Abstract
Multiple sclerosis, and its animal model experimental autoimmune encephalomyelitis, are neuroinflammatory diseases driven by autoreactive pathogenic TH cells which elicit demyelination and axonal damage. How TH cells acquire pathogenicity and communicate with myeloid cells and cells of the central nervous system remain unclear. IL-1β is recognized to play an important role in EAE and perhaps MS. Clinical EAE is significantly attenuated in IL-1 receptor-deficient and IL-1β-deficient mice, and IL-1β is found in the blood, cerebrospinal fluid, and CNS lesions of MS patients. Here, we will focus on new reports which elucidate the cellular sources of IL-1β and its actions during EAE, in both lymphoid tissues and within the CNS. Several immune cell types serve as critical producers of IL-1β during EAE, with the cytokine inducing responses in hematopoietic and non-hematopoietic cells. These findings from the EAE model should inspire efforts towards investigating the therapeutic potential of IL-1 blockade in MS.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the brain and spinal cord that presents clinically with different temporal and pathologic patterns and results in a variety of neurologic signs and symptoms. Immune cell invasion of the CNS in MS induces both demyelination and axon loss, and involves communication between the invading leukocytes and astrocytes, glia, and neurons. Autoreactive CD4+ T cells play an important role in driving MS pathology, although γδ T cells, CD8+ T cells, and B cells also appear to contribute to disease (1–5). How autoreactive TH cells acquire pathogenicity and how they mediate CNS damage remain important outstanding questions. Current MS therapies mainly target the functions of lymphocytes, but are not universally effective.
Experimental autoimmune encephalomyelitis (EAE) serves as an animal model of MS and can be elicited in several species through active immunization with myelin antigen or via adoptive transfer of T lymphocytes (passive EAE). In recent years the C57BL/6 mouse model of EAE has become the most popular, as it has allowed the use of knockout and transgenic mouse strains on this genetic background. In the most commonly used version of the C57BL/6 EAE model, mice are immunized with a Complete Freund’s Adjuvant (CFA)-based emulsion containing a peptide from murine myelin oligodendrocyte glycoprotein (MOG35-55) (6). Although this immunization elicits peptide-specific TH cells, mice must also be systemically injected with pertussis toxin (PTX) as a coadjuvant to induce a monophasic, paralytic clinical disease (7, 8). Following EAE induction, priming and differentiation of MOG-specific TH cells take place during the first week in secondary lymphoid organs. Over subsequent days, these TH cells and blood-derived myeloid cells traffic through the meninges and across the blood-brain barrier (BBB), with clinical signs of disease typically apparent by 10 days post-induction. TH cells are thought to re-encounter their cognate antigen (MOG35-55) in the context of MHC class II-expressing antigen presenting cells both in the meninges and the CNS parenchyma, with these interactions resulting in the production of pathogenic cytokines by the TH cells, notably granulocyte-macrophage colony-stimulating factor (GM-CSF) (9–11).
Established associations between IL-1 and autoimmune neuroinflammation
The IL-1 family of cytokines displays pleiotropic effects on a variety of hematopoietic and non-hematopoietic cells relevant to neuroinflammation. IL-1α is generated constitutively by epithelial cells and induced upon stimulation of most immune cell types (12). IL-1β is produced via both inflammasome-dependent and -independent pathways upon activation of a variety of leukocytes (13–17). Both of these IL-1 family members bind to a single activating receptor complex, composed of the IL-1R1 and IL-1RAcP (the IL-1R accessory protein, also called IL-1R3) chains, each containing a cytosolic Toll/interleukin-1 receptor homology (TIR) domain (18). Cytokine binding by this receptor engages the MyD88 signaling cascade, including IRAK1/2/4, TRAF6, and TAK1, to ultimately result in AP-1 and NF-κB activation and proinflammatory gene transcription. Separately, another IL-1 family member, IL-1Ra (also called IL-1RN), functions as a soluble receptor antagonist, capable of binding to IL-1R1.
Several members of the IL-1 family of cytokines have been studied in the context of EAE and MS, with initial work in EAE beginning in the late 1980s. In 1987, Symons et al. found increased levels of IL-1 activity, measured at the time by a mouse thymocyte proliferation assay, in the plasma and cerebrospinal fluid (CSF) of guinea pigs immunized with spinal cord homogenates to induce a chronic relapsing form of EAE (19). In the same year, using a rat model of passive EAE, Mannie et al. showed lymph node cells from EAE-induced rats treated with human IL-1β were more encephalitogenic, and suggested that this effect was via the action of IL-1β on T lymphocytes (20). Two studies later found evidence for IL-1α within the spinal cord of mice with EAE (21, 22), and one report showed that IL-1β protein could be detected in rats with EAE within meningeal macrophages, parenchymal infiltrating macrophages, and activated microglia (23). Subsequent studies in rats showed that recombinant human IL-1α treatment after EAE induction exacerbated clinical disease and that treatment with soluble recombinant murine IL-1 receptor or IL-1Ra could ameliorate disease (24–27). Schiffenbauer et al. first reported that IL-1R-deficient mice (on a mixed genetic background) were resistant to active EAE induction (28). Their results have been confirmed by several other groups using IL-1R-deficient mice on the C57BL/6 background (29–34), although the degree to which these mice were protected from clinical disease was somewhat variable. Despite one report to the contrary (35), IL-1β appears to be the critical mediator of EAE, rather than IL-1α, as IL-1β-deficient mice were seen to resist EAE by two groups (33, 36), while IL-1α-deficient mice remained susceptible (33, 35). For this reason, the remainder of this review will focus on IL-1β, although whether IL-1α plays any role in MS remains an open question. Consistent with a critical requirement for IL-1β for EAE susceptibility, mice deficient in the inflammasome components NLRP3 (37–41), ASC (36, 39, 42), caspase 1 (42, 43), and caspase 11 (44) were also at least partially resistant to EAE, as were mice treated with inhibitors of NLRP3 (45, 46) or caspase 1 (47). It is worth noting that in some reports (40–42), mice immunized with larger amounts of heat-killed Mycobacterium tuberculosis (Mtb) (usually greater than 300 micrograms per mouse) as part of the MOG35-55/CFA emulsion developed an NLPR3- and ASC-independent form of aggressive EAE. However, this form of EAE still appears to be IL-1β- and IL-1R-dependent, given that the experiments in IL-1β- and IL-1R-deficient mice which demonstrated EAE resistance were typically performed with large amounts of heat-killed Mtb (29–34, 36).
Beginning in 1990, reports emerged showing that IL-1β protein or IL1B transcript could be detected in the CSF (48, 49) or within CNS lesions of MS patients (50–55). More recently, these findings were extended by Seppi et al. who showed that CSF levels of IL-1β correlate with the number and volume of brain cortical demyelinating lesions (56), and by Rossi et al. who showed that relapsing-remitting MS (RRMS) patients with detectable IL-1β in the CSF at the time of clinical remission had a more severe course of disease (57). In addition, transcript levels of IL1B and two inflammasome components (CASP1 and NLRP3) were more highly expressed by peripheral blood mononuclear cells from MS patients compared to healthy controls (58–60). Collectively, while these results indicate that IL-1β expression in the CNS and blood is associated with disease activity in MS, they do not establish a causal role for the cytokine in disease pathogenesis. This review will therefore focus on the mouse model of EAE, where several groups have begun to elucidate the mechanism of action of IL-1β (Fig. 1).
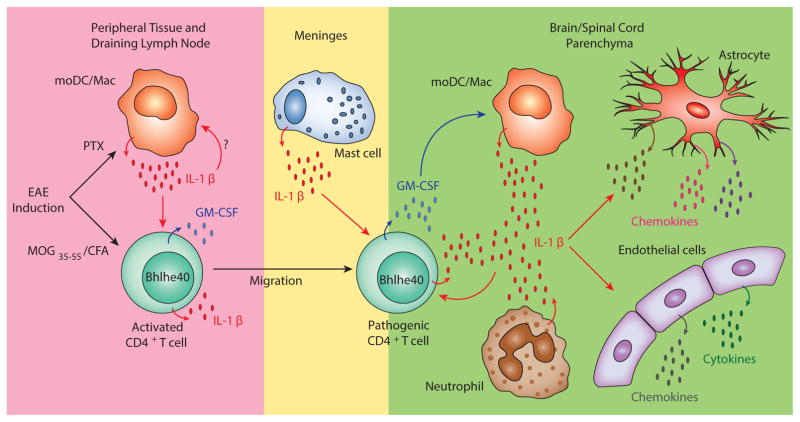
Figure 1. The Cellular Sources of IL-1β and Its Target Cells during EAE.
In the EAE model in C57BL/6 mice, animals are immunized with MOG35-55/CFA subcutaneously and treated with pertussis toxin (PTX) systemically. Five to eight days after immunization, IL-1β is primarily produced by CD11b+Ly6Cmid-hiMHC IIlo-hi monocyte-derived DC/macrophages (moDCs/Macs) in the peripheral DLNs. Activated CD4+ T cells have also been recently appreciated to be cellular sources of IL-1β. Although T cell-derived IL-1β is dispensable for CD4+ T cell priming in DLNs before disease onset, IL-1β derived from pathogenic T cells may be important to initiate inflammation within the CNS. IL-1β production from meningeal mast cells has also shown to be critical for EAE susceptibility and CD4+ T cell encephalitogenicity. Neutrophils and moDC/Macs also produce IL-1β in the CNS. Multiple cell types respond to IL-1β during EAE. IL-1β enhances Bhlhe40 expression and GM-CSF production by CD4+ T cells in both the DLN and the CNS and promotes T cell pathogenicity. Most of the myeloid cell subsets in the DLNs express IL-1R but whether they respond to IL-1β to facilitate disease progression is unclear. In the CNS, IL-1β can stimulate astrocytes to secrete chemokines which may recruit and activate leukocytes. Action of IL-1β on CNS endothelial cells facilitates cytokine production and neutrophil adhesion. Small arrows indicate cytokine or chemokine production. Large red and blue arrows indicate the actions of IL-1β and GM-CSF, respectively.
Cellular sources of IL-1β in EAE
Mononuclear phagocytes and neutrophils
Over two decades ago it was first suggested that CNS macrophages and microglia express IL-1β in rats with EAE (23), although more recent mouse experiments demonstrated that CNS-infiltrating Ly6C-positive macrophages but not resident microglia were producers of this cytokine (61). Examining IL-1β production in the CNS more carefully using pro-IL-1β reporter mice, Levesque et al. identified neutrophils and monocyte-derived macrophages as the primary cell subsets expressing IL-1β in the spinal cord after clinical disease onset (33). These authors also found that transmigration through the blood-spinal cord barrier triggered pro-IL-1β expression by neutrophils, and that at day seven after EAE induction, IL-1β-producing neutrophils were found in the blood. We and others also found IL-1β-producing myeloid cells in peripheral lymphoid organs at days five through eight following EAE induction (31, 32, 36, 62). These reports collectively identified a population of CD11b+Ly6Cmid-hiMHC IIlo-hi monocyte-derived DC/macrophages (moDCs/Macs) as the main source of IL-1β in draining lymph nodes (DLNs), and showed that these cells increase dramatically following MOG35-55/CFA immunization given with PTX coadjuvant. We and the laboratories of Sallusto and Waisman explicitly showed a requirement for PTX to yield IL-1β production from this DLN cell population, by comparing MOG35-55/CFA immunizations with and without PTX (32, 34, 36). While the details of how PTX induces DLN cells to generate IL-1β are not clear, Dumas et al. had previously shown that intraperitoneal PTX alone was sufficient to induce IL-1β production by macrophages and neutrophils in the peritoneum within a few hours after injection (63). It is noteworthy that in our experiments (32), we found that DLN cells collected at day seven from mice that were immunized with MOG35-55/CFA produced little IL-1β, even when re-exposed to the immunogenic component of CFA, heat-killed Mtb, ex vivo. In contrast, DLN cells collected from mice immunized with MOG35-55/CFA given with systemic administration of PTX produced a small amount of IL-1β ex vivo, which was greatly augmented by re-exposure to heat-killed Mtb. Overall, these results suggest that PTX is essential for the generation of IL-1β-producing cells in vivo in the C57BL/6 EAE model, and help to explain the requirement for this coadjuvant to yield clinical disease.
Another recent finding relevant to IL-1β production by myeloid cells came from Gao et al. (64) who confirmed that CD47-deficient mice were resistant to EAE (65) and showed that CD47’s role was hematopoietic cell-intrinsic. CD47 is a transmembrane protein of the immunoglobulin superfamily that can associate with integrins in cis and can bind the membrane ligand SIRPα in trans or the soluble ligand thrombospondin-1 (TSP-1). In vitro experiments showed that LPS plus ATP treatment resulted in less caspase 1 activation and less IL-1β production by CD47-deficient macrophages, and serum from CD47-deficient mice contained less IL-1β following EAE induction. Mechanistic studies demonstrated that CD47 appears to repress nitric oxide production in macrophages which in turn allows full inflammasome activation and mature IL-1β generation. These data highlight the fact that there are likely several signaling pathways which integrate to result in maximal IL-1β production by myeloid cells during EAE.
Mast cells
In 2000, Brown and colleagues showed that mast cells play an important role in the MOG35-55 model of EAE based on studies with mast cell-deficient WBB6F1-KitW/Wv mice, which displayed a reduced incidence and severity of disease (66). Selective reconstitution of meningeal mast cells by intracranial injection of bone marrow-derived mast cells (BMMCs) could restore EAE susceptibility in these mice, pointing to a particular role for mast cells at this anatomic location (67). Analysis of meningeal responses to EAE induction found that Il1b transcripts in the meninges of wild-type (WT) mice increased after EAE induction, with mast cells as a likely source, given that this increase was not seen in mast cell-deficient mice (68). More recently, the Brown group showed that reconstitution of meningeal mast cells with BMMCs from caspase 1-deficient mice could not restore EAE susceptibility or CD4+ T cell encephalitogenicity, likely indicating that IL-1β from these cells contributes to disease pathogenesis (69, 70). A recent in vitro study also showed that activated human mast cells secrete IL-1β and thus promote IL-17 production by CD4+ T cells (71).
T cells
IL-1β production by T cell has recently been appreciated. Using flow cytometry, McCandless et al. first showed that a majority of spinal cord-infiltrating CD4+ T cells, CD8+ T cells, and γδ T cells express pro-IL-1β during early EAE (72). A second study showed that in the cerebellum during EAE, CD3+ T cells expressed IL-1β as assessed by immunofluorescent microscopy, and that splenic T cells secreted IL-1β (73). TH cells from EAE-induced mice found in secondary lymphoid organs analyzed at day 7 after immunization produced IL-1β after antigen restimulation in the presence of either IL-12 or the combination of IL-23 and IL-1β (74). A true requirement for TH cell-intrinsic production of IL-1β during EAE was elegantly shown through the use of ASC conditional knockout mice (Ascfl/flLck-Cre), in which T cell-specific deletion of ASC resulted in almost complete resistance to active EAE (75). This was corroborated by the fact that Rag1-deficient mice reconstituted with Il1b-deficient CD4+ T cells also did not develop EAE after immunization. EAE induction in Ascfl/flLck-Cre mice resulted in normal CD4+ T cell priming and cytokine production in DLNs at day 10 after immunization, suggesting that non-T cell sources of IL-1β were sufficient before this time point to instruct priming. When in vivo primed Ascfl/flLck-Cre CD4+ T cells were cultured with MOG35-55 in TH1 polarizing conditions, they were fully capable of adoptively transferring EAE, but culture of these cells in TH17 conditions resulted in non-pathogenic cells. These results highlighted a surprising role for intrinsic IL-1β production specifically by TH17 cells during the effector phase of EAE, probably through autocrine action of this cytokine within the CNS. Interestingly, TH17 cell production of mature IL-1β was stimulated by ATP and required a caspase 8-containing inflammasome, rather than caspase 1. Taken all together, following EAE induction, multiple immune cell types at different times and anatomic locations must secrete IL-1β to promote clinical disease development.
IL-1β acts on TH cells to promote pathogenicity in EAE
Unlike naïve TH cells, in vitro polarized TH17 cells express high levels of the IL-1 receptor (TH1 and TH2 cells express only low levels) (76, 77). After EAE induction, IL-17A-producing as well as IFN-γ- and GM-CSF-producing CD4+ T cells in the DLN show higher IL-1R1 expression compared to Foxp3+ regulatory T cells (34). Because IL-1R1 knockout mice resisted active EAE induction but were susceptible to EAE when they served as recipients for adoptively transferred IL-1R-sufficient MOG-specific TH17 cells, Sutton et al. reasoned that TH17 cells themselves must be critical responders to IL-1β (29). Furthermore, Rag1-deficient mice reconstituted with IL-1R1-deficient CD4+ T cells (77) and mice with T cell-specific ablation of IL-1R1 (Il1r1fl/flCd4-Cre) developed milder clinical EAE after immunization (34), confirming a TH cell-intrinsic role for IL-1 responsiveness. Ghoreschi et al. demonstrated that IL-1β in combination with IL-6 and IL-23 was critically required for the generation of pathogenic TH17 cells using a system of adoptively transferred in vitro polarized MOG-specific 2D2 TCR transgenic TH cells (78). Tracking IL-17A-producing cells during EAE through a fate mapping approach confirmed that TH17 cells, but not TH1 cells, expressed IL-1R1, and that these cells responded to IL-1β by producing large amounts of IL-17A (79). Interestingly, this approach also revealed an abundant population of IL-1R1-expressing exTH17 cells that increased with disease progression and which no longer produced IL-17, but instead secreted IFN-γ in response to IL-1β.
Given that adoptive transfer studies had shown that neither IL-17 nor IFN-γ were absolutely required for passive EAE development (80, 81), the above studies did not provide a clear explanation for how IL-1 responsiveness led to TH cell encephalitogenicity. Two reports provided evidence that TH cell-derived GM-CSF was essential for pathogenicity in adoptive transfer EAE models, even in the combined absence of IL-17A and IFN-γ (80, 81), and one of these (81)showed that IL-1β strongly enhanced GM-CSF production by TH17 cells. Lukens et al. soon thereafter showed that TH and γδ T cells from EAE-induced IL-1R1-deficient mice (which were highly resistant to EAE) failed to produce GM-CSF (30), although it remained unproven that IL-1R signaling was cell-intrinsically required by TH cells for production of GM-CSF. This final point was made by Croxford et al. who showed that following EAE induction, IL-1R-deficient TH cells were less represented amongst the GM-CSF-producing TH cell population in IL-1R1 wildtype:IL-1R1-deficient mixed bone marrow chimeric mice (31).
Work from Jeffrey Bluestone’s group (82) and our own lab (83) has shown a TH cell-intrinsic requirement for the basic helix-loop-helix transcription factor Bhlhe40 (also known as DEC1, Stra13, Sharp2, and Bhlhb2) for EAE susceptibility. In each of these reports, Bhlhe40-deficient TH cells were found to produce less GM-CSF, consistent with the aforementioned work linking GM-CSF to TH cell encephalitogenicity. As a follow up to these studies, our lab analyzed the expression of Bhlhe40 in TH cells during EAE using Bhlhe40 BAC transgenic GFP reporter mice (Bhlhe40GFP) (32). GFP expression was notable in cytokine-producing TH cells within secondary lymphoid organs and the CNS during EAE, but was poorly induced if PTX was not administered at the time of immunization. In vitro cultures of TH17 cells revealed that IL-1β served as a strong stimulus for Bhlhe40 expression, and in vivo IL-1 neutralization or IL-1R1 deficiency impaired expression of the GFP reporter. More recently, to further test whether TH cell-intrinsic IL-1R signaling was required for optimal Bhlhe40 expression in autoreactive cells, we co-transferred congenically-marked 2D2.Bhlhe40GFP and 2D2.Bhlhe40GFP.Il1r1-/- TH cells to wildtype recipients and immunized these mice with MOG35-55/CFA given along with PTX (unpublished data). At seven days post-immunization, IL-1R1-deficient 2D2 cells expressed significantly less GFP than IL-1R1-sufficient cells in the DLN. Overall, these data indicate the presence of a pathway whereby PTX induces IL-1β which acts on autoreactive TH cells to induce Bhlhe40 expression and subsequent encephalitogenicity (Fig. 1).
Given the importance of IL-1R signaling for TH17 cell pathogenicity it is interesting to consider which pathways regulate expression of this receptor by these cells. Among the cytokines required for TH17 cell differentiation in vitro, IL-6 but not TGFβ or IL-23 strongly induced Il1r1 transcripts in naïve CD4+ T cells activated by anti-CD3 and anti-CD28 (77). Not surprisingly, IL-1R expression was severely impaired in TH cells lacking STAT3, the primary transcription factor downstream of IL-6 receptor signaling. Recent reports have identified three novel TH17 cell-intrinsic positive regulators of IL-1R expression, including the signaling regulator Spry4 (84), microRNA cluster miR-183-96-182 (85), and IL-1R signaling itself (64). Fukaya et al. found that Spry4-deficient mice were partially resistant to active EAE and that TH17 cells from these mice were unable to elicit passive EAE (84). Spry4-deficient TH17 cells expressed lower levels of IL-1R, and overexpression of Spry4 increased expression of the Il1r1 transcript. Spry4 may carry out this regulation via inhibition of MEK and PKC pathways. Ichiyama et al. found that the microRNA cluster miR-183-96-182 (miR-183C) was expressed specifically in TH17 cells cultured in conditions that favor encephalitogenicity (IL-1 + IL-6 + IL-23) (85). TH17 cells from miR-183C-deficient mice produced less IL-17 and GM-CSF and were less pathogenic. The 3′UTR of the transcription factor Foxo1 was found to be a target of all 3 miRs in this cluster such that miR-183C-deficient TH17 cells expressed increased levels of Foxo1, which in turn directly repressed expression of Il1r1. Lastly, Gao et al. found that addition of IL-1β to TH17 cells led to increased expression of Il1r1, supporting the existence of a feed forward circuit that reinforces TH17 cell pathogenicity (64).
IL-1β action on non-TH cells in EAE
Several lines of evidence indicate that cells other than TH cells also respond to IL-1β to promote EAE pathogenesis. Ronchi et al. tracked transferred 2D2 cells in MOG35-55/CFA-immunized hosts treated with systemic PTX and found that these autoreactive TH cells were less abundant and less frequently expressed both IFN-γ and GM-CSF in the DLNs of IL-1R-deficient recipients when compared to IL-1R1-sufficient recipients (36). Likewise, Mufazalov et al. found that mice globally deficient for IL-1R were more resistant to clinical EAE than those with only T cell-specific ablation of IL-1R1 (Il1r1fl/flCd4-Cre) (34). Finally, bone marrow chimera studies have shown that IL-1R signaling by radioresistant cells contributes to EAE pathogenesis (33, 72). Collectively, these studies suggest the possibility that hematopoietic non-TH cells, radioresistant microglia, and/or radioresistant non-hematopoietic cells all may respond to IL-1β to enhance neuroinflammation during EAE. Future studies using Il1r1fl/fl mice (34, 86–88) crossed to additional Cre-expressing strains should be useful to dissect the roles of the aforementioned cell types.
With regard to non-hematopoietic cell types, data exist supporting the notion that IL-1β acts directly on astrocytes and/or CNS endothelial cells to result in leukocyte recruitment and BBB or blood-spinal cord barrier disruption, processes that may contribute to neuroinflammation in EAE. Argaw et al. identified a pathway whereby IL-1β promoted BBB permeability, possibly through its action on astrocytes to induce their production of VEGF-A (54). More specific actions of IL-1β on astrocytes were also shown by others to include the stimulation of chemokine production (CCL2, CCL20, CXCL2) which might recruit and activate leukocytes (89, 90). Work from the groups of Quan and Lacroix, however, did not observe expression of IL-1R1 on GFAP-expressing astrocytes, and instead found its expression largely restricted to CNS endothelial cells (33, 91). Lacroix’s group further showed that this receptor was present specifically on venules at the pial venous plexus, corresponding to a site of myeloid cell infiltration during EAE (33, 92). Action of IL-1β on endothelial cells appears to make them receptive to firm adhesion with neutrophils (93) and induces their secretion of a variety of cytokines and chemokines (33). Specific knockdown of IL-1R1 on Tie2-positive endothelial cells attenuates clinical EAE, further supporting the pro-encephalitogenic action of IL-1β on these cells (94).
IL-1β in MS
While experiments in EAE have provided significant insight into the pathogenic role of IL-1β in this disease model, less is known about the contribution of this cytokine to the pathogenesis of MS. Numerous studies have detected IL-1β or its transcript in the brain, CSF, or blood of MS patients, although the cellular sources of IL-1β are not clear. Likewise, whether a specific environmental stimulus drives IL-1β production in MS, akin to how PTX acts in EAE, is unknown. Some reports have analyzed the actions of IL-1β on human cells, and these serve as a framework for understanding its potential pathogenic role in MS. IL-1β can act on subsets of human TH cells expressing the IL-1R (95), and receptor expression can be increased by IL-7, IL-15, and TGFβ (96). In vitro polarization of naïve human TH cells in a cocktail containing IL-1β induces IL17A, IL17F, IL21, IL22, IRF4, RORC, IL1R1, and IL23R expression in an IL-1R-dependent manner (97). Furthermore, IL-1β treatment of activated human TH17 cells increases the fraction of GM-CSF/IFN-γ/IL-17 multi-cytokine-producing cells (98), which are thought to play a pathogenic role in several autoimmune disorders including MS, rheumatoid arthritis, and inflammatory bowel disease. Recently, both naïve and memory TH cell subsets from MS patients were found to express higher levels of IL1R1 than cells from healthy controls, potentially suggesting increased responsiveness to this cytokine in MS (97). In addition, human astrocytes express CXCL12, IL6, CCL2, CCL5, PTGS2, and TLR2 in response to IL-1β treatment (99, 100). Future studies should aim to more fully determine whether IL-1β plays a pathogenic role in MS, perhaps in specific patient subsets or at specific stages in the disease process.
Many studies have observed that therapeutic agents used to treat MS affect IL-1Ra and/or IL-1β production. These include the commonly used drugs type I IFN, glatiramer acetate (GA), and natalizumab (anti-VLA-4). Treating RRMS and progressive MS patients with IFN-β significantly increased IL-1Ra in the serum (101–103). In vitro, IFN-β treatment of human PBMCs or monocytes promoted IL-1Ra but inhibited IL-1β production (104, 105). Using murine bone marrow-derived macrophages, Guarda et al. showed that IFN-β indirectly limited pro-IL-1β abundance by inducing IL-10 expression and directly prevented IL-1β maturation by repressing inflammasome activation (106). Inoue et al. also found an alternative mechanism whereby IFN-β reduced IL-1β maturation in macrophages, through induction of SOCS-1 and subsequent inhibition of Rac1 (40). Rac1 inhibition led to reduced ROS generation, and thus less activation of the NLRP3 inflammasome. Like IFN-β, GA increased serum IL-1Ra levels in RRMS patients, and stimulated IL-1Ra but suppressed IL-1β production when applied to human monocytes in vitro (107, 108). The regulation of IL-1Ra by GA in monocytes required both MEK/ERK and PI3Kδ pathways, while IFN-β relied on a MEK2/PI3Kδ pathway to induce IL-1Ra (109, 110). Natalizumab is a monoclonal antibody targeting the integrin very late activation antigen (VLA)-4 and thus prevents the entry of peripheral leukocytes into the CNS. Treating MS patients with natalizumab led to lower levels of IL-1β in the CSF (111, 112). Collectively, these findings suggest that regulating IL-1 family proteins may serve as one mechanism of action used by several currently approved treatments.
Numerous studies have shown that inflammasome inhibition or IL-1R signaling blockade ameliorates EAE severity and/or delays disease onset (24–27, 45, 46, 73, 93, 113). It has been proposed that drugs interrupting IL-1R signaling may be therapeutically useful as treatment for MS (92, 114). While no small molecule inhibitors have been generated to specifically block this signaling pathway, anakinra (recombinant IL-1Ra) has been approved by the U.S. Food and Drug Administration (FDA) to treat rheumatoid arthritis and appears to be relatively safe with few serious adverse reactions (115, 116). We suggest that targeting the production or actions of IL-1 in both secondary lymphoid organs and within the CNS be considered as new therapeutic opportunities for MS treatment.
Conclusions
This review has highlighted recent reports demonstrating the production of IL-1β in EAE by myeloid cells, mast cells, and T cells, and the response to this cytokine by both immune and non-hematopoietic cells. Several unanswered questions remain open to further experimentation. For example, how does PTX induce IL-1β production by monocytes and neutrophils, and what other stimuli can trigger IL-1β production by these cells? Also, it will be interesting to determine whether myeloid cells themselves respond to this cytokine in an autocrine and/or paracrine manner to facilitate disease progression. The anatomic location of these cells during different phases of disease should also be considered. As recent reports have shown T cells to produce IL-1β during EAE, it will be important to understand more fully the signals inducing this source of the cytokine. Lastly, many questions remain about the pathogenic role of IL-1β in MS and whether autoreactive subsets of human T cells respond to this cytokine to express GM-CSF or other proinflammatory cytokines.
Acknowledgments
This work was supported by National Institutes of Health Grant R01 AI113118 (to B.T. E.), a Burroughs Wellcome Fund Career Award for Medical Scientists (to B.T.E.), and the McDonnell International Scholars Academy at Washington University (to C.-C.L.).
References
- 1.Malik S, Want MY, Awasthi A. The emerging roles of gamma-delta T cells in tissue inflammation in experimental autoimmune encephalomyelitis. Front Immunol. 2016;7:14. doi: 10.3389/fimmu.2016.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salou M, Nicol B, Garcia A, Laplaud DA. Involvement of CD8(+) T cells in multiple sclerosis. Front Immunol. 2015;6:604. doi: 10.3389/fimmu.2015.00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinha S, Boyden AW, Itani FR, Crawford MP, Karandikar NJ. CD8(+) T-cells as immune regulators of multiple sclerosis. Front Immunol. 2015;6:619. doi: 10.3389/fimmu.2015.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hohlfeld R, Dornmair K, Meinl E, Wekerle H. The search for the target antigens of multiple sclerosis, part 2: CD8+ T cells, B cells, and antibodies in the focus of reverse-translational research. Lancet Neurol. 2016;15:317–331. doi: 10.1016/S1474-4422(15)00313-0. [DOI] [PubMed] [Google Scholar]
- 5.Claes N, Fraussen J, Stinissen P, Hupperts R, Somers V. B cells are multifunctional players in multiple sclerosis pathogenesis: insights from therapeutic interventions. Front Immunol. 2015;6:642. doi: 10.3389/fimmu.2015.00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nat Protoc. 2006;1:1810–1819. doi: 10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- 7.Levine S, Sowinski R. Experimental allergic encephalomyelitis in inbred and outbred mice. J Immunol. 1973;110:139–143. [PubMed] [Google Scholar]
- 8.Bettelli E, Das MP, Howard ED, Weiner HL, Sobel RA, Kuchroo VK. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J Immunol. 1998;161:3299–3306. [PubMed] [Google Scholar]
- 9.Codarri L, Greter M, Becher B. Communication between pathogenic T cells and myeloid cells in neuroinflammatory disease. Trends Immunol. 2013;34:114–119. doi: 10.1016/j.it.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Croxford AL, Spath S, Becher B. GM-CSF in neuroinflammation: licensing myeloid cells for tissue damage. Trends Immunol. 2015;36:651–662. doi: 10.1016/j.it.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Becher B, Tugues S, Greter M. GM-CSF: from growth factor to central mediator of tissue inflammation. Immunity. 2016;45:963–973. doi: 10.1016/j.immuni.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Di Paolo NC, Shayakhmetov DM. Interleukin 1alpha and the inflammatory process. Nat Immunol. 2016;17:906–913. doi: 10.1038/ni.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, March CJ, Kronheim SR, Druck T, Cannizzaro LA, et al. Molecular cloning of the interleukin-1 beta converting enzyme. Science. 1992;256:97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- 14.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 15.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 16.Piccioli P, Rubartelli A. The secretion of IL-1beta and options for release. Semin Immunol. 2013;25:425–429. doi: 10.1016/j.smim.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Netea MG, van de Veerdonk FL, van der Meer JW, Dinarello CA, Joosten LA. Inflammasome-independent regulation of IL-1-family cytokines. Annu Rev Immunol. 2015;33:49–77. doi: 10.1146/annurev-immunol-032414-112306. [DOI] [PubMed] [Google Scholar]
- 18.Boraschi D, Tagliabue A. The interleukin-1 receptor family. Semin Immunol. 2013;25:394–407. doi: 10.1016/j.smim.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 19.Symons JA, Bundick RV, Suckling AJ, Rumsby MG. Cerebrospinal fluid interleukin 1 like activity during chronic relapsing experimental allergic encephalomyelitis. Clin Exp Immunol. 1987;68:648–654. [PMC free article] [PubMed] [Google Scholar]
- 20.Mannie MD, Dinarello CA, Paterson PY. Interleukin 1 and myelin basic protein synergistically augment adoptive transfer activity of lymphocytes mediating experimental autoimmune encephalomyelitis in Lewis rats. J Immunol. 1987;138:4229–4235. [PubMed] [Google Scholar]
- 21.Baker D, O’Neill JK, Turk JL. Cytokines in the central nervous system of mice during chronic relapsing experimental allergic encephalomyelitis. Cell Immunol. 1991;134:505–510. doi: 10.1016/0008-8749(91)90321-2. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy MK, Torrance DS, Picha KS, Mohler KM. Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol. 1992;149:2496–2505. [PubMed] [Google Scholar]
- 23.Bauer J, Berkenbosch F, Van Dam AM, Dijkstra CD. Demonstration of interleukin-1 beta in Lewis rat brain during experimental allergic encephalomyelitis by immunocytochemistry at the light and ultrastructural level. J Neuroimmunol. 1993;48:13–21. doi: 10.1016/0165-5728(93)90053-2. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs CA, Baker PE, Roux ER, Picha KS, Toivola B, Waugh S, Kennedy MK. Experimental autoimmune encephalomyelitis is exacerbated by IL-1 alpha and suppressed by soluble IL-1 receptor. J Immunol. 1991;146:2983–2989. [PubMed] [Google Scholar]
- 25.Martin D, Near SL. Protective effect of the interleukin-1 receptor antagonist (IL-1ra) on experimental allergic encephalomyelitis in rats. J Neuroimmunol. 1995;61:241–245. doi: 10.1016/0165-5728(95)00108-e. [DOI] [PubMed] [Google Scholar]
- 26.Badovinac V, Mostarica-Stojkovic M, Dinarello CA, Stosic-Grujicic S. Interleukin-1 receptor antagonist suppresses experimental autoimmune encephalomyelitis (EAE) in rats by influencing the activation and proliferation of encephalitogenic cells. J Neuroimmunol. 1998;85:87–95. doi: 10.1016/s0165-5728(98)00020-4. [DOI] [PubMed] [Google Scholar]
- 27.Wiemann B, Van GY, Danilenko DM, Yan Q, Matheson C, Munyakazi L, Ogenstad S, Starnes CO. Combined treatment of acute EAE in Lewis rats with TNF-binding protein and interleukin-1 receptor antagonist. Exp Neurol. 1998;149:455–463. doi: 10.1006/exnr.1997.6723. [DOI] [PubMed] [Google Scholar]
- 28.Schiffenbauer J, Streit WJ, Butfiloski E, LaBow M, Edwards C, 3rd, Moldawer LL. The induction of EAE is only partially dependent on TNF receptor signaling but requires the IL-1 type I receptor. Clin Immunol. 2000;95:117–123. doi: 10.1006/clim.2000.4851. [DOI] [PubMed] [Google Scholar]
- 29.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lukens JR, Barr MJ, Chaplin DD, Chi H, Kanneganti TD. Inflammasome-derived IL-1beta regulates the production of GM-CSF by CD4(+) T cells and gammadelta T cells. J Immunol. 2012;188:3107–3115. doi: 10.4049/jimmunol.1103308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Croxford AL, Lanzinger M, Hartmann FJ, Schreiner B, Mair F, Pelczar P, Clausen BE, Jung S, Greter M, Becher B. The Cytokine GM-CSF drives the inflammatory signature of CCR2(+) monocytes and licenses autoimmunity. Immunity. 2015;43:502–514. doi: 10.1016/j.immuni.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Lin CC, Bradstreet TR, Schwarzkopf EA, Jarjour NN, Chou C, Archambault AS, Sim J, Zinselmeyer BH, Carrero JA, Wu GF, et al. IL-1-induced Bhlhe40 identifies pathogenic T helper cells in a model of autoimmune neuroinflammation. J Exp Med. 2016;213:251–271. doi: 10.1084/jem.20150568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levesque SA, Pare A, Mailhot B, Bellver-Landete V, Kebir H, Lecuyer MA, Alvarez JI, Prat A, de Rivero Vaccari JP, Keane RW, et al. Myeloid cell transmigration across the CNS vasculature triggers IL-1beta-driven neuroinflammation during autoimmune encephalomyelitis in mice. J Exp Med. 2016;213:929–949. doi: 10.1084/jem.20151437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mufazalov IA, Schelmbauer C, Regen T, Kuschmann J, Wanke F, Gabriel LA, Hauptmann J, Muller W, Pinteaux E, Kurschus FC, et al. IL-1 signaling is critical for expansion but not generation of autoreactive GM-CSF+ Th17 cells. EMBO J. 2017;36:102–115. doi: 10.15252/embj.201694615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuki T, Nakae S, Sudo K, Horai R, Iwakura Y. Abnormal T cell activation caused by the imbalance of the IL-1/IL-1R antagonist system is responsible for the development of experimental autoimmune encephalomyelitis. Int Immunol. 2006;18:399–407. doi: 10.1093/intimm/dxh379. [DOI] [PubMed] [Google Scholar]
- 36.Ronchi F, Basso C, Preite S, Reboldi A, Baumjohann D, Perlini L, Lanzavecchia A, Sallusto F. Experimental priming of encephalitogenic Th1/Th17 cells requires pertussis toxin-driven IL-1beta production by myeloid cells. Nat Commun. 2016;7:11541. doi: 10.1038/ncomms11541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gris D, Ye Z, Iocca HA, Wen H, Craven RR, Gris P, Huang M, Schneider M, Miller SD, Ting JP. NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J Immunol. 2010;185:974–981. doi: 10.4049/jimmunol.0904145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jha S, Srivastava SY, Brickey WJ, Iocca H, Toews A, Morrison JP, Chen VS, Gris D, Matsushima GK, Ting JP. The inflammasome sensor, NLRP3, regulates CNS inflammation and demyelination via caspase-1 and interleukin-18. J Neurosci. 2010;30:15811–15820. doi: 10.1523/JNEUROSCI.4088-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inoue M, Williams KL, Gunn MD, Shinohara ML. NLRP3 inflammasome induces chemotactic immune cell migration to the CNS in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2012;109:10480–10485. doi: 10.1073/pnas.1201836109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inoue M, Williams KL, Oliver T, Vandenabeele P, Rajan JV, Miao EA, Shinohara ML. Interferon-beta therapy against EAE is effective only when development of the disease depends on the NLRP3 inflammasome. Sci Signal. 2012;5:ra38. doi: 10.1126/scisignal.2002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inoue M, Chen PH, Siecinski S, Li QJ, Liu C, Steinman L, Gregory SG, Benner E, Shinohara ML. An interferon-beta-resistant and NLRP3 inflammasome-independent subtype of EAE with neuronal damage. Nat Neurosci. 2016;19:1599–1609. doi: 10.1038/nn.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaw PJ, Lukens JR, Burns S, Chi H, McGargill MA, Kanneganti TD. Cutting edge: critical role for PYCARD/ASC in the development of experimental autoimmune encephalomyelitis. J Immunol. 2010;184:4610–4614. doi: 10.4049/jimmunol.1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furlan R, Martino G, Galbiati F, Poliani PL, Smiroldo S, Bergami A, Desina G, Comi G, Flavell R, Su MS, et al. Caspase-1 regulates the inflammatory process leading to autoimmune demyelination. J Immunol. 1999;163:2403–2409. [PubMed] [Google Scholar]
- 44.Hisahara S, Yuan J, Momoi T, Okano H, Miura M. Caspase-11 mediates oligodendrocyte cell death and pathogenesis of autoimmune-mediated demyelination. J Exp Med. 2001;193:111–122. doi: 10.1084/jem.193.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shao BZ, Wei W, Ke P, Xu ZQ, Zhou JX, Liu C. Activating cannabinoid receptor 2 alleviates pathogenesis of experimental autoimmune encephalomyelitis via activation of autophagy and inhibiting NLRP3 inflammasome. CNS Neurosci Ther. 2014;20:1021–1028. doi: 10.1111/cns.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lalor SJ, Dungan LS, Sutton CE, Basdeo SA, Fletcher JM, Mills KH. Caspase-1-processed cytokines IL-1beta and IL-18 promote IL-17 production by gammadelta and CD4 T cells that mediate autoimmunity. J Immunol. 2011;186:5738–5748. doi: 10.4049/jimmunol.1003597. [DOI] [PubMed] [Google Scholar]
- 48.Hauser SL, Doolittle TH, Lincoln R, Brown RH, Dinarello CA. Cytokine accumulations in CSF of multiple sclerosis patients: frequent detection of interleukin-1 and tumor necrosis factor but not interleukin-6. Neurology. 1990;40:1735–1739. doi: 10.1212/wnl.40.11.1735. [DOI] [PubMed] [Google Scholar]
- 49.Dujmovic I, Mangano K, Pekmezovic T, Quattrocchi C, Mesaros S, Stojsavljevic N, Nicoletti F, Drulovic J. The analysis of IL-1 beta and its naturally occurring inhibitors in multiple sclerosis: The elevation of IL-1 receptor antagonist and IL-1 receptor type II after steroid therapy. J Neuroimmunol. 2009;207:101–106. doi: 10.1016/j.jneuroim.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Wucherpfennig KW, Newcombe J, Li H, Keddy C, Cuzner ML, Hafler DA. T cell receptor V alpha-V beta repertoire and cytokine gene expression in active multiple sclerosis lesions. J Exp Med. 1992;175:993–1002. doi: 10.1084/jem.175.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woodroofe MN, Cuzner ML. Cytokine mRNA expression in inflammatory multiple sclerosis lesions: detection by non-radioactive in situ hybridization. Cytokine. 1993;5:583–588. doi: 10.1016/s1043-4666(05)80008-0. [DOI] [PubMed] [Google Scholar]
- 52.Cannella B, Raine CS. The adhesion molecule and cytokine profile of multiple sclerosis lesions. Ann Neurol. 1995;37:424–435. doi: 10.1002/ana.410370404. [DOI] [PubMed] [Google Scholar]
- 53.McGuinness MC, Powers JM, Bias WB, Schmeckpeper BJ, Segal AH, Gowda VC, Wesselingh SL, Berger J, Griffin DE, Smith KD. Human leukocyte antigens and cytokine expression in cerebral inflammatory demyelinative lesions of X-linked adrenoleukodystrophy and multiple sclerosis. J Neuroimmunol. 1997;75:174–182. doi: 10.1016/s0165-5728(97)00020-9. [DOI] [PubMed] [Google Scholar]
- 54.Argaw AT, Zhang Y, Snyder BJ, Zhao ML, Kopp N, Lee SC, Raine CS, Brosnan CF, John GR. IL-1beta regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J Immunol. 2006;177:5574–5584. doi: 10.4049/jimmunol.177.8.5574. [DOI] [PubMed] [Google Scholar]
- 55.Burm SM, Peferoen LA, Zuiderwijk-Sick EA, Haanstra KG, t Hart BA, van der Valk P, Amor S, Bauer J, Bajramovic JJ. Expression of IL-1beta in rhesus EAE and MS lesions is mainly induced in the CNS itself. J Neuroinflammation. 2016;13:138. doi: 10.1186/s12974-016-0605-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seppi D, Puthenparampil M, Federle L, Ruggero S, Toffanin E, Rinaldi F, Perini P, Gallo P. Cerebrospinal fluid IL-1beta correlates with cortical pathology load in multiple sclerosis at clinical onset. J Neuroimmunol. 2014;270:56–60. doi: 10.1016/j.jneuroim.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 57.Rossi S, Studer V, Motta C, Germani G, Macchiarulo G, Buttari F, Mancino R, Castelli M, De Chiara V, Weiss S, et al. Cerebrospinal fluid detection of interleukin-1beta in phase of remission predicts disease progression in multiple sclerosis. J Neuroinflammation. 2014;11:32. doi: 10.1186/1742-2094-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Furlan R, Filippi M, Bergami A, Rocca MA, Martinelli V, Poliani PL, Grimaldi LM, Desina G, Comi G, Martino G. Peripheral levels of caspase-1 mRNA correlate with disease activity in patients with multiple sclerosis; a preliminary study. J Neurol Neurosurg Psychiatry. 1999;67:785–788. doi: 10.1136/jnnp.67.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heidary M, Rakhshi N, Pahlevan Kakhki M, Behmanesh M, Sanati MH, Sanadgol N, Kamaladini H, Nikravesh A. The analysis of correlation between IL-1B gene expression and genotyping in multiple sclerosis patients. J Neurol Sci. 2014;343:41–45. doi: 10.1016/j.jns.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 60.Peelen E, Damoiseaux J, Muris AH, Knippenberg S, Smolders J, Hupperts R, Thewissen M. Increased inflammasome related gene expression profile in PBMC may facilitate T helper 17 cell induction in multiple sclerosis. Mol Immunol. 2015;63:521–529. doi: 10.1016/j.molimm.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 61.Vainchtein ID, Vinet J, Brouwer N, Brendecke S, Biagini G, Biber K, Boddeke HW, Eggen BJ. In acute experimental autoimmune encephalomyelitis, infiltrating macrophages are immune activated, whereas microglia remain immune suppressed. Glia. 2014;62:1724–1735. doi: 10.1002/glia.22711. [DOI] [PubMed] [Google Scholar]
- 62.Ko HJ, Brady JL, Ryg-Cornejo V, Hansen DS, Vremec D, Shortman K, Zhan Y, Lew AM. GM-CSF-responsive monocyte-derived dendritic cells are pivotal in Th17 pathogenesis. J Immunol. 2014;192:2202–2209. doi: 10.4049/jimmunol.1302040. [DOI] [PubMed] [Google Scholar]
- 63.Dumas A, Amiable N, de Rivero Vaccari JP, Chae JJ, Keane RW, Lacroix S, Vallieres L. The inflammasome pyrin contributes to pertussis toxin-induced IL-1beta synthesis, neutrophil intravascular crawling and autoimmune encephalomyelitis. PLoS Pathog. 2014;10:e1004150. doi: 10.1371/journal.ppat.1004150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao Q, Zhang Y, Han C, Hu X, Zhang H, Xu X, Tian J, Liu Y, Ding Y, Liu J, et al. Blockade of CD47 ameliorates autoimmune inflammation in CNS by suppressing IL-1-triggered infiltration of pathogenic Th17 cells. J Autoimmun. 2016;69:74–85. doi: 10.1016/j.jaut.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 65.Han MH, Lundgren DH, Jaiswal S, Chao M, Graham KL, Garris CS, Axtell RC, Ho PP, Lock CB, Woodard JI, et al. Janus-like opposing roles of CD47 in autoimmune brain inflammation in humans and mice. J Exp Med. 2012;209:1325–1334. doi: 10.1084/jem.20101974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Secor VH, Secor WE, Gutekunst CA, Brown MA. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med. 2000;191:813–822. doi: 10.1084/jem.191.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sayed BA, Christy AL, Walker ME, Brown MA. Meningeal mast cells affect early T cell central nervous system infiltration and blood-brain barrier integrity through TNF: a role for neutrophil recruitment? J Immunol. 2010;184:6891–6900. doi: 10.4049/jimmunol.1000126. [DOI] [PubMed] [Google Scholar]
- 68.Christy AL, Walker ME, Hessner MJ, Brown MA. Mast cell activation and neutrophil recruitment promotes early and robust inflammation in the meninges in EAE. J Autoimmun. 2013;42:50–61. doi: 10.1016/j.jaut.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 69.Russi AE, Walker-Caulfield ME, Guo Y, Lucchinetti CF, Brown MA. Meningeal mast cell-T cell crosstalk regulates T cell encephalitogenicity. J Autoimmun. 2016;73:100–110. doi: 10.1016/j.jaut.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Russi AE, Walker-Caulfield ME, Brown MA. Mast cell inflammasome activity in the meninges regulates EAE disease severity. Clin Immunol. 2016 doi: 10.1016/j.clim.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 71.Suurmond J, Habets KL, Dorjee AL, Huizinga TW, Toes RE. Expansion of Th17 Cells by Human Mast Cells Is Driven by Inflammasome-Independent IL-1beta. J Immunol. 2016;197:4473–4481. doi: 10.4049/jimmunol.1502640. [DOI] [PubMed] [Google Scholar]
- 72.McCandless EE, Budde M, Lees JR, Dorsey D, Lyng E, Klein RS. IL-1R signaling within the central nervous system regulates CXCL12 expression at the blood-brain barrier and disease severity during experimental autoimmune encephalomyelitis. J Immunol. 2009;183:613–620. doi: 10.4049/jimmunol.0802258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mandolesi G, Musella A, Gentile A, Grasselli G, Haji N, Sepman H, Fresegna D, Bullitta S, De Vito F, Musumeci G, et al. Interleukin-1beta alters glutamate transmission at purkinje cell synapses in a mouse model of multiple sclerosis. J Neurosci. 2013;33:12105–12121. doi: 10.1523/JNEUROSCI.5369-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murphy AC, Lalor SJ, Lynch MA, Mills KH. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav Immun. 2010;24:641–651. doi: 10.1016/j.bbi.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 75.Martin BN, Wang C, Zhang CJ, Kang Z, Gulen MF, Zepp JA, Zhao J, Bian G, Do JS, Min B, et al. T cell-intrinsic ASC critically promotes T(H)17-mediated experimental autoimmune encephalomyelitis. Nat Immunol. 2016;17:583–592. doi: 10.1038/ni.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guo L, Wei G, Zhu J, Liao W, Leonard WJ, Zhao K, Paul W. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci U S A. 2009;106:13463–13468. doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 81.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martinez-Llordella M, Esensten JH, Bailey-Bucktrout SL, Lipsky RH, Marini A, Chen J, Mughal M, Mattson MP, Taub DD, Bluestone JA. CD28-inducible transcription factor DEC1 is required for efficient autoreactive CD4+ T cell response. J Exp Med. 2013;210:1603–1619. doi: 10.1084/jem.20122387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin CC, Bradstreet TR, Schwarzkopf EA, Sim J, Carrero JA, Chou C, Cook LE, Egawa T, Taneja R, Murphy TL, et al. Bhlhe40 controls cytokine production by T cells and is essential for pathogenicity in autoimmune neuroinflammation. Nat Commun. 2014;5:3551. doi: 10.1038/ncomms4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fukaya T, Someya K, Hibino S, Okada M, Yamane H, Taniguchi K, Yoshimura A. Loss of Sprouty4 in T cells ameliorates experimental autoimmune encephalomyelitis in mice by negatively regulating IL-1beta receptor expression. Biochem Biophys Res Commun. 2014;447:471–478. doi: 10.1016/j.bbrc.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 85.Ichiyama K, Gonzalez-Martin A, Kim BS, Jin HY, Jin W, Xu W, Sabouri-Ghomi M, Xu S, Zheng P, Xiao C, et al. The MicroRNA-183–96–182 Cluster Promotes T Helper 17 Cell Pathogenicity by Negatively Regulating Transcription Factor Foxo1 Expression. Immunity. 2016;44:1284–1298. doi: 10.1016/j.immuni.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Robson MJ, Zhu CB, Quinlan MA, Botschner DA, Baganz NL, Lindler KM, Thome JG, Hewlett WA, Blakely RD. Generation and Characterization of Mice Expressing a Conditional Allele of the Interleukin-1 Receptor Type 1. PLoS One. 2016;11:e0150068. doi: 10.1371/journal.pone.0150068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abdulaal WH, Walker CR, Costello R, Redondo-Castro E, Mufazalov IA, Papaemmanouil A, Rothwell NJ, Allan SM, Waisman A, Pinteaux E, et al. Characterization of a conditional interleukin-1 receptor 1 mouse mutant using the Cre/LoxP system. Eur J Immunol. 2016;46:912–918. doi: 10.1002/eji.201546075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mufazalov IA, Regen T, Schelmbauer C, Kuschmann J, Muratova AM, Nikolaev A, Muller W, Pinteaux E, Waisman A. Generation of a Novel T Cell Specific Interleukin-1 Receptor Type 1 Conditional Knock Out Mouse Reveals Intrinsic Defects in Survival, Expansion and Cytokine Production of CD4 T Cells. PLoS One. 2016;11:e0161505. doi: 10.1371/journal.pone.0161505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Y, Jin S, Sonobe Y, Cheng Y, Horiuchi H, Parajuli B, Kawanokuchi J, Mizuno T, Takeuchi H, Suzumura A. Interleukin-1beta induces blood-brain barrier disruption by downregulating Sonic hedgehog in astrocytes. PLoS One. 2014;9:e110024. doi: 10.1371/journal.pone.0110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rothhammer V, Quintana FJ. Control of autoimmune CNS inflammation by astrocytes. Semin Immunopathol. 2015;37:625–638. doi: 10.1007/s00281-015-0515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu X, Yamashita T, Chen Q, Belevych N, McKim DB, Tarr AJ, Coppola V, Nath N, Nemeth DP, Syed ZW, et al. Interleukin 1 type 1 receptor restore: a genetic mouse model for studying interleukin 1 receptor-mediated effects in specific cell types. J Neurosci. 2015;35:2860–2870. doi: 10.1523/JNEUROSCI.3199-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pare A, Mailhot B, Levesque SA, Lacroix S. Involvement of the IL-1 system in experimental autoimmune encephalomyelitis and multiple sclerosis: Breaking the vicious cycle between IL-1beta and GM-CSF. Brain Behav Immun. 2016 doi: 10.1016/j.bbi.2016.07.146. [DOI] [PubMed] [Google Scholar]
- 93.Aube B, Levesque SA, Pare A, Chamma E, Kebir H, Gorina R, Lecuyer MA, Alvarez JI, De Koninck Y, Engelhardt B, et al. Neutrophils mediate blood-spinal cord barrier disruption in demyelinating neuroinflammatory diseases. J Immunol. 2014;193:2438–2454. doi: 10.4049/jimmunol.1400401. [DOI] [PubMed] [Google Scholar]
- 94.Li Q, Powell N, Zhang H, Belevych N, Ching S, Chen Q, Sheridan J, Whitacre C, Quan N. Endothelial IL-1R1 is a critical mediator of EAE pathogenesis. Brain Behav Immun. 2011;25:160–167. doi: 10.1016/j.bbi.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shirakawa F, Tanaka Y, Ota T, Suzuki H, Eto S, Yamashita U. Expression of interleukin 1 receptors on human peripheral T cells. J Immunol. 1987;138:4243–4248. [PubMed] [Google Scholar]
- 96.Lee WW, Kang SW, Choi J, Lee SH, Shah K, Eynon EE, Flavell RA, Kang I. Regulating human Th17 cells via differential expression of IL-1 receptor. Blood. 2010;115:530–540. doi: 10.1182/blood-2009-08-236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sha Y, Markovic-Plese S. Activated IL-1RI Signaling Pathway Induces Th17 Cell Differentiation via Interferon Regulatory Factor 4 Signaling in Patients with Relapsing-Remitting Multiple Sclerosis. Front Immunol. 2016;7:543. doi: 10.3389/fimmu.2016.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Duhen T, Campbell DJ. IL-1beta promotes the differentiation of polyfunctional human CCR6+CXCR3+ Th1/17 cells that are specific for pathogenic and commensal microbes. J Immunol. 2014;193:120–129. doi: 10.4049/jimmunol.1302734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Calderon TM, Eugenin EA, Lopez L, Kumar SS, Hesselgesser J, Raine CS, Berman JW. A role for CXCL12 (SDF-1alpha) in the pathogenesis of multiple sclerosis: regulation of CXCL12 expression in astrocytes by soluble myelin basic protein. J Neuroimmunol. 2006;177:27–39. doi: 10.1016/j.jneuroim.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 100.Mayo L, Trauger SA, Blain M, Nadeau M, Patel B, Alvarez JI, Mascanfroni ID, Yeste A, Kivisakk P, Kallas K, et al. Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat Med. 2014;20:1147–1156. doi: 10.1038/nm.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nicoletti F, Patti F, DiMarco R, Zaccone P, Nicoletti A, Meroni P, Reggio A. Circulating serum levels of IL-1ra in patients with relapsing remitting multiple sclerosis are normal during remission phases but significantly increased either during exacerbations or in response to IFN-beta treatment. Cytokine. 1996;8:395–400. doi: 10.1006/cyto.1996.0054. [DOI] [PubMed] [Google Scholar]
- 102.Perini P, Tiberio M, Sivieri S, Facchinetti A, Biasi G, Gallo P. Interleukin-1 receptor antagonist, soluble tumor necrosis factor-alpha receptor type I and II, and soluble E-selectin serum levels in multiple sclerosis patients receiving weekly intramuscular injections of interferon-beta1a. Eur Cytokine Netw. 2000;11:81–86. [PubMed] [Google Scholar]
- 103.Comabella M, Julia E, Tintore M, Brieva L, Tellez N, Rio J, Lopez C, Rovira A, Montalban X. Induction of serum soluble tumor necrosis factor receptor II (sTNF-RII) and interleukin-1 receptor antagonist (IL-1ra) by interferon beta-1b in patients with progressive multiple sclerosis. J Neurol. 2008;255:1136–1141. doi: 10.1007/s00415-008-0855-1. [DOI] [PubMed] [Google Scholar]
- 104.Coclet-Ninin J, Dayer JM, Burger D. Interferon-beta not only inhibits interleukin-1beta and tumor necrosis factor-alpha but stimulates interleukin-1 receptor antagonist production in human peripheral blood mononuclear cells. Eur Cytokine Netw. 1997;8:345–349. [PubMed] [Google Scholar]
- 105.Jungo F, Dayer JM, Modoux C, Hyka N, Burger D. IFN-beta inhibits the ability of T lymphocytes to induce TNF-alpha and IL-1beta production in monocytes upon direct cell-cell contact. Cytokine. 2001;14:272–282. doi: 10.1006/cyto.2001.0884. [DOI] [PubMed] [Google Scholar]
- 106.Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Forster I, Farlik M, Decker T, Du Pasquier RA, Romero P, et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–223. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 107.Burger D, Molnarfi N, Weber MS, Brandt KJ, Benkhoucha M, Gruaz L, Chofflon M, Zamvil SS, Lalive PH. Glatiramer acetate increases IL-1 receptor antagonist but decreases T cell-induced IL-1beta in human monocytes and multiple sclerosis. Proc Natl Acad Sci U S A. 2009;106:4355–4359. doi: 10.1073/pnas.0812183106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Caragnano M, Tortorella P, Bergami A, Ruggieri M, Livrea P, Specchio LM, Martino G, Trojano M, Furlan R, Avolio C. Monocytes P2X7 purinergic receptor is modulated by glatiramer acetate in multiple sclerosis. J Neuroimmunol. 2012;245:93–97. doi: 10.1016/j.jneuroim.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 109.Carpintero R, Brandt KJ, Gruaz L, Molnarfi N, Lalive PH, Burger D. Glatiramer acetate triggers PI3Kdelta/Akt and MEK/ERK pathways to induce IL-1 receptor antagonist in human monocytes. Proc Natl Acad Sci U S A. 2010;107:17692–17697. doi: 10.1073/pnas.1009443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Carpintero R, Burger D. IFNbeta and glatiramer acetate trigger different signaling pathways to regulate the IL-1 system in multiple sclerosis. Commun Integr Biol. 2011;4:112–114. doi: 10.4161/cib.4.1.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mellergard J, Edstrom M, Vrethem M, Ernerudh J, Dahle C. Natalizumab treatment in multiple sclerosis: marked decline of chemokines and cytokines in cerebrospinal fluid. Mult Scler. 2010;16:208–217. doi: 10.1177/1352458509355068. [DOI] [PubMed] [Google Scholar]
- 112.Mellergard J, Tisell A, Dahlqvist Leinhard O, Blystad I, Landtblom AM, Blennow K, Olsson B, Dahle C, Ernerudh J, Lundberg P, et al. Association between change in normal appearing white matter metabolites and intrathecal inflammation in natalizumab-treated multiple sclerosis. PLoS One. 2012;7:e44739. doi: 10.1371/journal.pone.0044739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Furlan R, Bergami A, Brambilla E, Butti E, De Simoni MG, Campagnoli M, Marconi P, Comi G, Martino G. HSV-1-mediated IL-1 receptor antagonist gene therapy ameliorates MOG(35–55)-induced experimental autoimmune encephalomyelitis in C57BL/6 mice. Gene Ther. 2007;14:93–98. doi: 10.1038/sj.gt.3302805. [DOI] [PubMed] [Google Scholar]
- 114.Warabi Y. Role of IL-1 and potential therapies in multiple sclerosis. Drug Discovery Today. 2007;4:19–24. [Google Scholar]
- 115.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schett G, Dayer JM, Manger B. Interleukin-1 function and role in rheumatic disease. Nat Rev Rheumatol. 2016;12:14–24. doi: 10.1038/nrrheum.2016.166. [DOI] [PubMed] [Google Scholar]