Abstract
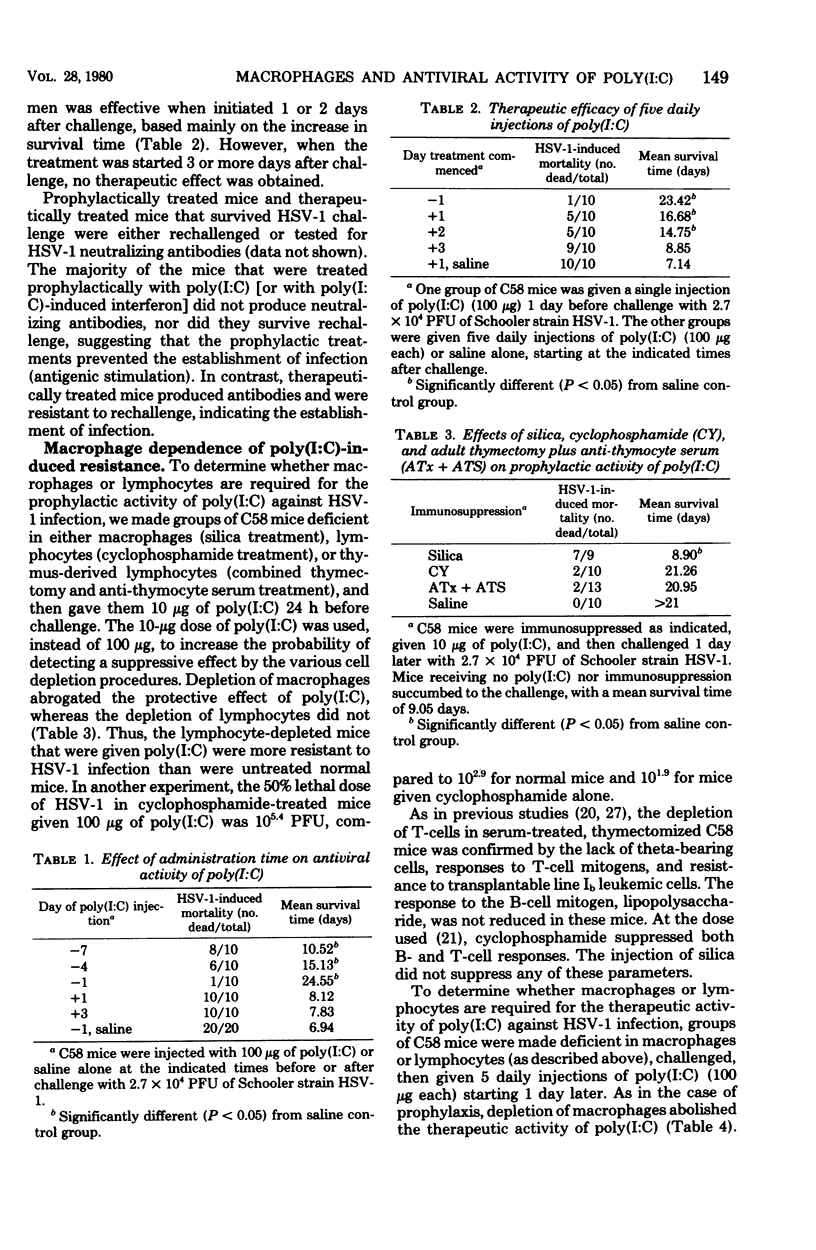
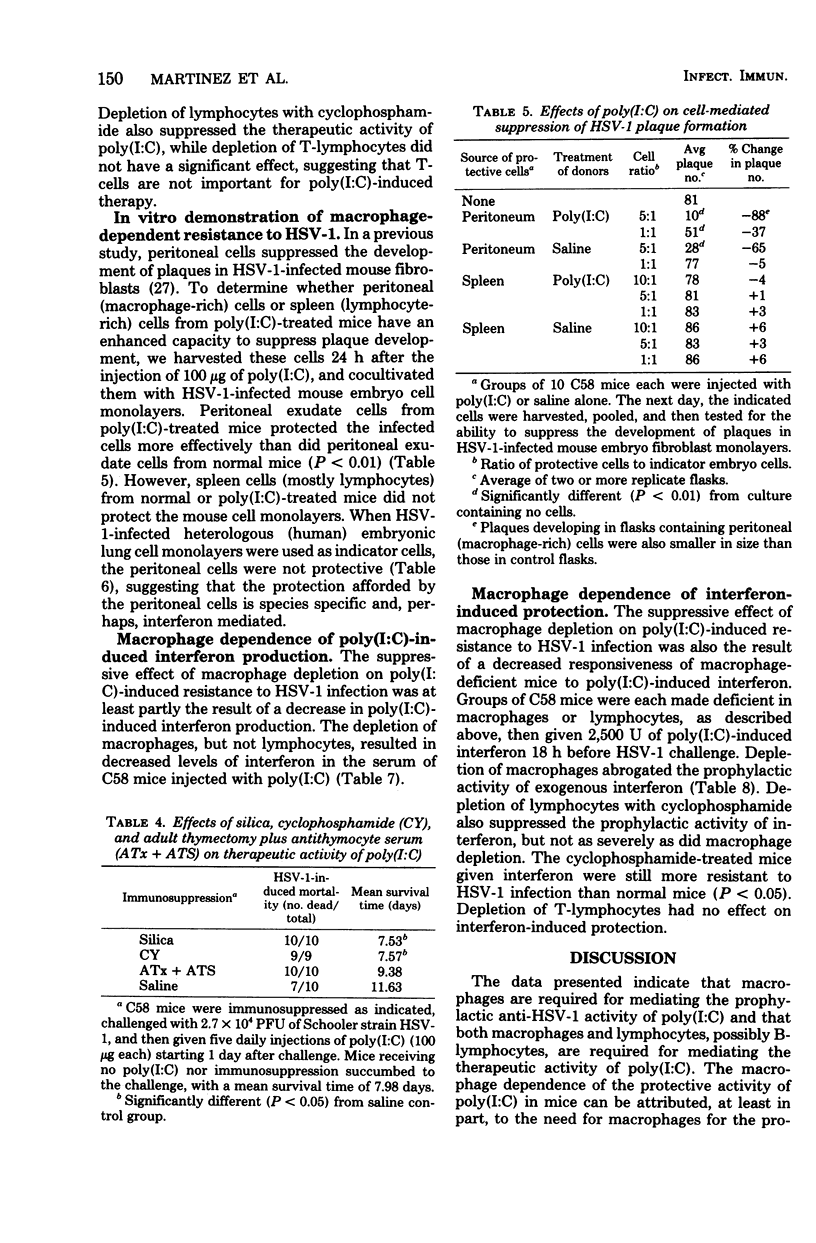
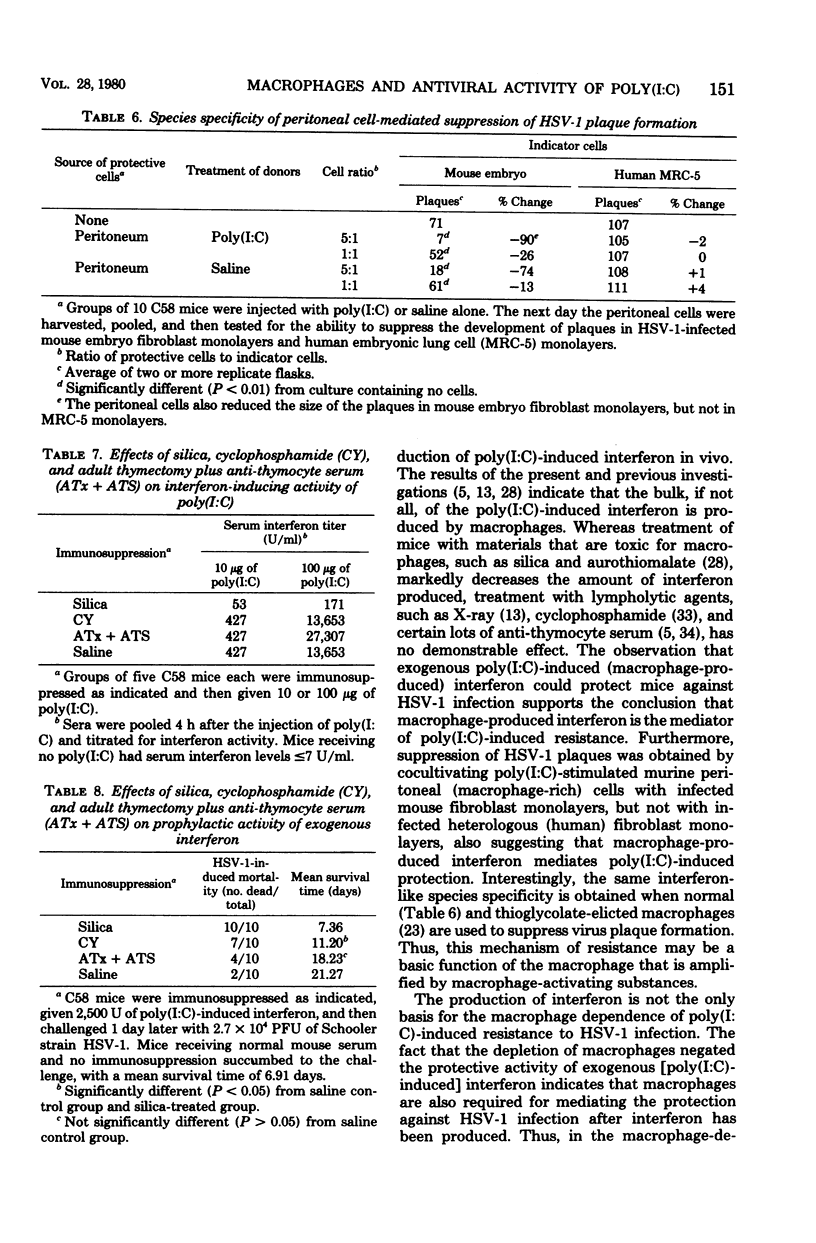
The relative contributions of macrophages and lymphocytes to the induction of resistance to primary herpes simplex virus type 1 (HSV-1) infection by polyriboinosinic-polyribocytidylic acid complex [poly (I:C)] were investigated in C58 mice. The induction of resistance was found to be strongly dependent on macrophages compared to lymphocytes. Macrophage-deficient (silica-treated) mice produced less interferon and were not as responsive to prophylactic treatment of HSV-1 infections with poly (I:C) as were either normal, lymphocyte-deficient (cyclophosphamide-treated), or T-lymphocyte-deficient (anti-thymocyte serum-treated, adult-thymectomized) mice. Silica and cyclophosphamide treatments reduced the therapeutic activity of poly (I:C), whereas T-cell depletion did not have a significant effect. Similarly, the protection of mice with exogenous interferon was markedly reduced in silica-treated mice and moderately reduced in cyclophosphamide-treated mice, but unaffected in T-cell-deficient mice. Furthermore, suppression of HSV-1 plaque formation was obtained by cocultivation of infected mouse fibroblast monolayers with peritoneal (macrophage-rich) cells, but not with splenic (lymphocyte-rich) cells, from poly (I:C)-treated mice. Peritoneal cells did not protect heterologous (human) fibroblasts, suggesting that the protection of mouse embryo fibroblasts is mediated by interferon. Collectively, the data indicate that macrophages are required for the production of poly (I:C)-induced interferon and that macrophages and perhaps B-lymphocytes are important for mediating the protection against HSV-1 infection after interferon has been produced.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen L. B., Cochran K. W. Target-organ treatment of neurotropic virus disease with interferon inducers. Infect Immun. 1972 Nov;6(5):819–823. doi: 10.1128/iai.6.5.819-823.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison A. C. On the role of mononuclear phagocytes in immunity against viruses. Prog Med Virol. 1974;18(0):15–31. [PubMed] [Google Scholar]
- Catalano L. W., Jr, London W. T., Rice J. M., Sever J. L. Prophylactic and therapeutic use of poly(I)-poly(C) (poly-D-lysine) against herpesvirus encephalitis in mice. Proc Soc Exp Biol Med. 1972 May;140(1):66–71. doi: 10.3181/00379727-140-36396. [DOI] [PubMed] [Google Scholar]
- Field A. K., Tytell A. A., Lampson G. P., Hilleman M. R. Inducers of interferon and host resistance, V. In vitro studies. Proc Natl Acad Sci U S A. 1968 Sep;61(1):340–346. doi: 10.1073/pnas.61.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. K., Young C. W., Krakoff I. H., Tytell A. A., Lampson G. P., Nemes M. M., Hilleman M. R. Induction of interferon in human subjects by poly I:C. Proc Soc Exp Biol Med. 1971 Apr;136(4):1180–1186. doi: 10.3181/00379727-136-35454. [DOI] [PubMed] [Google Scholar]
- Galin M. A., Chowchuvech E., Kronenberg B. Therapeutic use of inducers of interferon on Herpes simplex keratitis in humans. Ann Ophthalmol. 1976 Jan;8(1):72–76. [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. II. A CELLULAR BASIS FOR THE DEVELOPMENT OF RESISTANCE WITH AGE. J Exp Med. 1964 Sep 1;120:359–374. doi: 10.1084/jem.120.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullien P., De Maeyer-Guignard J., De Maeyer E. Interferon synthesis in x-irradiated animals v. Origin of mouse serum interferon induced by polyinosinic-polycytidylic Acid and encephalomyocarditis virus. Infect Immun. 1974 Nov;10(5):1023–1028. doi: 10.1128/iai.10.5.1023-1028.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern E. R., Overall J. C., Jr, Glasgow L. A. Herpesvirus hominis infection in newborn mice: treatment with interferon inducer polyinosinic-polycytidylic acid. Antimicrob Agents Chemother. 1975 Jun;7(6):793–800. doi: 10.1128/aac.7.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner H., Hirt H. M., Munk K. Protection against herpes simplex virus infection in mice by Corynebacterium parvum. Infect Immun. 1977 Apr;16(1):9–11. doi: 10.1128/iai.16.1.9-11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. S., Levy H. B. Phase I-II trials of poly IC stabilized with poly-L-lysine. Cancer Treat Rep. 1978 Nov;62(11):1907–1912. [PubMed] [Google Scholar]
- Liddell F. D. Evaluation of survival in challenge experiments. Microbiol Rev. 1978 Mar;42(1):237–249. doi: 10.1128/mr.42.1.237-249.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan T., Santos G. W., Quinn R. P. Immunosuppressive drugs. Pharmacol Rev. 1970 Jun;22(2):189–247. [PubMed] [Google Scholar]
- Martinez D., Field A. K., Schwam H., Tytell A. A., Hilleman M. R. Failure of thymopoietin, ubiquitin and synthetic serum thymic factor to restore immunocompetence in T-cell deficient mice. Proc Soc Exp Biol Med. 1978 Nov;159(2):195–200. doi: 10.3181/00379727-159-40313. [DOI] [PubMed] [Google Scholar]
- Martinez D., Lukasewycz O. A., Murphy W. H. Immune mechanisms in leukemia: suppression of cellular immunity by drugs and x-irradiation. J Immunol. 1975 Sep;115(3):724–729. [PubMed] [Google Scholar]
- Mogensen S. C. Macrophages and age-dependent resistance to hepatitis induced by herpes simplex virus type 2 im mice. Infect Immun. 1978 Jan;19(1):46–50. doi: 10.1128/iai.19.1.46-50.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morahan P. S., Glasgow L. A., Crane J. L., Jr, Kern E. R. Comparison of antiviral and antitumor activity of activated macrophages. Cell Immunol. 1977 Feb;28(2):404–415. doi: 10.1016/0008-8749(77)90122-8. [DOI] [PubMed] [Google Scholar]
- Morahan P. S., McCord R. S. Resistance to herpes simplex type 2 virus induced by an immunopotentiator (pyran) in immunosuppressed mice. J Immunol. 1975 Jul;115(1):311–313. [PubMed] [Google Scholar]
- Rager-Zisman B., Allison A. C. Mechanism of immunologic resistance to herpes simplex virus 1 (HSV-1) infection. J Immunol. 1976 Jan;116(1):35–40. [PubMed] [Google Scholar]
- Romano A., Kaplinsky C., Frand M., Rotem Y., Stein R., Blumenthal M. Systemic and topical use of poly I.C. in treatment of generalized neonatal herpes simplex infection with severe ocular involvement. J Pediatr Ophthalmol Strabismus. 1978 Jul-Aug;15(4):239–245. doi: 10.3928/0191-3913-19780701-13. [DOI] [PubMed] [Google Scholar]
- Schlabach A. J., Martinez D., Field A. K., Tytell A. A. Resistance of C58 mice to primary systemic herpes simplex virus infection: macrophage dependence and T-cell independence. Infect Immun. 1979 Nov;26(2):615–620. doi: 10.1128/iai.26.2.615-620.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbing N., Dawson K. M., Lindley I. J. Requirement for macrophages for interferon to be effective against encephalomyocarditis virus infection of mice. Infect Immun. 1978 Jan;19(1):5–11. doi: 10.1128/iai.19.1.5-11.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Restriction of herpes simplex virus by macrophages. An analysis of the cell-virus interaction. J Exp Med. 1971 Jan 1;133(1):19–38. doi: 10.1084/jem.133.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman G. D., Heim L. R., South M. A., Trentin J. J. Differential effects of cyclophosphamide on the B and T cell compartments of adult mice. J Immunol. 1973 Jan;110(1):277–282. [PubMed] [Google Scholar]
- Tarro G., Sabin A. B. Virus-specific, labile, nonvirion antigen in herpesvirus-infected cells. Proc Natl Acad Sci U S A. 1970 Mar;65(3):753–760. doi: 10.1073/pnas.65.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk J. L., Parker D. The effect of cyclophosphamide on the immune response. J Immunopharmacol. 1979;1(2):127–137. doi: 10.3109/08923977909026368. [DOI] [PubMed] [Google Scholar]
- Worthington M. G., Conliffe M., Williams J. Treatment of fatal disseminated herpes simplex virus, type 1, infection in immunosuppressed mice (39899). Proc Soc Exp Biol Med. 1977 Oct;156(1):168–172. doi: 10.3181/00379727-156-39899. [DOI] [PubMed] [Google Scholar]
- Worthington M., Baron S. Effectiveness of an interferon stimulator in immunosuppressed mice. Proc Soc Exp Biol Med. 1971 Feb;136(2):349–353. doi: 10.3181/00379727-136-35262. [DOI] [PubMed] [Google Scholar]
- Zisman B., Hirsch M. S., Allison A. C. Selective effects of anti-macrophage serum, silica and anti-lymphocyte serum on pathogenesis of herpes virus infection of young adult mice. J Immunol. 1970 May;104(5):1155–1159. [PubMed] [Google Scholar]
- de Maeyer-Guignard J., de Maeyer E. Effect of antilymphocytic serum on circulating interferon in mice as a function of the inducer. Nat New Biol. 1971 Feb 17;229(7):212–214. doi: 10.1038/newbio229212a0. [DOI] [PubMed] [Google Scholar]


