Abstract
Global incidence of acute heart failure (AHF) has reached pandemic proportions and hospitalization for decompensated AHF portends a poor prognosis. Identification of new biomarkers to identify AHF patients at high risk of mortality is an area of unmet clinical need. Circulating microRNAs (miRNAs, miRs) have recently been characterized as novel biomarkers for heart disease; however their use as prognostic biomarkers for clinical outcomes has not been fully delineated. Recently, we reported that the baseline level of circulating miR-30d was associated with left ventricular remodeling in response to cardiac resynchronization therapy in patients with advanced chronic heart failure. However, the role of circulating miR-30d as a prognostic marker of survival in patients with AHF has not been explored. Here a total of 95 patients clinically diagnosed with AHF were enrolled and followed for up for 1 year in this study. Serum miR-30d levels were determined by quantitative reverse transcription polymerase chain reactions. Serum miR-30d was significantly lower in AHF patients who expired in the one year follow-up period compared to those who survided. Univariate logistic regression analysis yielded 18 variables that were associated with all-cause mortality in AHF patients, while the multivariate logistic regression analysis identified 4 variables including heart rate, hemoglobin, serum sodium, and serum miR-30d level associated with mortality. ROC curve analysis showed that hemoglobin, heart rate and serum sodium displayed poor prognostic value for AHF (AUCs not higher than 0.700) compared to miR-30d level (AUC = 0.806). Kaplan–Meier survival analysis confirmed that patients with higher serum miR-30d levels had significantly lower mortality (P=0.001). Collectively, this study shows evidence for the predictive value of circulating miR-30d as 1-year all-cause mortality in AHF patients. Large multicentre studies are further needed to validate our findings and accelerate the transition to clinical utilization.
Keywords: Circulating microRNAs, miR-30d, acute heart failure, survival
Introduction
Acute heart failure (AHF) constitutes a leading public health burden in both developed and developing countries1–3. In the global context, AHF is responsible for over 1,100,000 admissions and 60,000 deaths annually4, 5, is a major cause of hospital admission in patients older than 65 years old, and is associated with increased morbidity and mortality5, 6. Despite utilization of guideline-mandated therapy, clinical outcomes of AHF patients after hospitalization remains poor2. Identification of novel prognostic biomarkers will assist in tailoring of appropriate therapy for at-risk AHF patients1, 2, 8.
MicroRNAs (miRNAs, miRs) are a class of endogenous, non-coding RNAs of 19–25 nucleotides in length9, 10. As central regulators of gene networks, miRNAs participate in several biological processes including proliferation, hypertrophy, inflammation, apoptosis and stress response11–14. Deregulation of miRNAs play a critical role in many cardiovascular diseases including heart failure15–17. Interestingly, miRNAs are present in the systemic circulation in a stable form, and the profile of plasma miRNAs can reflect cardiovascular disease18–22. A circulating miRNA signature for AHF patients has been reported, and more recently, the admission level of one of these AHF-associated miRNA miR-423-5p, has been shown to predict 1-year mortality in AHF patients23, 24. We have recently described that baseline levels of plasma miR-30d in patients with advanced chronic HF,, was associated with left ventricular remodeling in response to cardiac resynchronization therapy (CRT)25. Interestingly, miR-30d appears to play a functional role in remodeling, activating protective signaling pathways associated with adaptive hypertrophy of primary cardiomyocytes and could protecting against tumor necrosis factor-α (TNF-α)-induced cardiomyocytes apoptosis25. However, levels of circulating miR-30d could also translate to meaningful clinical outcomes in patients with AHF is unclear.
Here a total of 95 patients with clinically diagnosed AHF were enrolled in this study and followed for up to 1 year. Serum miR-30d levels were determined by quantitative reverse transcription polymerase chain reactions (qRT-PCRs) and correlated to clinical outcomes. Our results suggest that levels of serum miR-30d in AHF correlated with mortality at 1 year even after adjustment for important clinical variables in multivariate logistic models. Collectively, this study suggests a prognostic role for circulating miR-30d in predicting 1-year mortality in AHF patients. Large multicenter investigations to validate these findings may expedite the clinical utilization of circulating miR-30d to identify at-risk AHF patients.
Methods
Study cohort
All human investigations conformed to the principles outlined in the Declaration of Helsinki and were approved by the institutional review committees of Nanjing Medical University. The AHF cohort used in this study consisted of 95 patients admitted to the cardiac care unit between March 2012 and April 2015. The diagnosis of AHF was confirmed by two senior physicians in the Department of Cardiology based on the clinical and biological parameters (including N-terminal pro-B-type Natriuretic Peptide (NT-proBNP)) according to the guidelines of American Heart Association26, 27. Eligible patients were those hospitalized with new-onset or worsening preexisting heart failure as the primary cause of admission. Patients were excluded if they were diagnosed with malignant tumors, cognitive dysfunction or dementia, severe mental illness, or other uncontrolled systemic disease. Patients were also excluded if they had a history of PTE/DVT, or anti-coagulation therapy within 3 months. All participants gave written informed consent before enrollment in the study. This study was registered at http://www.chictr.org/cn/. (ChiCTR–ONC-12001 944).
Follow-up and endpoints
Typical clinical follow-up was performed in the outpatient setting during monthly visits or via telephone interview. The endpoints of the study were defined as all cause of death within 1 year from the time they were diagnosed with AHF. During the 1-year follow-up, all enrolled AHF patients received standard treatment and management. Information concerning the death was obtained from the hospital medical record, the patient’s physician, or family members.
Measurement of circulating miRNAs
Venous blood was collected in serum tubes and after a two-step centrifugation (4°C at 820 × g for 10 min, then 4°C at 16000 × g for 10 min), the supernatant was transferred to RNase/DNase-free tubes and stored at -80°C. Total RNA was isolated from the serum samples using the mirVana PARIS isolation kit (Ambion, Austin, Texas) according to the manufacturer’s instructions for serum samples without enrichment for small RNAs. Caenorhabditis elegans miR-39 (cel-miR-39) of 50 pmol/L was added as the spike-in control after the equal volume of denaturing solution was added. The Bulge-LoopTM miRNA qPCR Primer Set (RiboBio) was used to determine the expression levels of miRNAs by qRT-PCRs with Takara SYBR Premix Ex Taq™ (TliRNaseH Plus) in ABI-7900 Real-Time PCR Detection System. Cel-miR-39 was used as an internal control. The relative expression level was calculated using the 2−ΔΔCt method.
Statistical analysis
Statistical Package for the Social Sciences (SPSS, version 20.0, Chicago, IL) was used in all statistical analysis. Quantitative variables in normal distribution were expressed as mean ± SD, and categorical variables as count (percentage). miR-30d was dichotomized into 2 categories with categorical analysis including higher than median and lower than median. Groups were compared using two independent sample t test. To calculate the predictive value of variables including miR-30d for AHF, miR-30d relative expression values were calculated with the ΔCt method (mean Ct cel-miR-39—Ct target miRNA) and log-transformed by taking the base 2 logarithm, and a logistic regression models using forward stepwise were constructed. Unordered categorical variables were analyzed by applying dummy variables, odds ratios and 95% CI’s were calculated for the parameters. The survival curve was drawn by the Kaplan-Meier method and survival analysis used the log-rank test. Receiver-operator characteristic (ROC) curve was established to calculate the area under the curve (AUC) of miR-30d level for diagnosing AHF patients. MedCalc software (MedCalc Software, Mariakerke, Belgium) was used to compare the areas under every ROC curves. A difference of P < 0.05 (two-sided) was considered statistically significant.
Results
Baseline characteristics
A total of 95 patients with clinically diagnosed AHF were enrolled in this study and followed for up to 1 year. The mean age of enrolled patients was 61±16, with relative high prevalence of cardiovascular risk factors including hypertension (49.0%), atrial fibrillation (37.5%), myocardiosis (32.3%), diabetes (26.0%), ischemic heart disease (18.8%), and valvular heart disease (22.9%) (Table 1). During the 1-year follow up, 17 patients died (17.9%). Patients that died within 1 year after AHF had lower levels of serum sodium and haemoglobin, higher levels of cystatin and uric acid, and larger red blood cell distribution width (Table 2).
Table 1.
Characteristics of patients with acute heart failure enrolled in the study
| Age (years, mean±SD) | 61±16 |
| Sex (No. (%)) | |
| Male | 60 (62.5%) |
| Female | 36 (37.5%) |
| Heart rate (times/min, mean±SD) | 80±17 |
| ASA class (No. (%)) | |
| I | 1 (1.0%) |
| II | 17 (17.7%) |
| III | 51 (53.1%) |
| IV | 27 (28.1%) |
| Ejection Fraction (%) | 47±17 |
| SBP (mmHg, mean±SD) | 126±25 |
| DBP (mmHg, mean±SD) | 75±14 |
| Co-morbidities (No. (%)) | |
| Atrial fibrillation | 36 (37.5%) |
| Hypertension | 47 (49.0%) |
| Acute coronary syndromes | 8 (8.3%) |
| Ischemic heart disease | 18 (18.8%) |
| Myocardiosis | 31 (32.3%) |
| Valvular disease of the heart | 22 (22.9%) |
| Pulmonary heart disease | 3 (3.1%) |
| Pneumonia | 17 (17.7%) |
| Hyperthyreosis | 3 (3.1%) |
| Congenital heart diseases | 8 (8.3%) |
| Diabetes mellitus | 25 (26.0%) |
| Renal insufficiency | 3 (3.1%) |
ASA: American Society of Anesthesiologists; SBP: systolic blood pressure; DBP: diastolic blood pressure;
Table 2.
Acute heart failure patient characteristics with different clinical outcomes (mean±SD)
| Survival (n=79) | Death (n=17) | P value | |||||
|---|---|---|---|---|---|---|---|
| Age (years) | 61 | ± | 16 | 62 | ± | 17 | 0.919 |
| Male sex (No.) | 52 | 8 | 0.173 | ||||
| NT-proBNP (ng/mL) | 2129.23 | ± | 3925.41 | 2408.14 | ± | 1306.59 | 0.794 |
| D-Dimer (mg/L) | 1.59 | ± | 3.96 | 1.36 | ± | 1.50 | 0.832 |
| Plasma potassium (mmol/L) | 3.95 | ± | 0.61 | 4.12 | ± | 0.60 | 0.297 |
| Serum sodium (mmol/L) | 140.53 | ± | 3.40 | 136.88 | ± | 6.22 | 0.031 |
| CK-MB (IU/L) | 42.46 | ± | 183.10 | 18.73 | ± | 17.54 | 0.671 |
| Scr (μmoI/L) | 95.29 | ± | 47.01 | 101.29 | ± | 37.25 | 0.624 |
| Hemoglobin (g/L) | 134.63 | ± | 20.78 | 116.12 | ± | 28.60 | 0.003 |
| RDW (%) | 14.03 | ± | 2.11 | 16.76 | ± | 2.08 | <0.001 |
| Cystatinc C (mg/L) | 1.37 | ± | 0.65 | 2.00 | ± | 1.27 | 0.006 |
| Uric acid (umol/L) | 470.01 | ± | 160.44 | 562.88 | ± | 190.40 | 0.040 |
| Ejection Fraction (%) | 47.30 | ± | 16.42 | 48.41 | ± | 18.02 | 0.806 |
| LVDD (mm) | 59.63 | ± | 13.83 | 53.56 | ± | 13.87 | 0.116 |
RDW: Red blood cell distribution width; LVDD: Left ventricular end diastolic diameter
Serum miR-30d level and all cause of death within 1 year
Serum miR-30d levels in the AHF patients who survived was compared to the patients who met the primary end-point of mortality in the follow-up period. The expression level of miR-30d in serum was significantly lower in those patients that expired compared to patients who survived during the 1 year follow up (Figure 1), indicating that serum miR-30d level may correlate with survival in AHF patients.
Figure 1. Expression of serum miR-30d in acute heart failure patients survive during 1 year follow up comparing to those died.
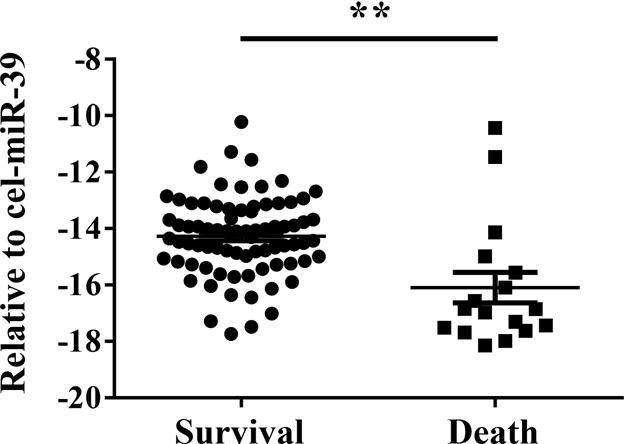
**, P <0.01.
Serum miR-30d level in relation to baseline characteristics
The AHF cohort was divided into 2 groups according to the median of serum miR-30d levels. These two groups of AHF patients had similar age and sex characteristics, and received standard treatment and management for the whole course from admission to discharge. Binary logistic regression showed that miR-30d was not associated with baseline clinical characteristics (Tables 3), suggesting that serum miR-30d was not suggest a surrogate marker of other comorbidities in this cohort.
Table 3.
Association of the serum level of miR-30d with baseline characteristics
| Variable | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Age | 0.989 | 0.963–1.015 | 0.398 |
| Sex | 0.565 | 0.681–3.594 | 0.291 |
| ASA class | 0.972 | 0.549–1.722 | 0.923 |
| Heart rate | 0.987 | 0.964–1.010 | 0.271 |
| Pneumonia | 0.707 | 0.247–2.022 | 0.518 |
| Acute coronary syndromes | 0.833 | 0.196–3.546 | 0.805 |
| Hyperthyreosis | 0.412 | 0.036–4.700 | 0.412 |
| Hypertension | 0.998 | 0.986–1.010 | 0.742 |
| Congenital heart diseases | 0.833 | 0.196–3.546 | 0.805 |
| Diabetes mellitus | 0.575 | 0.229–1.442 | 0.238 |
| Renal insufficiency | 1.720 | 0.151–19.632 | 0.662 |
ASA: American Society of Anesthesiologists
Serum miR-30d as a prognostic indicator for AHF
In univariate logistic regression analysis, the variable selection process yielded 18 variables that closely related to the outcomes of AHF (Table 4). Of the 18 variables, factors including heart rate, serum sodium, blood urea nitrogen, hemoglobin, cystatin, uric acid and serum miR-30d level were chosen as independent variables. These variables were then included in the multivariate logistic regression analysis. Our results showed that higher hemoglobin, serum sodium and miR-30d level were associated with a reduced risk of death caused in AHF patients (OR: 0.962 [0.930 – 0.995], 0.759 [0.610 to 0.944] and 0.610 [0.409 to 0.911], respectively, all P<0.05), while higher heart rate correlated with increased mortality after AHF (OR: 1.048 [1.000 – 1.098], P=0.048) (Table 5).
Table 4.
Prediction of death (univariate analysis)
| Variable | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Age | 1.002 | 0.969–1.036 | 0.918 |
| Sex | 0.444 | 0.154–1.286 | 0.135 |
| NT-proBNP/SD | 1.073 | 0.665–1.731 | 0.772 |
| D-Dimer | 0.981 | 0.819–1.173 | 0.830 |
| Heart rate | 1.038 | 1.005–1.072 | 0.025 |
| Serum potassium | 1.606 | 0.662–3.899 | 0.295 |
| Serum sodium | 0.826 | 0.724–0.941 | 0.004 |
| CK-MB | 0.998 | 0.986–1.010 | 0.742 |
| Blood urea nitrogen | 1.104 | 1.009–1.208 | 0.032 |
| Scr | 1.003 | 0.992–1.013 | 0.622 |
| Hemoglobin | 0.968 | 0.945–0.991 | 0.007 |
| ALT | 1.003 | 1.000–1.007 | 0.053 |
| AST | 1.003 | 0.999–1.006 | 0.160 |
| Cystatinc C | 2.189 | 1.117–4.291 | 0.022 |
| Uric acid | 1.003 | 1.000–1.006 | 0.047 |
| Ejection Fraction | 1.004 | 0.972–1.037 | 0.803 |
| LVDD | 0.967 | 0.926–1.009 | 0.118 |
| miR-30d | 0.472 | 0.316–0.705 | <0.001 |
LVDD: Left ventricular end diastolic diameter
Table 5.
Prediction of death (multivariate analysis)
| Variable | Odds ratio | 95%CI | P value |
|---|---|---|---|
| Hemoglobin | 0.962 | 0.930–0.995 | 0.025 |
| Heart rate | 1.048 | 1.000–1.098 | 0.049 |
| Serum sodium | 0.759 | 0.610–0.944 | 0.049 |
| miR-30d | 0.610 | 0.409–0.911 | 0.016 |
We further evaluated the predictive power of these 4 variables to the outcomes of AHF by ROC curve analysis. Hemoglobin, heart rate and serum sodium displayed only moderate prognostic values for AHF (AUCs ≤ 0.700) compared to miR-30d level (AUC = 0.806) (Figure 2). Combination of two or three variables (excluding miR-30d) also did not improve the clinically prognostic value (Figure 3 and Figure 4). While AUC for combination of all four variables reached 0.872, which was significantly improved over the AUC areas of the single variables; this combination was not statistically different compared to serum miR-30d (P>0.05) (Figure 4), indicating that serum miR-30d could be a prognostic indicator for AHF. Finally, Kaplan–Meier survival analysis confirmed that patients with higher serum miR-30d levels had significantly lower mortality (P=0.001) (Figure 5). Taken together, these results extend our previous studies that suggested an association between circulating miR-30d and cardiac remodeling to suggest a potential association with a clinically meaningful endpoint (mortality) in patients with AHF.
Figure 2. The receiver-operator characteristic (ROC) curves for acute heart failure patients survive during 1 year follow up comparing to those died with single variable.
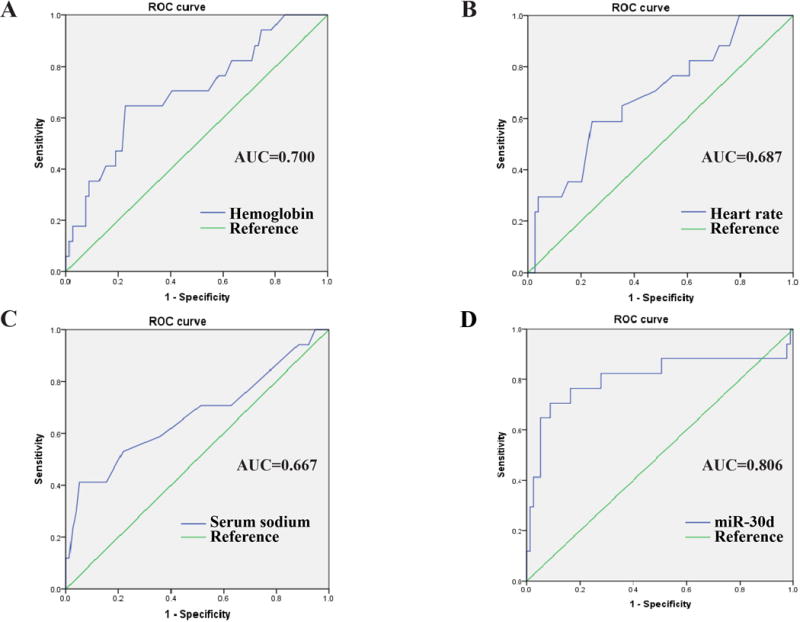
The receiver-operator characteristic (ROC) curves for distinguishing acute heart failure patients survive during 1 year follow up from to those died by hemoglobin (A), heart rate (B), serum sodium (C) and serum miR-30d level (D), respectively. AUC, area under the ROC curve.
Figure 3. The receiver-operator characteristic (ROC) curves for acute heart failure patients survive during 1 year follow up comparing to those died with two combined variables.
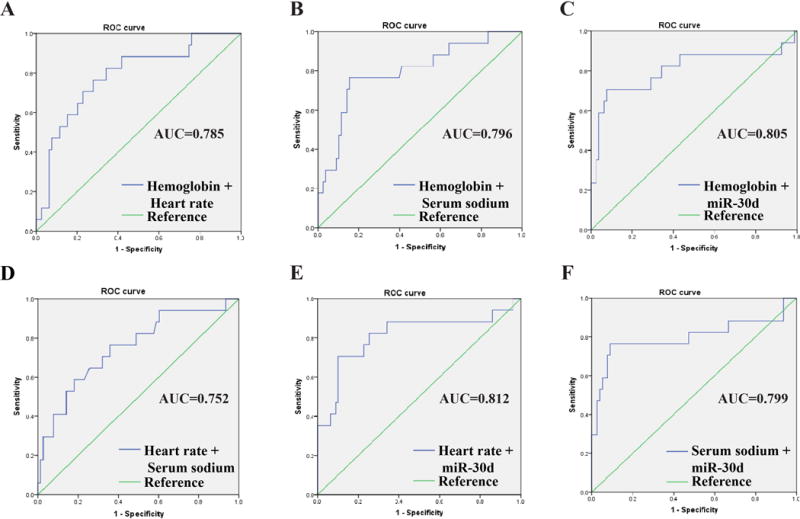
The receiver-operator characteristic (ROC) curves for distinguishing acute heart failure patients survive during 1 year follow up from to those died by hemoglobin+heart rate (A), hemoglobin+ serum sodium (B), hemoglobin+ serum miR-30d (C), heart rate + serum sodium (D), heart rate + serum miR-30d (E), and serum sodium + serum miR-30d (F), respectively. AUC, area under the ROC curve.
Figure 4. The receiver-operator characteristic (ROC) curves for acute heart failure patients survive during 1 year follow up comparing to those died with three or four combined variables.

The receiver-operator characteristic (ROC) curves for distinguishing acute heart failure patients survive during 1 year follow up from to those died by hemoglobin + heart rate + serum sodium (A), hemoglobin+serum sodium+ serum miR-30d (B), heart rate + serum sodium+ serum miR-30d (C), and hemoglobin +heart rate + serum sodium+ serum miR-30d (D), respectively. AUC, area under the ROC curve.
Figure 5. Kaplan–Meier cumulative survival analysis according to serum miR-30d levels.
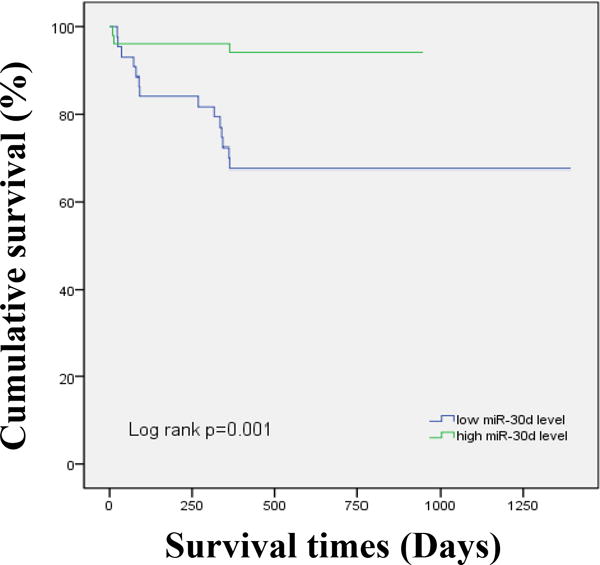
The acute heart failure patients in this study was divided into two groups according to the median of miR-30d (lower than median vs higher than median). Survival rate free of all cause of death based on serum miR-30d level.
Discussion
Identification of AHF patients who are at high risk of mortality following discharge is of critical importance to guide therapeutic decision making for physicians1. Identification of high-risk AHF patients could facilitate allocation of resources including ventricular assist device support, cardiac transplantation, and enrollment into AHF management programs2, 28. Nevertheless, accurate risk assessment remains a challenging task29. Thus, exploring novel biomarkers for prediction of survival in AHF patients is greatly needed28.
Several biomarkers measured during an AHF admission have been reported to independently predict death after discharge, including sST2, galectin-3, copeptin, and mid-regional pro-adrenomedullin etc1, 30–32. miRNAs have been found to exist in circulation in a consistent and reproducible manner, making them attractive candidates for biomarker development in AHF patients23, 24. Growing evidence has showed that several miRNAs can predict outcome in AHF patients, including miR-423-5p, miR-18a-5p, and miR-652-3p23, 24. Recently, we have reported that circulating miR-30d was associated with response to cardiac resynchronization therapy in HF25. miR-30d was found to have a higher diagnostic value than cTnI for the early diagnosis of acute myocardial infarction (AMI), but it was not associated with 6 month cardiovascular events in AMI patients33. In the present study, we have found that AHF patients with higher serum miR-30d levels had significantly lower mortality even after adjustment for clinical and biochemical variable, thereby providing evidence that circulating miR-30d may be a novel prognostic biomarker for survival in AHF patients.
Our previous study reported a cardiac origin for circulating miR-30d, and cardiomyocyte as the possible source of the extracellular miR-30d25. In another study, miR-30d was reported to be absorbed by the failing heart34. We have previously shown that miR-30d acts as a ‘protecteomir’, with its upregulation associated with molecular features of adaptive hypertrophy, and cardioprotection against TNF-α mediated apoptosis25. Although in other models of cardiovascular diseases, miR-30d has been associated with cardiomyocyte pyroptosis35, this study supports our findings that higher levels of circulating miR-30d are likely cardioprotective. Nonetheless, definitive proof of the functional role of miR-30d can only come from gain-of-function and loss-of-function experiments in animal models.
Several limitations of the present study should be highlighted. Importantly, rather than performing microarrays or RNA sequencing, we only measured miR-30d based on our previous study25. As has been conventionally accepted, we normalized miR-30d levels to an exogenous spike-in. The issue of normalization of biofluid extracellular RNAs remains a hotly debated topic in the field, and we agree that this remains a limitation in the field. Nonetheless, we took care to use non-hemolyzed specimens and measured miR-30d in the same amount of sample for each patient.
Our sample size was limited to 95 patients, and undoubtedly our findings will need to be validated in larger studies and other populations. Finally, while we measured several clinical and biochemical variables, we did not correlate miR-30d levels with other conventional biomarkers such as cTnT, galectin, and cystatin due to lack of availability of these data. Finally, it remains to be determined if miR-30d could response to treatment and if miR-30d guided therapy in AHF could achieve additional benefits.
In conclusion, our study provides novel insights into the predictive value of circulating miR-30d for 1-year mortality in AHF patients. Large multicenter investigations to validate our findings would be highly desired to expedite the transition to clinical utilization.
Acknowledgments
This work was supported by the grants from National Natural Science Foundation of China (81570362 and 81200169 to JJ Xiao, 81370332 and 81170201 to XL Li, 81270314 and 81470515 to JH Xu, 81400647 to Y Bei), Shanghai Medical Guide Project from Shanghai Science and Technology Committee (134119a3000 to JH Xu and 14411971600 to JF Jiang), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD20102013 to XL Li) and the National Institutes of Health (NCATS grant UH3 TR000901 to S Das). Dr XL Li is an Associate Fellow at the Collaborative Innovation Center For Cardiovascular Disease Translational Medicine.
Footnotes
Author Contributions
J.X., S.D., X.L. designed the study, instructed all experiments and drafted the manuscript. J.X., R.G., Y.B., J.Z., J.X., and J.J. performed the experiments and analyzed the data. R.G., Y.Z., H.Z., and X.L. enrolled all patients and conducted the follow up.
Competing Financial Interests
The authors declare no competing financial interests.
References
- 1.Maisel AS, Choudhary R. Biomarkers in acute heart failure–state of the art. Nat Rev Cardiol. 2012;9:478–490. doi: 10.1038/nrcardio.2012.60. [DOI] [PubMed] [Google Scholar]
- 2.Jumean MF, Kiernan MS. Determinants of survival following hospitalization for acute heart failure. Curr Heart Fail Rep. 2014;11:201–211. doi: 10.1007/s11897-014-0190-z. [DOI] [PubMed] [Google Scholar]
- 3.Yanavitski M, Givertz MM. Novel biomarkers in acute heart failure. Curr Heart Fail Rep. 2011;8:206–211. doi: 10.1007/s11897-011-0065-5. [DOI] [PubMed] [Google Scholar]
- 4.Maisel AS, Richards AM, Pascual-Figal D, Mueller C. Serial st2 testing in hospitalized patients with acute heart failure. Am J Cardiol. 2015;115:32B–37B. doi: 10.1016/j.amjcard.2015.01.038. [DOI] [PubMed] [Google Scholar]
- 5.Pascual-Figal DA, Caballero L, Sanchez-Mas J, Lax A. Prognostic markers for acute heart failure. Expert Opin Med Diagn. 2013;7:379–392. doi: 10.1517/17530059.2013.814638. [DOI] [PubMed] [Google Scholar]
- 6.Givertz MM, Teerlink JR, Albert NM, Westlake Canary CA, Collins SP, Colvin-Adams M, Ezekowitz JA, Fang JC, Hernandez AF, Katz SD, Krishnamani R, Stough WG, Walsh MN, Butler J, Carson PE, Dimarco JP, Hershberger RE, Rogers JG, Spertus JA, Stevenson WG, Sweitzer NK, Tang WH, Starling RC. Acute decompensated heart failure: Update on new and emerging evidence and directions for future research. J Card Fail. 2013;19:371–389. doi: 10.1016/j.cardfail.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Januzzi JL, Mebazaa A, Di Somma S. St2 and prognosis in acutely decompensated heart failure: The international st2 consensus panel. Am J Cardiol. 2015;115:26B–31B. doi: 10.1016/j.amjcard.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 8.Vasile VC, Jaffe AS. Emerging biomarkers for acute heart conditions. Curr Opin Cardiol. 2014;29:312–318. doi: 10.1097/HCO.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 9.Melman YF, Shah R, Das S. Micrornas in heart failure: Is the picture becoming less mirky? Circulation. Heart failure. 2014;7:203–214. doi: 10.1161/CIRCHEARTFAILURE.113.000266. [DOI] [PubMed] [Google Scholar]
- 10.Bruno N, ter Maaten JM, Ovchinnikova ES, Vegter EL, Valente MA, van der Meer P, de Boer RA, van der Harst P, Schmitter D, Metra M, O’Connor CM, Ponikowski P, Teerlink JR, Cotter G, Davison B, Cleland JG, Givertz MM, Bloomfield DM, Dittrich HC, Pinto YM, van Veldhuisen DJ, Hillege HL, Berezikov E, Voors AA. Micrornas relate to early worsening of renal function in patients with acute heart failure. Int J Cardiol. 2016;203:564–569. doi: 10.1016/j.ijcard.2015.10.217. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Bei Y, Liu Q, Lv D, Xu T, He Y, Chen P, Xiao J. Microrna-221 is required for proliferation of mouse embryonic stem cells via p57 targeting. Stem cell reviews. 2015;11:39–49. doi: 10.1007/s12015-014-9543-y. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Xiao J, Zhu H, Wei X, Platt C, Damilano F, Xiao C, Bezzerides V, Bostrom P, Che L, Zhang C, Spiegelman BM, Rosenzweig A. Mir-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell metabolism. 2015;21:584–595. doi: 10.1016/j.cmet.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Bei Y, Shi J, Xiao J, Kong X. Non-coding rnas in cardiac aging. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2015;36:1679–1687. doi: 10.1159/000430141. [DOI] [PubMed] [Google Scholar]
- 14.Xiao J, Liang D, Zhang H, Liu Y, Zhang D, Liu Y, Pan L, Chen X, Doevendans PA, Sun Y, Liang X, Sluijter JP, Chen YH. Microrna-204 is required for differentiation of human-derived cardiomyocyte progenitor cells. Journal of molecular and cellular cardiology. 2012;53:751–759. doi: 10.1016/j.yjmcc.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 15.Xiao J, Liang D, Zhang Y, Liu Y, Zhang H, Liu Y, Li L, Liang X, Sun Y, Chen YH. Microrna expression signature in atrial fibrillation with mitral stenosis. Physiological genomics. 2011;43:655–664. doi: 10.1152/physiolgenomics.00139.2010. [DOI] [PubMed] [Google Scholar]
- 16.Lv D, Liu J, Zhao C, Sun Q, Zhou Q, Xu J, Xiao J. Targeting micrornas in pathological hypertrophy and cardiac failure. Mini reviews in medicinal chemistry. 2015;15:475–478. doi: 10.2174/1389557515666150324124751. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Bei Y, Shen S, Huang P, Shi J, Zhang J, Sun Q, Chen Y, Yang Y, Xu T, Kong X, Xiao J. Mir-21-3p controls sepsis-associated cardiac dysfunction via regulating sorbs2. Journal of molecular and cellular cardiology. 2016;94:43–53. doi: 10.1016/j.yjmcc.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Xiao J, Jing ZC, Ellinor PT, Liang D, Zhang H, Liu Y, Chen X, Pan L, Lyon R, Liu Y, Peng LY, Liang X, Sun Y, Popescu LM, Condorelli G, Chen YH. Microrna-134 as a potential plasma biomarker for the diagnosis of acute pulmonary embolism. Journal of translational medicine. 2011;9:159. doi: 10.1186/1479-5876-9-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jansen F, Yang X, Proebsting S, Hoelscher M, Przybilla D, Baumann K, Schmitz T, Dolf A, Endl E, Franklin BS, Sinning JM, Vasa-Nicotera M, Nickenig G, Werner N. Microrna expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J Am Heart Assoc. 2014;3:e001249. doi: 10.1161/JAHA.114.001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J, Zhao J, Evan G, Xiao C, Cheng Y, Xiao J. Circulating micrornas: Novel biomarkers for cardiovascular diseases. Journal of molecular medicine (Berlin, Germany) 2012;90:865–875. doi: 10.1007/s00109-011-0840-5. [DOI] [PubMed] [Google Scholar]
- 21.Xiao J, Shen B, Li J, Lv D, Zhao Y, Wang F, Xu J. Serum microrna-499 and microrna-208a as biomarkers of acute myocardial infarction. International journal of clinical and experimental medicine. 2014;7:136–141. [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Xu J, Cheng Y, Wang F, Song Y, Xiao J. Circulating micrornas as mirrors of acute coronary syndromes: Miracle or quagmire? Journal of cellular and molecular medicine. 2013;17:1363–1370. doi: 10.1111/jcmm.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seronde MF, Vausort M, Gayat E, Goretti E, Ng LL, Squire IB, Vodovar N, Sadoune M, Samuel JL, Thum T, Solal AC, Laribi S, Plaisance P, Wagner DR, Mebazaa A, Devaux Y, network G Circulating micrornas and outcome in patients with acute heart failure. PloS one. 2015;10:e0142237. doi: 10.1371/journal.pone.0142237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ovchinnikova ES, Schmitter D, Vegter EL, Ter Maaten JM, Valente MA, Liu LC, van der Harst P, Pinto YM, de Boer RA, Meyer S, Teerlink JR, O’Connor CM, Metra M, Davison BA, Bloomfield DM, Cotter G, Cleland JG, Mebazaa A, Laribi S, Givertz MM, Ponikowski P, van der Meer P, van Veldhuisen DJ, Voors AA, Berezikov E. Signature of circulating micrornas in patients with acute heart failure. European journal of heart failure. 2016;18:414–423. doi: 10.1002/ejhf.332. [DOI] [PubMed] [Google Scholar]
- 25.Melman YF, Shah R, Danielson K, Xiao J, Simonson B, Barth A, Chakir K, Lewis GD, Lavender Z, Truong QA, Kleber A, Das R, Rosenzweig A, Wang Y, Kass DA, Singh JP, Das S. Circulating microrna-30d is associated with response to cardiac resynchronization therapy in heart failure and regulates cardiomyocyte apoptosis: A translational pilot study. Circulation. 2015;131:2202–2216. doi: 10.1161/CIRCULATIONAHA.114.013220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, Dickstein K, Drazner MH, Fonarow GC, Jaarsma T, Jondeau G, Sendon JL, Mebazaa A, Metra M, Nieminen M, Pang PS, Seferovic P, Stevenson LW, van Veldhuisen DJ, Zannad F, Anker SD, Rhodes A, McMurray JJ, Filippatos G. Assessing and grading congestion in acute heart failure: A scientific statement from the acute heart failure committee of the heart failure association of the european society of cardiology and endorsed by the european society of intensive care medicine. European journal of heart failure. 2010;12:423–433. doi: 10.1093/eurjhf/hfq045. [DOI] [PubMed] [Google Scholar]
- 27.Mebazaa A. Current esc/esicm and accf/aha guidelines for the diagnosis and management of acute heart failure in adults–are there differences? Polskie Archiwum Medycyny Wewnetrznej. 2009;119:569–573. [PubMed] [Google Scholar]
- 28.Cohen-Solal A, Laribi S, Ishihara S, Vergaro G, Baudet M, Logeart D, Mebazaa A, Gayat E, Vodovar N, Pascual-Figal DA, Seronde MF. Prognostic markers of acute decompensated heart failure: The emerging roles of cardiac biomarkers and prognostic scores. Arch Cardiovasc Dis. 2015;108:64–74. doi: 10.1016/j.acvd.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 29.McCullough PA, Jefferies JL. Novel markers and therapies for patients with acute heart failure and renal dysfunction. Am J Med. 2015;128:312 e311–322. doi: 10.1016/j.amjmed.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 30.van der Velde AR, Meijers WC, de Boer RA. Biomarkers for risk prediction in acute decompensated heart failure. Curr Heart Fail Rep. 2014;11:246–259. doi: 10.1007/s11897-014-0207-7. [DOI] [PubMed] [Google Scholar]
- 31.Heil B, Tang WH. Biomarkers: Their potential in the diagnosis and treatment of heart failure. Cleve Clin J Med. 2015;82:S28–35. doi: 10.3949/ccjm.82.s2.05. [DOI] [PubMed] [Google Scholar]
- 32.Peacock WF. Novel biomarkers in acute heart failure: Mr-pro-adrenomedullin. Clin Chem Lab Med. 2014;52:1433–1435. doi: 10.1515/cclm-2014-0222. [DOI] [PubMed] [Google Scholar]
- 33.Jia K, Shi P, Han X, Chen T, Tang H, Wang J. Diagnostic value of mir-30d-5p and mir-125b-5p in acute myocardial infarction. Mol Med Rep. 2016;14:184–194. doi: 10.3892/mmr.2016.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marques FZ, Vizi D, Khammy O, Mariani JA, Kaye DM. The transcardiac gradient of cardio-micrornas in the failing heart. European journal of heart failure. 2016 doi: 10.1002/ejhf.517. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Du N, Zhang Q, Li J, Chen X, Liu X, Hu Y, Qin W, Shen N, Xu C, Fang Z, Wei Y, Wang R, Du Z, Zhang Y, Lu Y. Microrna-30d regulates cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic cardiomyopathy. Cell Death Dis. 2014;5:e1479. doi: 10.1038/cddis.2014.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morishima M, Iwata E, Nakada C, Tsukamoto Y, Takanari H, Miyamoto S, Moriyama M, Ono K. Atrial fibrillation-mediated upregulation of mir-30d regulates myocardial electrical remodeling of the g-protein-gated k(+) channel, ik.Ach. Circ J. 2016;80:1346–1355. doi: 10.1253/circj.CJ-15-1276. [DOI] [PubMed] [Google Scholar]


