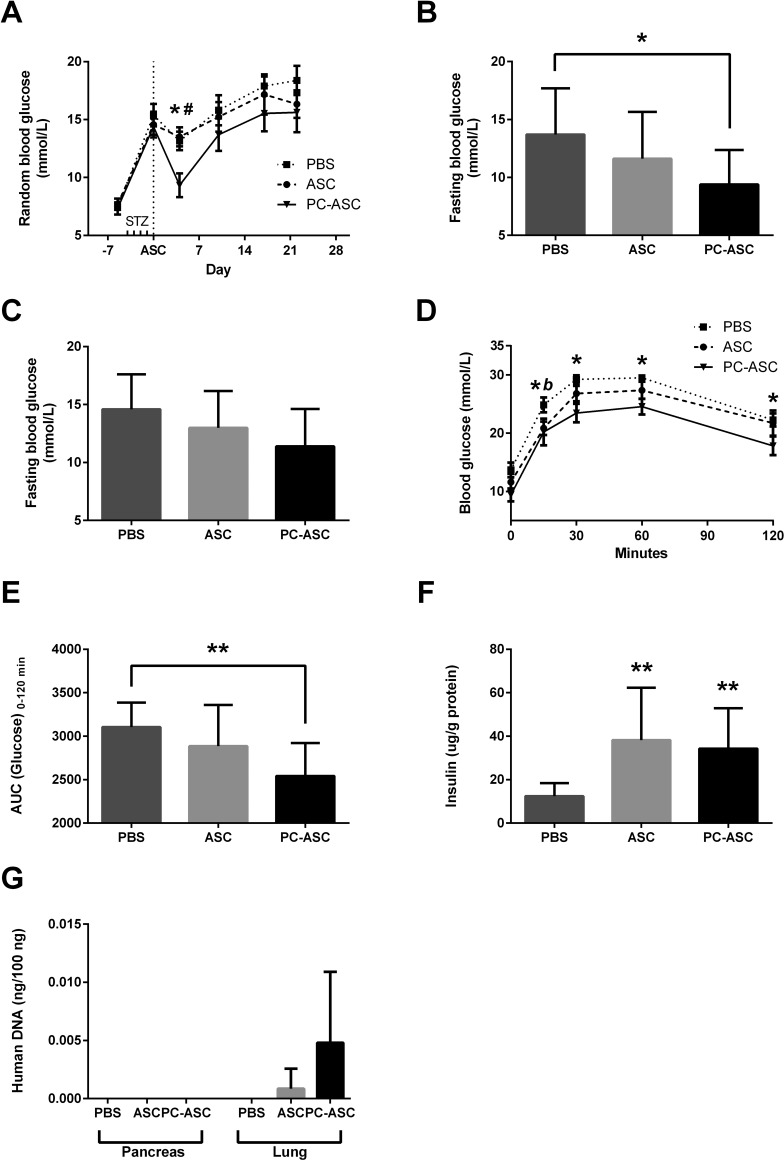
To investigate if hypoxic preconditioning of ASCs would translate into improved protection against STZ-induced insulitis and hyperglycemia, diabetic mice were randomized into three groups and received 1 day after the last STZ injection an intravenous injection of either 0.2 ml of PBS (PBS) (n = 10), or dissolved in 0.2 ml PBS containing either 0.8 × 106 ASCs (ASC) (n = 8) or 0.8 × 106 ASCs preconditioned by 48 h of incubation in hypoxia prior to infusion (PC-ASC) (n = 7). Four days after the ASC injection, we measured a significantly reduced nonfasting blood glucose in the group injected with PC-ASCs, compared to both the ASC and the PBS groups (p = 0.007 and p = 0.01) (Fig. 5A). Fasting glucose prior to OGTT performed at days 13–16 was significantly lower in the mice injected with PC-ASCs compared to PBS ( p = 0.02) (Fig. 5B). Fasting glucose at days 21–23 showed a similar tendency, although the difference did not reach statistical significance (p = 0.06) (Fig. 5C). During OGTT, significantly lower blood glucose levels were measured in the group injected with PC-ASCs at all time points compared to PBS, while the group injected with ASCs had significantly reduced blood glucose compared to PBS at 15 min (Fig. 5D). Area under the curve (AUC) analysis of the OGTT showed a significantly lower AUC in the group injected with PC-ASCs compared to PBS (p = 0.007) (Fig. 5E). To determine the remaining β-cell mass on harvest, protein-adjusted insulin in the harvested pancreas was measured. Pancreata from both the ASC and PC-ASC groups had significantly higher protein-adjusted insulin content compared to the PBS group (p = 0.003 and p = 0.003) (Fig. 5F). In order to track the ASCs after in vivo injections in the mice, levels of human DNA in harvested pancreata and lungs were determined by PCR. Human DNA was undetectable in the pancreas for all groups, while in the lungs, human DNA was detectable in two of eight animals injected with ASCs (mean: 0.0009 ng/ng total DNA), compared to four of seven animals injected with PC-ASCs (mean: 0.0048 ng/ng total DNA). Human DNA was not detected in any of the 10 animals injected with PBS.
Figure 5.
Hypoxic preconditioning potentiates antidiabetic effect of ASCs in mouse streptozotocin (STZ)-induced insulitis model. Balb/c recombination activating gene 1-deficient (Rag 1–/–) immunodeficient mice were intravenously injected with STZ (50 mg/kg) for 4 consecutive days. One day after the last STZ injection, mice received an intravenous injection with either 0.2 ml of PBS (PBS) (n = 10 mice), 0.8 × 106 nonpreconditioned ASCs (ASC) (n = 8 mice), or 0.8 × 106 ASCs preconditioned for 48 h in 1% O2 (PC-ASC) (n = 7 mice). Random glucose measured weekly (A). Fasting glucose measured on days 13–16 (B) and 21–23 after ASC injection (C). Oral glucose tolerance test performed on days 13–16 after ASC injection (D) with an area under the curve (AUC) analysis (E). Protein-adjusted insulin content of pancreas after harvest on days 21–23 after ASC injection was analyzed by insulin ELISA (F). Human DNA detected by PCR in harvested pancreata or lung homogenate as described in Materials and Methods. (G). Results are presented as mean ± SEM for line graphs and mean ± SD for bar graphs. *p < 0.05, **p < 0.01 for PC-ASC versus PBS. #p < 0.05 versus ASC, b p < 0.05 for ASC versus PBS. Dotted line for PBS, dashed line for ASCs, and solid line for PC-ASCs.
DISCUSSION
In this study, we show that incubating ASCs for 48 h in hypoxia is well tolerated. We describe significant differences in the soluble factors measured in 21% O2 CM and 1% O2 CM, and that the factors released in hypoxia improve human islet function, reduce apoptosis during culture, and protect against cytokine-induced apoptosis in vitro. Finally, we show that preconditioning ASCs in hypoxia potentiates their ability to protect against STZ-induced diabetes in vivo.
There are several reports describing the islet protective effects of both ASCs and BM-MSCs3,4,8. Although some articles describe how BM-MSCs influence islet viability and function through soluble factors in experiments using Transwell inserts18,19, others suggest that direct cell-to-cell contact is necessary20,21. Human ASCs have been shown to influence islets cultured in Transwell inserts8,22, and paracrine crosstalk between islets and ASCs has been suggested to improve secretion of trophic factors8. Our results show that when exposed to hypoxia, the ASCs release factors that can benefit the islets in vitro without the need for any stimulus from islets. Additionally, as most of the research on the islet protective effects of ASCs have been done on animal islets23, confirming the ability of human ASC CM to positively influence human islets is an important finding.
Of the 48 different factors we measured in the CM, we found a significant increase in VEGF-A (eight-plex array and multiplex), bNGF (eight-plex array and multiplex), and FGF-2 (eight-plex array only) in 1% O2 CM compared to 21% O2 CM. This is similar to previously published reports on altered secretion of soluble factors by ASCs incubated in reduced oxygen tension12,13. The beneficial effect of VEGF-A on islets has been shown in vitro by incubating porcine islets with VEGF-A and confirmed by blocking the effect with bevacizumab22, as well as in vivo by cotransplanting islets with human embryonic MSCs overexpressing VEGF-A24. bNGF has been shown to improve islet viability in vitro in a dose-dependent manner25. FGF-2 preserves islet function measured by GSIS over time26. While these trophic factors were found in higher concentrations in 1% O2 CM, we found HGF, IL-8, and CXCL1 to be significantly reduced in 1% O2 CM. The reduction in HGF was surprising, given that there are several reports describing how HGF is beneficial for islets3,27. The beneficial effect of HGF therefore cannot explain our in vitro islet results. IL-8 is a potent mediator of inflammation, which is highly upregulated in islets after isolation28. Although the levels of IL-8 did not change significantly in hypoxia when measuring CM from six different donors, we measured a significant reduction in the CM used in our islet experiments. One possible explanation for this could be that there is a variation between donors in the release of IL-8 in hypoxia and that the two donors selected for production of CM for islet experiments responded with a stronger reduction of IL-8 than expected. Thus, both donor and environmental factors seem to influence the secretory capacity of ASCs. To ensure that we describe factors relevant for our islet results, the 48-factor profile described in our article was measured in the actual CM used for islet experiments. The factor most strongly reduced in 1% O2 CM was CXCL1, a protein that has been shown to increase in type 1 diabetic patients29. It has also been shown to be upregulated during culture of human islets30 and to be detrimental to islet function in combination with CXCL531. CXCL1 receptor-deficient mice have better outcome after syngeneic islet transplantation, and medical blockade by reparixin has shown to improve islet graft survival and function, leading to an ongoing phase 3 clinical trial for this approach30. Interestingly, both IL-8 and CXCL1 act on the same receptors, CXCR1 and CXCR2. The increased levels of TGF-β1 in 1% O2 CM did not reach statistical significance, and neither IDO nor PD-L1 was detected in our samples. Although we cannot rule out effects of factors we have not measured, we believe that increased levels of islet trophic factors and lower levels of islet detrimental factors contribute to our in vitro findings of reduced apoptosis both in culture and when exposed to proinflammatory cytokines.
Finally, we describe the potential of hypoxic preconditioning of ASCs in relation to islet damage in vivo. Multiple studies have shown that intravenous injection of ASCs can limit islet injury in STZ-induced diabetic mouse models7,8. Hypoxic preconditioning of mouse BM-MSCs for 24 h in 1% O2 increases in vivo BM-MSC survival and improves therapeutic efficiency in a mouse limb ischemia model 14. Our results show that an injection of 0.8 × 106 PC-ASCs significantly improves nonfasting glucose, fasting glucose, and OGTT, compared to PBS. A lack of similar results in the ASC group and the fact that the number of ASCs injected in our experiments is lower than the dose of 1–2 × 106 used by others in similar experiments8,32,33 suggest that hypoxic preconditioning can potentiate the ASCs. When measuring insulin levels in the pancreas, we found significantly higher insulin content in both the ASC and the PC-ASC groups compared to the PBS group, indicating that ASCs still provide a degree of protection against STZ injury independent of hypoxic preconditioning. Whether the ASCs exert their effect in vivo through the secretion of factors as suggested by Kono et al.8, or whether it is due some direct cell-to-cell interactions, as described by Bassi et al.32, is unknown. In order to track our ASC in vivo, we measured human DNA in tissue after harvest. Homing of MSCs to a STZ-injured pancreas has been described34, and although some homing at levels below the sensitivity of our assay may have occurred, we did not detect human DNA in the pancreas in either of the groups. However, we did detect human DNA in the lungs of 57% of the animals injected with PC-ASCs, compared to 25% of the mice injected with ASCs, and in none of the animals injected with only PBS. Although not conclusive, these results seem to support the hypothesis of increased ASC viability after injection when preconditioned in hypoxia. Having shown that ASC CM can influence islets, we hypothesize that ASCs exert their effect through soluble factors transported through the circulation, rather than by cell-to-cell interaction.
In summary, we show that human ASCs tolerate 48 h of incubation in hypoxia and that hypoxic incubation increases the levels of islet trophic factors in CM, while islet detrimental factors are reduced. The sum of these changes improves islet function and reduces apoptosis induced by culture or proinflammatory cytokines when islets are exposed to hypoxic CM in vitro. Preconditioning ASCs in hypoxia improves their therapeutic efficiency in vivo, allowing us to observe the islet-protective effects in STZ-induced diabetes at lower doses than other reports. Further studies are needed to determine the molecular mechanisms responsible for our results and to investigate if hypoxic preconditioning of ASCs translates to improved therapeutic potential in other settings, such as islet transplantation.
ACKNOWLEDGMENTS
Human islets were provided through the JDRF award 31-2008-413 (ECIT Islet for Basic Research program). The authors are grateful to the Nordic Network for Clinical Islet Transplantation and the islet isolation teams in Uppsala, Sweden, and Oslo, Norway. The study was supported by the European Union’s 7th Framework Programme (Grant No. 222741, METOXIA) and by grants from the South-Eastern Norway Regional Health Authority (Project Nos. 2006240 and 2012031), The Norwegian Diabetes Association, and NovoNordisk (Project No. 36772).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–8. [DOI] [PubMed] [Google Scholar]
- 2. Barton FB, Rickels MR, Alejandro R, Hering BJ, Wease S, Naziruddin B, Oberholzer J, Odorico JS, Garfinkel MR, Levy M, Pattou F, Berney T, Secchi A, Messinger S, Senior PA, Maffi P, Posselt A, Stock PG, Kaufman DB, Luo X, Kandeel F, Cagliero E, Turgeon NA, Witkowski P, Naji A, O’Connell PJ, Greenbaum C, Kudva YC, Brayman KL, Aull MJ, Larsen C, Kay TW, Fernandez LA, Vantyghem MC, Bellin M, Shapiro AM. Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care 2012;35(7):1436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yeung TY, Seeberger KL, Kin T, Adesida A, Jomha N, Shapiro AM, Korbutt GS. Human mesenchymal stem cells protect human islets from pro-inflammatory cytokines. PLoS One 2012;7(5):e38189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu H, Wen D, Mahato RI. Third-party mesenchymal stem cells improved human islet transplantation in a humanized diabetic mouse model. Mol Ther. 2013;21(9):1778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zuk P, Zhu M, Mizuno H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7(2):211–28. [DOI] [PubMed] [Google Scholar]
- 6. Veronesi F, Maglio M, Tschon M, Aldini NN, Fini M. Adipose-derived mesenchymal stem cells for cartilage tissue engineering: State-of-the-art in in vivo studies. J Biomed Mater Res Part A. 2014;102(7):2448–66. [DOI] [PubMed] [Google Scholar]
- 7. Ohmura Y, Tanemura M, Kawaguchi N, Machida T, Tanida T, Deguchi T, Wada H, Kobayashi S, Marubashi S, Eguchi H, Takeda Y, Matsuura N, Ito T, Nagano H, Doki Y, Mori M. Combined transplantation of pancreatic islets and adipose tissue-derived stem cells enhances the survival and insulin function of islet grafts in diabetic mice. Transplantation 2010;90(12):1366–73. [DOI] [PubMed] [Google Scholar]
- 8. Kono TM, Sims EK, Moss DR, Yamamoto W, Ahn G, Diamond J, Tong X, Day KH, Territo PR, Hanenberg H, Traktuev DO, March KL, Evans-Molina C. Human adipose-derived stromal/stem cells protect against STZ-induced hyperglycemia: Analysis of hASC-derived paracrine effectors. Stem Cells 2014;32(7):1831–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cai L, Johnstone BH, Cook TG, Liang Z, Traktuev D, Cornetta K, Ingram D a, Rosen ED, March KL. Suppression of hepatocyte growth factor production impairs the ability of adipose-derived stem cells to promote ischemic tissue revascularization. Stem Cells 2007;25(12):3234–43. [DOI] [PubMed] [Google Scholar]
- 10. Wei X, Zhao L, Zhong J, Gu H, Feng D, Johnstone BH, March KL, Farlow MR, Du Y. Adipose stromal cells-secreted neuroprotective media against neuronal apoptosis. Neurosci Lett. 2009;462(1):76–9. [DOI] [PubMed] [Google Scholar]
- 11. Goossens GH, Blaak EE. Adipose tissue oxygen tension. Curr Opin Clin Nutr Metab Care. 2012;15(6):539–46. [DOI] [PubMed] [Google Scholar]
- 12. Liu L, Gao J, Yuan Y, Chang Q, Liao Y, Lu F. Hypoxia preconditioned human adipose derived mesenchymal stem cells enhance angiogenic potential via secretion of increased VEGF and bFGF. Cell Biol Int. 2013;37(6):551–60. [DOI] [PubMed] [Google Scholar]
- 13. Roemeling-van Rhijn M, Mensah FK, Korevaar SS, Leijs MJ, van Osch GJ, Ijzermans JN, Betjes MG, Baan CC, Weimar W, Hoogduijn MJ. Effects of hypoxia on the immunomodulatory properties of adipose tissue-derived mesenchymal stem cells. Front Immunol. 2013;4:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu H, Sun A, Zou Y, Ge J. Inducible metabolic adaptation promotes mesenchymal stem cell therapy for ischemia: A hypoxia-induced and glycogen-based energy prestorage strategy. Arterioscler Thromb Vasc Biol. 2014;34(4):870–6. [DOI] [PubMed] [Google Scholar]
- 15. Fraser JK, Hicok KC, Shanahan R, Zhu M, Miller S, Arm DM. The Celution(®) System: Automated processing of adipose-derived regenerative cells in a functionally closed system. Adv Wound Care 2014;3(1):38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause DS, Deans RJ, Keating A, Prockop DJ, Horwitz EM. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8(4):315–17. [DOI] [PubMed] [Google Scholar]
- 17. Friberg AS, Ståhle M, Brandhorst H, Korsgren O, Brandhorst D. Human islet separation utilizing a closed automated purification system. Cell Transplant. 2008;17(12):1305–13. [DOI] [PubMed] [Google Scholar]
- 18. Park KS, Kim YS, Kim JH, Choi B, Kim SH, Tan AH, Lee MS, Lee MK, Kwon CH, Joh JW, Kim SJ, Kim KW. Trophic molecules derived from human mesenchymal stem cells enhance survival, function, and angiogenesis of isolated islets after transplantation. Transplantation 2010;9(5):509–17. [DOI] [PubMed] [Google Scholar]
- 19. Scuteri A, Donzelli E, Rodriguez-Menendez V, Ravasi M, Monfrini M, Bonandrini B, Figliuzzi M, Remuzzi A, Tredici G. A double mechanism for the mesenchymal stem cells’ positive effect on pancreatic islets. PLoS One 2014;9(1):e84309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jung E-J, Kim S-C, Wee Y-M, Kim Y-H, Choi MY, Jeong S-H, Lee J, Lim D-G, Han D-J. Bone marrow-derived mesenchymal stromal cells support rat pancreatic islet survival and insulin secretory function in vitro. Cytotherapy 2011;13(1):19–29. [DOI] [PubMed] [Google Scholar]
- 21. Rackham CL, Dhadda PK, Chagastelles PC, Simpson SJ, Dattani AA, Bowe JE, Jones PM, King AJ. Pre-culturing islets with mesenchymal stromal cells using a direct contact configuration is beneficial for transplantation outcome in diabetic mice. Cytotherapy 2013;15(4):449–59. [DOI] [PubMed] [Google Scholar]
- 22. Yamada S, Shimada M, Utsunomiya T, Ikemoto T, Saito Y, Morine Y, Imura S, Mori H, Arakawa Y, Kanamoto M, Iwahashi S. Trophic effect of adipose tissue-derived stem cells on porcine islet cells. J Surg Res. 2014;187(2):667–72. [DOI] [PubMed] [Google Scholar]
- 23. Dietrich I, Crescenzi A, Chaib E, D’Albuquerque LAC. Trophic effects of adipose derived stem cells on Langerhans islets viability—Review. Transplant Rev. 2015;29(3):121–6. [DOI] [PubMed] [Google Scholar]
- 24. Hajizadeh-Saffar E, Tahamtani Y, Aghdami N, Azadmanesh K, Habibi-Anbouhi M, Heremans Y, De Leu N, Heimberg H, Ravassard P, Shokrgozar M A, Baharvand H. Inducible VEGF expression by human embryonic stem cell-derived mesenchymal stromal cells reduces the minimal islet mass required to reverse diabetes. Sci Rep. 2015;5:9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saito Y, Chan NK, Sakata N, Hathout E. Nerve growth factor is associated with islet graft failure following intraportal transplantation. Islets 2012;4(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rivas-Carrillo JD, Navarro-Alvarez N, Soto-Gutierrez A, Okitsu T, Chen Y, Tabata Y, Misawa H, Noguchi H, Matsumoto S, Tanaka N, Kobayashi N. Amelioration of diabetes in mice after single-donor islet transplantation using the controlled release of gelatinized FGF-2. Cell Transplant. 2006;15(10):939–44. [DOI] [PubMed] [Google Scholar]
- 27. García-Ocaña A, Vasavada RC, Cebrian A, Reddy V, Takane KK, López-Talavera J-C, Stewart AF. Transgenic overexpression of hepatocyte growth factor in the β-cell markedly improves islet function and islet transplant outcomes in mice. Diabetes 2001;50:2752–62. [DOI] [PubMed] [Google Scholar]
- 28. Negi S, Jetha A, Aikin R, Hasilo C, Sladek R, Paraskevas S. Analysis of beta-cell gene expression reveals inflammatory signaling and evidence of dedifferentiation following human islet isolation and culture. PLoS One 2012;7(1):e30415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takahashi K, Ohara M, Sasai T, Homma H, Nagasawa K, Takahashi T, Yamashina M, Ishii M, Fujiwara F, Kajiwara T, Taneichi H, Takebe N, Satoh. SerumCXCL1 concentrations are elevated in type 1 diabetes mellitus, possibly reflecting activity of anti-islet autoimmune activity. Diabetes Metab Res Rev. 2011;27(8):830–3. [DOI] [PubMed] [Google Scholar]
- 30. Citro A, Cantarelli E, Maffi P, Nano R, Melzi R, Mercalli A, Dugnani E, Sordi V, Magistretti P, Daffonchio L, Ruffini PA, Allegretti M, Secchi A, Bonifacio E, Piemonti L. CXCR1/2 inhibition enhances pancreatic islet survival after transplantation. J Clin Invest. 2012;122(10):3647–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nunemaker CS, Chung HG, Verrilli GM, Corbin KL, Upadhye A, Sharma PR. Increased serum CXCL1 and CXCL5 are linked to obesity, hyperglycemia, and impaired islet function. J Endocrinol. 2014;222(2):267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bassi EJ, Moraes-Vieira PM, Moreira-Sa CS, Almeida DC, Vieira LM, Cunha CS, Hiyane MI, Basso AS, Pacheco-Silva A, Camara NO. Immune regulatory properties of allogeneic adipose-derived mesenchymal stem cells in the treatment of experimental autoimmune diabetes. Diabetes 2012;61(10):2534–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li YY, Liu HH, Chen HL, Li YP. Adipose-derived mesenchymal stem cells ameliorate STZ-induced pancreas damage in type 1 diabetes. Biomed Mater Eng. 2012;22(1–3):97–103. [DOI] [PubMed] [Google Scholar]
- 34. Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, Prockop DJ. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA. 2006;103(46):17438–43. [DOI] [PMC free article] [PubMed] [Google Scholar]