Abstract
Although renin cells are crucial for blood pressure homeostasis, little is known about their nature. We now know that renin cells are precursors that appear early and in multiple tissues during embryonic development. They participate in morphogenetic events, vascular development and injury, tissue repair and regeneration. When confronted to a homeostatic threat, renin cell descendants have the capability to switch the renin gene on or off. This poorly understood switch or molecular memory enables the organism to maintain constancy of the internal milieu and tissue perfussion. Here, we discuss briefly the major events that govern the acquisition and maintenace of renin cell identity and how manipulations that alter the fate of renin cells can lead to serious disease. We also advance the concept that renin cells are at the center of an ancestral sytem of defense linking the endocrine, the immune and repair responses of the organism.
The renin angiotensin system
The renin-angiotensin system (RAS) is crucial in the regulation of blood pressure and fluid electrolyte homeostasis.1;2 In the traditional view of the RAS, renin is released by the kidney juxtaglomerular (JG) cells and upon reaching the circulation it acts on its only known substrate, angiotensinogen, produced mainly in the liver to yield angiotensin I (Ang I), a decapeptide and Des-Ang I- angiotensinogen, a large molecule of unclear function. Thereafter, Ang I is hydrolyzed by angiotensin-converting enzyme (ACE) to yield the octapeptide Ang II, a fast acting and very powerful vasoconstrictor that regulates peripheral vascular resistance, renal hemodynamics and sodium reabsorption via several mechanisms including the stimulation of aldosterone secretion by the adrenal glands. Most of the known cardiovascular and renal actions of the RAS are achieved by the actions of Ang II on its receptors, mainly AT1 receptors. It should be noted that for the system to operate properly, it needs to respond accurately and rapidly to changes in the composition and volume of the extracellular fluid and to variations in systemic blood pressure. The key regulated event in this enzymatic cascade is the tightly controlled, minute-to-minute regulation of renin release by the JG cells. This is possible because JG cells are sensors strategically located in the juxtaglomerular apparatus (JGA) where they receive and interpret signals that convey the composition and volume of the extracellular fluid and the level of perfusion pressure. The JGA is composed of the afferent and efferent arterioles, the macula densa and the extra-glomerular mesangium or polkissen.1;3;4 In the adult unstressed mammalian kidney, JG cells are located in the afferent arteriole at the entrance to the glomerulus where they make contact with macula densa cells, extra-glomerular mesangial cells and other renin and smooth muscle cells along the arteriole.3, 4. JG cells have a myo-epithelioid appearance, they are densely innervated by sympathetic terminals arising from the renal nerve, they contain granules from where renin is stored and released in response to a diverse number of stimuli emanating from nearby cells, sympathetic terminal and/or from the circulation.5 Three major mechanisms control renin release by JG cells: 1) the renal baroreceptor mechanism whereby renin release is elicited by a decrease in renal perfusion pressure as it occurs during hypotension, shock, hemorrhage, or cardiac failure. The nature of the renal baroreceptor has not been determined since its original 1959 description by Tobian and colleagues,6 (2) the macula densa mechanism whereby renin release is stimulated by a decrease in sodium chloride in the distal tubule as it occurs during sodium depletion and 3) the beta-receptor mediated mechanism whereby stimulation of beta-receptors elicited by sympathetic terminals or via circulating catecholamines such as during hypoxia results in increased renin release. Interestingly, the renal baroreceptor mechanism continues to function in the absence of the other two mechanisms: in the denervated, non-filtering kidney the baroreceptor mechanism continues to operate suggesting that the renal baroreceptor mechanism is independent from the influence of the macula densa or the beta-receptor.2 Under normal circumstances, however, these mechanisms operate together to finely regulate renin output. For instance, the beta receptor mechanism, the baroreceptor mechanism and the macula densa mechanism are all activated during hemorrhage a situation where there is decreased perfusion pressure, decreased delivery of sodium chloride to the macula densa and stimulation of the sympathetic system. It should be noted that Ang II exerts a negative feedback on renin release, a typical case where the byproduct of an enzymatic reaction controls its own production, in this case governed by the underlying physiological status of the animal. When angiotensin production and/or its actions are limited such as when animals are exposed to ACE inhibitors or AT1 receptor blockers, renin synthesis and release is increased. This is accomplished in great part by an increase in the number of cells that synthesize and release renin as described below.7–9
JG cells and other cells that manufacture renin
Although a fair amount of knowledge has accumulated about renin as a hormone and an enzyme, much less is known about the cells that manufacture it. In fact, over the years several conceptual misunderstandings have accrued and amplified over time by simple repetition, without clear data.
In describing renin-expressing cells, most portrayals are limited to the JG cells, which, although better known, they are nonetheless poorly understood. In addition, it is important to consider that renin cells exist on other renal and extra renal sites, beyond the JGA. Moreover, because JG cells constitute a small fraction (about 0.01%) of the total kidney cells mass and because they produce a hormone, the cells have been considered as terminally differentiated. However, the JG area is not the only place where renin-producing cells are found. In fact, renin cells appear early in the mammalian embryo, before organogenesis has taken place. Thereafter, renin cell progenitors emerge in different embryonic tissues including skin, nervous system, bone marrow, pancreas, spleen, testis, eyes, sympathetic ganglia and many other tissues.10–12 The appearance of renin cells in the kidney is a late event in the history of these cells preceded by their advent in the adrenal gland. Thus, throughout development, renin progenitors are broadly distributed in numerous tissues and organs. In addition, during kidney development, the distribution of renin-expressing cells is not limited to their circumscribed site in the JGA as found in the adult mammal. In fact, during early kidney development the cells are widely distributed throughout the renal arterial tree as described in more detail below.4;13–15 As the kidneys develop, the distribution of renin expression shifts from large arteries to a more circumscribed juxtaglomerular position at the tip of the renal arterioles in the adult animal. This shifting pattern of renin expression is highly conserved and it has been confirmed in numerous mammals including humans, pigs, rats, sheep and mice.1;16–19 Further, this pattern is also found during evolution: as the animal species develop from fish to mammals renin localization switches from a broad arterial distribution in fish to more restricted JG localization in mammals. We now know that such distribution of renin expression is linked to renal vascular development as described below.15
The progenitors for renin cells in the kidney
The progenitors for renin cells in the kidney have now been clearly identified20.
Renin cells descend from Foxd1+ stromal cells.21;22 Foxd1 precursors give rise to renin precursor cells (RPCs), which in turn give rise to mesangial cells, smooth muscle cells and interstitial pericytes. RPCs contribute directly to the assembly and branching of the kidney arterioles. These progenitors are distinct from the SCL+ progenitors that give rise to the endothelial cells of the glomeruli, arterioles and interstitial capillaries.23 The distribution of renin cells in the developing arterioles follows a stereotypical pattern both in rats and mice: every time that a new arteriolar branch is forming, a small group of renin cells coalesce around where the vessel will sprout.15 This is followed by budding and elongation of the nascent vessel, which is covered by renin cells. As the vessel matures, renin cells differentiate into smooth muscle cells, except for the JG cells that retain expression of renin. Every time a new branch is generated, the process described above is repeated numerous times, in a fractal-like pattern until the entire arterial tree is completed. Therefore, throughout fetal and early postnatal development there is a continuous stereotypical changing distribution of renin. Overall, during this period of rapid arteriolar development, the distribution of renin changes continuously and is extensive throughout the renal arterial tree. When arteriolar development is completed, renin-expressing cells are confined to the classical JG localization as usually seen in the adult unstressed mammal. Interestingly, a variable percentage of of the arterioles ranging from 20 to 30 %, may not express renin in the adult, although those vessels retain such capability under conditions of physiological stress.15;24
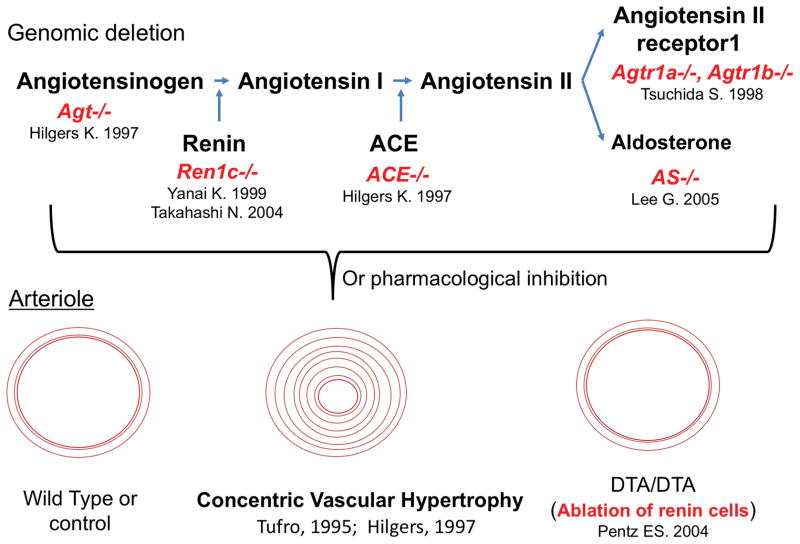
The above findings suggested that renin cells participate directly or indirectly in the morphogenesis and branching of the kidney arteriolar tree. In fact, pharmacological inhibition of the RAS in early life or knock out of any of the RAS genes results in severe vascular defects25;25–29 as shown in Figure 1. The arterioles are not only fewer but they are also shorter and thicker. The arteriolar thickening (concentric vascular hypertrophy) is at first difficult to interpret from a pathophysiological point of view because the hypertrophy resembles the lesion found in animals, including humans, with severe and chronic hypertension whereas animals subjected to knock out or pharmacological inhibition of RAS genes have low arterial pressure. This concentric vascular hypertrophy has also been described in humans with renal tubular dysgenesis resulting from mutations of RAS genes.30
Figure 1. Inactivation of the RAS in early life leads to marked abnormalities in kidney vascular development.
A central feature in all the manipulations is the presence of concentric vascular hypertrophy of the renal arterioles. The lesion does not occur if the renin cells are ablated with diphtheria toxin subunit A (DTA) targeted to the renin gene suggesting that renin cells per se are responsible for the pathology. The same vascular alteration is also seen in humans with renin gene mutations, see text for details. Agt, angiotensinogen; Ren1c−/−, renin gene deletion, ACE−/−, angiotensin converting enzyme deletion,; Agtr1a−/−, Agtr1b−/−, double deletion of angiotensin receptors subtypes A and B. AS−/−, aldosterone synthase KO
A breakthrough in our understanding came about when we ablated the renin cells in mice by targeting diphtheria toxin subunit A into the renin gene.31 Although these animals had renal abnormalities, the arteriolar hypertrophy was prevented (Figure 1), indicating that the hypertrophy occurred because the cells themselves directly or by the production of a growth factor(s) contributed to the vascular lesions. Given that the lesions are prevented when the renin cells are not present and similar lesions are found in renin KO mice, the lesion could not be ascribed to the lack of renin. In fact as described below, in renin KO mice the coding region of the renin gene is inactivated but the regulatory regions are left intact and (we now know) respond normally to physiological stimuli. Thus, in renin KO mice, the cells in charge of making renin do not disappear, they actually increase in number, continue to encircle the blood vessels and although they cannot synthesize the enzyme, they retain the molecular program of the renin phenotype which involves a much wider repertoire of genes besides renin. Overall, the results did suggest that renin cells per se contribute directly to the vascular hypertrophy. To address this question, we examined the distribution of renin cells along the arterioles of mice or rats with either pharmacological or genetic ablation of the RAS. We found that in all the conditions mentioned above, the renin cells distributed extensively along and around the thickened vessels. Because renin KO mice do not make renin, we used a surrogate marker to locate the cells: aldo-keto-reductase 1B7 (AKR1B7) an enzyme expressed by cells programed for the renin phenotype. Indeed, AKR1b7 also extended along and encircled the renal arterioles of renin KO mice. These findings strengthened the idea that renin cells participated in the vascular lesion. However, further proof was needed. This came in the form of lineage tracing. We traced the fate of renin cells using a cre-lox system (driving cre recombinase by the renin promoter) whereby all cells that ever expressed renin could be marked either with beta- galactosidase (LacZ) or green fluorescent protein. The results indicated that in fact cells from the renin lineage had not died or disappeared but instead they migrated within the vessel wall and physically participated in the vascular hypertrophy. These results do not exclude that the cells from the renin lineage produce some factor(s) that stimulated the growth of adjacent smooth muscle cells. In fact from previous microarray data, we know that cells that are so stimulated produce at least fourteen different types of growth factors with the capability to induce smooth muscle growth.32 Further work in this area is necessary, but what might the possible mechanisms underlying such seemingly non-homeostatic response from renin cells be?
We hypothesize that in response to a threat to homeostasis such as low blood pressure, the cells activate the molecular machinery that drive the renin phenotype. This molecular program in the early stages of the homeostatic threat includes increased synthesis of renin by JG cells and when the crisis persists, reactivation of renin expression by smooth muscle cells along the arterioles. Those recruited cells, which descended from renin precursors, retain the memory of the renin phenotype and have the plasticity to switch phenotypes on and off. If the blood pressure is normalized, the recruited cells switch the renin gene off and regain their full smooth muscle phenotype8;12;33;34. However, if the crisis is not resolved as it cannot be in the case of the renin KO animals, the molecular program of the renin phenotype persists and acquires additional characteristics that resemble those observed in embryonic life. In embryonic life the renin cells as mentioned above have angiogenic capabilities contributing to the branching and elongation of the renal arterial tree. Although speculative, the same molecular program that controls the renin gene may also trigger the synthesis of growth factors and angiogenic compounds known to be present in renin cells. Although more work in this area is needed, we hypothesize that as time goes on; the cells adopt or retain their embryonic nature. Further work will be needed to define whether the repertoire of genes expressed by these renin KO cells is similar to the pattern found in fetal-embryonic cells.
The phenomenon of recruitment
Under normal physiological circumstances, renin secretion by existent JG cells is sufficient to respond to minute-to-minute variations in arterial blood pressure and to changes in the status of the extracellular fluid. However, if an adult animal is dehydrated or hypotensive, or receives a diet low in sodium, or receives chronic treatment with RAS inhibitors, additional cells along the kidney vasculature (and sometimes in the mesangium and/or the renal interstitium) are recruited to produce renin.4;7–9;35;36 When this process occurs, the smooth muscle cells along the kidney arterioles change morphology, become epithelioid, make granules that contain renin and express genes characteristic of the renin phenotype, including Akr1b7 an enzyme recently identified as characteristic of the renin endocrine phenotype.32;37 The cells also express miR-330, a microRNA that is only expressed in JG cells when they are subjected to stimuli that demand high production of renin.38
The cell recruitment is due in great part to the reenactment of the renin phenotype by cells that descended from the renin cell precursors described above. Thus, the recruited cells have retained the memory -or plasticity- to re-express renin when the physiological need for more renin arises. As homeostasis is re-established the cells return to their smooth muscle phenotype but may be called back into action if the threat to homeostasis recurs.12 Thus, preservation of the memory of the renin phenotype may be a crucial mechanism to rapidly respond to a homeostatic threat without the extra energy needed for cell proliferation and/or migration. The mechanism takes advantage of a previous developmental process whereby renin precursor cells differentiate into smooth muscle cells leaving an imprint, the molecular memory of the renin phenotype, primed for action when needed. It is likely that such imprint resides in the organization of chromatin, which through posttranslational epigenetic modifications renders the DNA open for transcription. Our laboratory is currently investigating the epigenetic marks present in renin cells throughout development and those that may be utilized to activate transcription of the renin gene in adult animals when a physiological challenge occurs. Although this developmental mechanism seems to be the major mechanism underlying recruitment, it does not exclude other possibilities such that a few renin-expressing cells may arise de novo by neogenesis of cells that have never expressed renin. The nature of these potential precursors remains to be investigated. It should be noted, however, that the phenomenon of recruitment is not exclusive of renin cells. A similar process has been observed in ventricular cardiac cells, which are capable or re-expressing atrial natriuretic peptide in a rat model of aortocaval fistula.39 A similar mechanism has also been documented in pancreatic beta cells, thyroid cells, and in albumin synthesizing-hepatocytes.40–42 In all of these situations, the adult recruited cells had been able to express the hormone/protein in embryonic or fetal life. This strongly suggests that the adult cells retain the memory of the embryonic phenotype, which is re-enacted when the organism is under physiological stress. Efforts directed to understand where this memory resides, how it is constructed and how it is retained throughout cell replication are under way in our laboratories.
Major mechanisms that regulate renin cell identity
The cAMP pathway
cAMP mediates the actions of multiple physiological signals conveyed among others by β-adrenergic receptors, adenosine, prostaglandins, and low calcium that regulate renin secretion. cAMP stimulates renin gene transcription and renin release.43–45 We found that when there is an increase in renin release it is mainly due to an increased number of renin-secreting cells with minor changes in the amount of renin secreted by individual cells.46 In addition, using cells harboring yellow fluorescent protein (YFP) driven by the renin promoter, we showed an increase in the number of renin-expressing cells in response to manipulations that increase intracellular cAMP levels.34 These results in vitro corroborate numerous reports in whole animals indicating that the control of hormone availability is achieved by regulating the number of cells that express renin.
The renin gene contains a cAMP responsive element (CRE), which is critical for renin expression.47–49 Creb and its associated coactivators CBP/p300 associate with it at the CRE site to control renin gene expression. Histone Acetyl Transferases (HATs) such as CBP/p300 introduce activating acetyl marks in histones resulting in local relaxation of chromatin and nucleosomal displacement thus facilitating access of transcription factors important for transcription. In fact, conditional deletion of both HATs in renin cells led to reduction in the number of renin+ cells, diminished renin expression and abnormal arteriolar development indicating that CBP and p300 are necessary for the maintenance of renin cell identity and integrity of the kidney vasculature.50 Further, mice with deletion of both HATs cannot recruit renin-expressing cells or increase circulating renin in response to a challenge to homeostasis.
Confirming the importance of the cAMP pathway in renin regulation, conditional deletion of more proximal components of the pathway such as Gsa or beta adrenergic receptors in the renin cells, results in a reduction of renin expression in the kidney vasculature accompanied by arteriolar branching defects and/or renal failure.51–53 These results underscore the decisive role of the cAMP pathway in regulating renin cell fate and plasticity.
Cell-to-cell communication systems: the Notch pathway
As indicated above, renin cells communicate with one another and with adjacent cells including smooth muscle, mesangial cells and perivascular interstitial cells. Disruption of such communication alters the renin phenotype demonstrating that the identity of these cells is heavily dependent on the cell’s context, particularly their interaction with other cell types.
The Notch pathway is an ancestral, highly conserved, cell-to-cell communication system involved in cell fate decisions during development and in response to physiological challenges.54;55 Notch receptors, their ligands, and their final transcriptional effector, RBP-J, are expressed in renin cells.32 We found that disruption of the Notch pathway either by inhibition of gamma secretase (unpublished) or conditional deletion of RBP-J in renin cells56 results in severe reduction in the number of renin cells, low circulating renin and decreased blood pressure. Further, mutant mice are unable to recruit renin cells in response to a homeostatic challenge indicating that this pathway is necessary to maintain the memory of the renin phenotype. Further studies using mice harboring a bacterial artificial chromosome (BAC) driving enhanced (e)GFP showed that RBP-J regulates the renin promoter directly both during the basal state and in response to physiological challenges indicating that RBP-J is directly involved in the ability of smooth muscle cells along the arteriole to re-acquire the renin phenotype.57
The actions of RBP-J are not limited to the renin gene. In fact, RBP-J governs a network of genes that ultimately define the renin cell identity.57 RBP-J regulates AKR1b7, an enzyme that is co-expressed with renin during normal development and in response to physiological challenges. The functions of AKR1B7 in renin cells are still unclear, but it is known that aldo-keto reductases play a role in detoxification of cells. We can only speculate that AKR1b7 may transform harmful aldehydes into less toxic alcohols thus maintaining renin cell vitality. AKR1b7 is part of the genetic program of the renin phenotype that RBP-J controls. In fact, mice with deletion of RBP-J have significantly fewer AKR1b7+ cells. 57;57 This is accompanied, as expected, by a frank decrease in the number of renin granules indicating that RBP-J controls several components of the endocrine renin phenotype. The renin phenotype is not limited to the endocrine capabilities of the cell. JG cells retain the ability to contract and express a variety of smooth muscle genes. RBP-J controls the expression of several smooth muscle genes (calponin, SM myosin heavy chain, alpha smooth muscle actin) as well as their master regulators (such as SRF, miR145, CRIP1) indicating that RBP-J/Notch controls a genetic program that maintains the well balanced endocrine-contractile phenotype of the renin cell.57
Cell fate tracing studies showed that the marked decrease in the number of renin+/AKR1b7+ cells in RBP-J cKO mice was not due to cell death or a decrease endowment of renin progenitors. Instead, it was due to phenotypic switching of former renin expressing cells to another cell type.57 The dually negative cells did not express endothelial, epithelial or stem cell markers. However, they expressed genes characteristic of hematopoietic cells, indicating that in renin cells, RBP-J normally suppresses the ectopic expression of genes from other lineages.57 The mechanisms involved in RBP-J suppression of genes from undesirable lineages remain to be studied. In summary, RBP-J controls the identity of the renin cell by activating genes involved in the dual endocrine-contractile phenotype of the renin cell and by preventing the ectopic expression of genes from other lineages, which could have severe consequences for the control of homeostasis.
The effects of RBP-J deletion are heightened if the mutation occurs at an early stage of differentiation. Whereas deletion of RBP-J in the renin cells leads to a remarkable change in renin cell identity without overt morphological changes, deletion of RBP-J in their Foxd1+ stromal precursors results in a decreased endowment of renin cells, mesangial cells, and smooth muscle cells accompanied by inadequate vascular formation and glomerular aneurysms.22 Thus, deletion of RBP-J in the early progenitor leads to a decrease of descendants whereas deletion in the more differentiated RCPs results in a significant change in cell identity. These results indicate that the effects of RBP-J depend on the degree of differentiation of the target cells.
Novel functions of renin cells
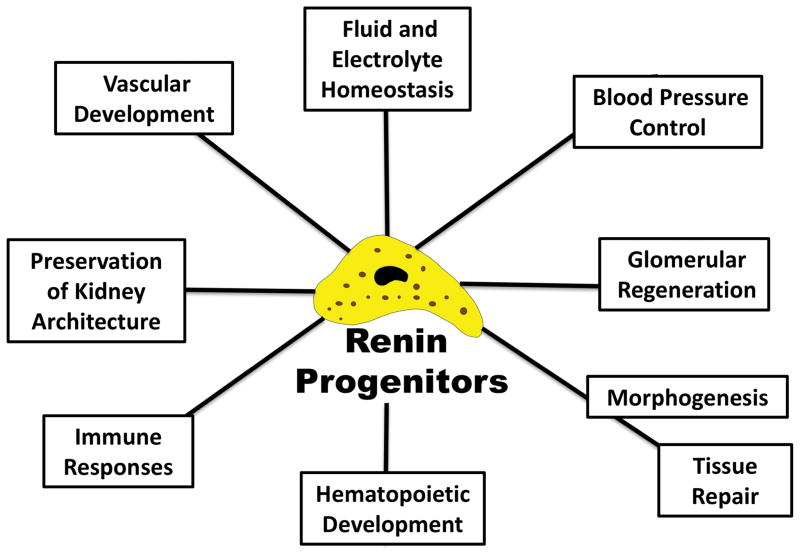
As described above, renin cells are progenitor cells that in the kidney are intimately linked to the development of the renal arterial tree. In addition, renin cells may have a role in renal repair and/or regeneration (Figure 2).
Figure 2. Renin cells participate in multiple defense mechanisms.
In addition to control of fluid and electrolytes and blood pressure homeostasis, the cells are involved in morphogenetic events and kidney vascular development. They play a role in the regeneration of injured glomeruli and preserve kidney structure. Their presence in hematopoietic tissues suggest a link between the endocrine and immune systems.
Recently, two groups of investigators working independently demonstrated that renin cells likely from the JG area participated in the regeneration of podocytes and mesangial cells in two different models of glomerular injury.58;59 In an experimental model closely resembling focal segmental glomerular sclerosis Pippin and collaborators tracked the fate of cells from the renin lineage and observed that, after podocyte injury and depletion induced with a cytotoxic anti-podocyte antibody, cells of the renin lineage repopulated a significant fraction of the parietal epithelial cells of the Bowman’s capsule and of the podocytes.58 Thus, under pathological situations, renin cells presumably from the JGA are able to regenerate affected glomerular cells. Podocytes are not believed to regenerate from remaining uninjured podocytes, are unrelated to the renin lineage and do not descend from renin cells. Thus the finding poses a very interesting question as to how podocytes can be regenerated from a different cell type. Although it remains to be studied, it is possible that, under the stress of injury, direct conversion or trans-differentiation from one cell type to the other may occur. The process will require a significant change in the molecular factors that control renin cell identity requiring significant changes in chromatin architecture and establishment of new transcriptional factories so that new sets of genes could be expressed to conform to a new podocyte identity. This situation also implies that the available JG cells maintain progenitor capabilities that can be employed when injury occurs. We have seen that renin cells are capable of switching phenotype but usually among lineage related cells. In this case the total conversion from one cell type to the other is intriguing and exciting implying a more powerful and ample role of renin cells in tissue regeneration. These findings do not exclude that parietal epithelial cells, a fraction of which seem to express renin during development, may also give rise to podocytes.12 In this case, instead of trans-differentiation a reenactment of an embryological program, as in the case of the mesangial cells discussed below, may take place.
As mentioned above, during embryonic and early postnatal nephrogenesis, renin precursor cells give rise to mesangial cells. Therefore, Starke and colleagues recently tested whether renin cells in the adult animal could regenerate the glomerular mesangium in an experimental model of mesangiolysis resembling mesangial proliferative glomerulonephritis.59
Using mice engineered with inducible reporters to track the fate of renin cells, they found that a significant fraction (around sixty percent) of the regenerating glomeruli were repopulated by cells derived from the renin lineage. Mesangial cells presumably arose from the nearby renin-containing JG cells. Upon differentiation, as it occurs during development, the newly differentiated cells acquired mesangial markers such as alpha8-integrin and platelet-derived growth factor receptor beta, and stopped making renin. It would be important to determine whether or not regeneration of mesangial cells in this instance results from the reenactment of the yet to be discovered developmental program used during differentiation of mesangial cells from renin precursors during normal kidney development. Altogether, these experiments indicate that renin cells are a progenitor niche with the ability to regenerate injured cells from the glomerulus.
Both sets of experiments also suggest that the renin cells may need to migrate to reach their glomerular locus. Whether and how this happens remains to be investigated but it is the subject of intense work in several prominent laboratories. Further, these interesting findings open new and exciting questions. The molecular program(s) responsible for this reparative process remains to be understood. Similarly, the signals used by seemingly migrating JG cells to arrive at the locus of injury and replace injured cells remain to be identified. It would be important to determine whether the replenishment of cells is either a transient or a permanent phenomenon, a transient reaction to injury or a true reparative process with long-term clinically relevant consequences such as lasting improvement in kidney function.
Renin progenitors in hematopoietic tissues
Hematopoietic tissues possess unique renin progenitors which harbor fundamental clues for our understanding of normal and neoplastic hematopoiesis. While studying the effects of RBP-J deletion in the kidney, we found that as the mice aged, they developed signs of a highly penetrant and devastating form of pre-B-cell leukemia.60 Therefore, we performed an extensive series of experiments to characterize not only the disease but also to identify its cell of origin. We found that cells from the bone marrow, spleen, Peyer’s patches, and lymph nodes normally contain a group of primitive lymphocytes that synthezise and release renin. These renin cells with a lymphocyte pedigree display some developmental, biochemical, and transcriptional similarities with the more distant kidney JG cell.60 Similar to JG cells, renin-bearing lymphocytes in the bone marrow and spleen decrease in number as development progresses. Remarkably, at all ages examined, these cells are proportionally ten times more abundant than juxtaglomerular cells. Furthermore, the cells are responsive to physiological and pharmacological stimuli: they become more abundant when animals are treated with ACE inhibitors. Brenin cells and JG cells also have in common the expression of some transcription factors such as EBF1, a well known regulator of lymphocyte development, Ikaros, EPAS1 (HIF2alpha), Notch receptors, and RBP-J.60 This suggests a conservation of a portion of the transcriptional machinery and a possible lineage relationship between hematopoietic and kidney renin cells. Both cell types require RBP-J to differentiate properly. Deficiency of RBP-J in the kidney as mentioned above leads to a significant change in the identity of renin cells. Brenin cells lacking RBP-J do not differentiate normally; they are held as precursors, lose control of their cell cycle, proliferate, and become neoplastic. In both cases, RBP-J controls cell fate.60
The function of renin cells in hematopoietic tissues needs to be studied. In analogy with their role in the kidney, it is possible that Brenin cells control bone marrow structural morphogenesis and/or hematopoietic development. From an integrative physiological point of view, it is tempting to speculate that hematopoietic renin cells are part of a systemic defense response coordinated not only to sustain blood pressure and fluid/electrolyte homeostasis but also to provide a rapid response to infections and/or to foreign antigens. Overall, if these cells play a role in immune defense, it will widen the defense functions of renin cells linking the endocrine and immune systems, two major ancestral mechanisms to maintain homeostasis. This exciting possibility is being pursued in our laboratories.
Renin Cell Fate and Disease
Finally, it should be noted that renin cells, which emerged over 400 million years ago, might have retained many of their original properties.61 On superficial inspection, the morphological characteristics and distribution of the renin cells seem to have been well conserved. Furthermore, it seems that they have also maintained some ancestral functions such as their involvement in vascular development and branching,18 and other fundamental homeostatic functions such as the control of fluid-electrolyte homeostasis and blood pressure regulation.19 It is also possible that the function of these cells depends on the site where they reside (kidney versus bone marrow) and on the main phenotype (pericyte,62 JG cell, tubular epithelial cell,63;64 lymphocyte60) they adopt. The answers to these questions will likely open new avenues of understanding and opportunities for new and exciting research and therapeutics.
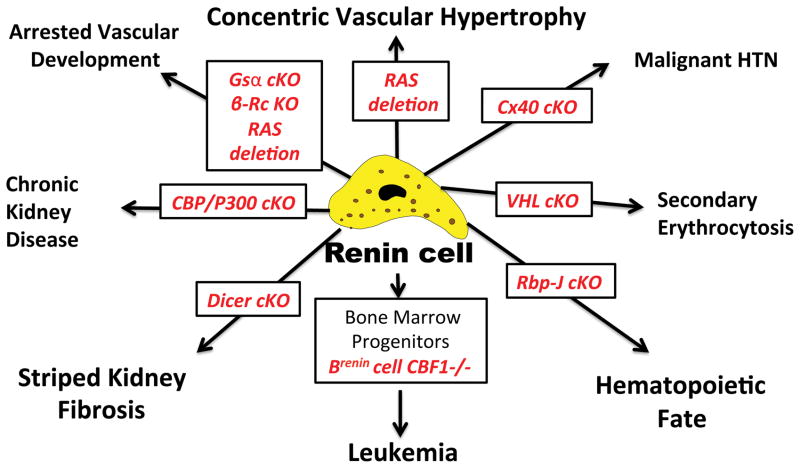
Equally important, the understanding of renin cell fate is not a luxurious academic exercise: manipulations that alter the identity and fate of renin cells can and do frequently lead to serious disease. Although space constraints do not permit to discuss each example in detail, Figure 3 provides some examples, which illustrate the point. As mentioned above, deletion of RBP-J in renin cells results in a significant change in the phenotype of the cells in the kidney: the cells lose their dual endocrine-contractile phenotype and cannot respond properly to physiological threats such as sodium depletion and angiotensin converting enzyme inhibitors.57 When this deletion occurs in the bone marrow a highly penetrant type of pre-B leukemia develops.60 Deletion of the enzyme Dicer that is responsible for the processing of mature microRNAS results in striped kidney fibrosis, which resembles among other things toxicity by immunosupressants of the calcineurin inhibitor class such as tacrolimus and cyclosporine.65 A similar type of lesion has been described in hyperuricemia and hyperhomocysteinemia66;67 Deletion of the histone acetyl transferases (HATs) p300 and CBP results in chronic kidney disease resembling the old textbook descriptions of Bright’s disease.50 Deletion of several components of the cAMP pathway including deletion of the beta-adrenergic receptor results in arrested vascular development and/or alterations in renin secretion.51;68;69 Lack of connexin 40 (Cx40) in renin cells leads to malignant hypertension70 and deletion of the Von Hippel Lindau (VHL) gene in renin cells leads to a remarkable phenotypic switch: the former renin producing cells stop making renin and transcribe erythropoietin.71 As a result the animals display secondary erythrocytosis. Because the renin-angiotensin system is at the crux of our armamentarium to treat hypertension and other disorders, understanding the mechanisms involved in normal and abnormal renin cell fate may help design new strategies to prevent and better treat our patients with hypertension, cardiovascular and renal diseases.
Figure 3. Alterations in renin cell fate lead to disease.
Conditional deletion of key molecules within renin cells results in in cell fate change leading to renal and systemic pathology. B-RcKO, deletion of the beta-adrenergic receptor; Cx40, connexin 40; VHL, Von Hippel Lindau gene; RBP-J, recombination signal binding protein for immunoglobulin Kappa J. CBF1, human homolog of RBP-J.
Acknowledgments
This award is the result of the work of many individuals that contributed throughout the years. Special recognition thus to my colleague Dr. Maria Luisa S. Sequeira Lopez (MLSSL) for many meaningful contributions and discussions, and to past and current members of our joint laboratories. Also many thanks to our colleagues around the world with whom we continue to join efforts to understand what makes renin cells who they are. I am greatly indebted to those who taught me: Elvira Arrizurieta and Alfredo Lanari (Instituto de Investigaciones Médicas, Buenos Aires, Argentina), Jean Robillard (University of Iowa), Malcolm Holliday (UCSF), and Drs. Robert M Carey, and Michael J. Peach (University of Virginia). The facilities of the Child Health Research Center and the resources of the Pediatric Center of Excellence in Nephrology are greatly appreciated.
Sources of funding:
The work presented here was supported by NIH grants R37HL066242, P50DK096373, RO1HL096735 (to RAG), and DK091330, DK096373 (to MLSSL).
Footnotes
Disclosures: None
Reference List
- 1.Taugner R, Hackenthal E. The Juxtaglomerular Apparatus: structure and function. Heidelberg: Springer Verlag; 1989. pp. 104–126. [Google Scholar]
- 2.Keeton TK, Campbell WB. The pharmacologic alteration of renin release. Pharmacol Rev. 1980;32:81–227. [PubMed] [Google Scholar]
- 3.Gomez RA, Chevalier RL, Sturgill BC, Johns DW, Peach MJ, Carey RM. Maturation of the intrarenal renin distribution in Wistar-Kyoto rats. Journal of Hypertension. 1986;4:s31–s33. [Google Scholar]
- 4.Gomez RA, Chevalier RL, Carey RM, Peach MJ. Molecular biology of the renal renin-angiotensin system. Kidney Int Suppl. 1990;30:S18–S23. [PubMed] [Google Scholar]
- 5.Pupilli C, Gomez RA, Tuttle JB, Peach MJ, Carey RM. Spatial association of renin-containing cells and nerve fibers in developing rat kidney. Pediatr Nephrol. 1991;5:690–695. doi: 10.1007/BF00857873. [DOI] [PubMed] [Google Scholar]
- 6.TOBIAN L, TOMBOULIAN A, JANECEK J. The effect of high perfusion pressures on the granulation of juxtaglomerular cells in an isolated kidney. J Clin Invest. 1959;38:605–610. doi: 10.1172/JCI103838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez RA, Lynch KR, Chevalier RL, Everett AD, Johns DW, Wilfong N, Peach MJ, Carey RM. Renin and angiotensinogen gene expression and intrarenal renin distribution during ACE inhibition. Am J Physiol. 1988;254:F900–F906. doi: 10.1152/ajprenal.1988.254.6.F900. [DOI] [PubMed] [Google Scholar]
- 8.Gomez RA, Chevalier RL, Everett AD, Elwood JP, Peach MJ, Lynch KR, Carey RM. Recruitment of renin gene-expressing cells in adult rat kidneys. Am J Physiol. 1990;259:F660–F665. doi: 10.1152/ajprenal.1990.259.4.F660. [DOI] [PubMed] [Google Scholar]
- 9.Kim HS, Maeda N, Oh GT, Fernandez LG, Gomez RA, Smithies O. Homeostasis in mice with genetically decreased angiotensinogen is primarily by an increased number of renin-producing cells. J Biol Chem. 1999;274:14210–14217. doi: 10.1074/jbc.274.20.14210. [DOI] [PubMed] [Google Scholar]
- 10.Gomez RA, Sequeira-Lopez ML. Novel Functions of Renin Precursors in Homeostasis and Disease. Physiology (Bethesda) 2016;31:25–33. doi: 10.1152/physiol.00039.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones CA, Sigmund CD, McGowan RA, Kane-Haas CM, Gross KW. Expression of murine renin genes during fetal development. Mol Endocrinol. 1990;4:375–383. doi: 10.1210/mend-4-3-375. [DOI] [PubMed] [Google Scholar]
- 12.Sequeira Lopez ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell. 2004;6:719–728. doi: 10.1016/s1534-5807(04)00134-0. [DOI] [PubMed] [Google Scholar]
- 13.Gomez RA, Lynch KR, Chevalier RL, Wilfong N, Everett A, Carey RM, Peach MJ. Renin and angiotensinogen gene expression in maturing rat kidney. Am J Physiol. 1988;254:F582–F587. doi: 10.1152/ajprenal.1988.254.4.F582. [DOI] [PubMed] [Google Scholar]
- 14.Gomez RA, Lynch KR, Sturgill BC, Elwood JP, Chevalier RL, Carey RM, Peach MJ. Distribution of renin mRNA and its protein in the developing kidney. Am J Physiol. 1989;257:F850–F858. doi: 10.1152/ajprenal.1989.257.5.F850. [DOI] [PubMed] [Google Scholar]
- 15.Reddi V, Zaglul A, Pentz ES, Gomez RA. Renin-expressing cells are associated with branching of the developing kidney vasculature. J Am Soc Nephrol. 1998;9:63–71. doi: 10.1681/ASN.V9163. [DOI] [PubMed] [Google Scholar]
- 16.Egerer G, Taugner R, Tiedemann K. Renin immunohistochemistry in the mesonephros and metanephros of the pig embryo. Histochemistry. 1984;81:385–390. doi: 10.1007/BF00514334. [DOI] [PubMed] [Google Scholar]
- 17.Celio MR, Groscurth P, Inagami T. Ontogeny of renin immunoreactive cells in the human kidney. Anat Embryol (Berl) 1985;173:149–155. doi: 10.1007/BF00316297. [DOI] [PubMed] [Google Scholar]
- 18.Rider SA, Mullins LJ, Verdon RF, MacRae CA, Mullins JJ. Renin expression in developing zebrafish is associated with angiogenesis and requires the Notch pathway and endothelium. Am J Physiol Renal Physiolajprenal. 2015;309:F531–F539. doi: 10.1152/ajprenal.00247.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishimura H, Ogawa M, Sawyer WH. Renin-angiotensin system in primitive bony fishes and a holocephalian. Am J Physiol. 1973;224:950–956. doi: 10.1152/ajplegacy.1973.224.4.950. [DOI] [PubMed] [Google Scholar]
- 20.Sequeira Lopez ML, Gomez RA. Development of the renal arterioles. J Am Soc Nephrol. 2011;22:2156–2165. doi: 10.1681/ASN.2011080818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sequeira-Lopez ML, Lin EE, Li M, Hu Y, Sigmund CD, Gomez RA. The earliest metanephric arteriolar progenitors and their role in kidney vascular development. Am J Physiol Regul Integr Comp Physiol. 2015;308:R138–R149. doi: 10.1152/ajpregu.00428.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin EE, Sequeira-Lopez ML, Gomez RA. RBP-J in FOXD1+ renal stromal progenitors is crucial for the proper development and assembly of the kidney vasculature and glomerular mesangial cells. Am J Physiol Renal Physiol. 2014;306:F249–F258. doi: 10.1152/ajprenal.00313.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu Y, Li M, Gothert JR, Gomez RA, Sequeira-Lopez ML. Hemovascular Progenitors in the Kidney Require Sphingosine-1-Phosphate Receptor 1 for Vascular Development. J Am Soc Nephrol. 2016;27:1984–1995. doi: 10.1681/ASN.2015060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee G, Makhanova N, Caron K, Lopez ML, Gomez RA, Smithies O, Kim HS. Homeostatic responses in the adrenal cortex to the absence of aldosterone in mice. Endocrinology. 2005;146:2650–2656. doi: 10.1210/en.2004-1102. [DOI] [PubMed] [Google Scholar]
- 25.Hilgers KF, Reddi V, Krege JH, Smithies O, Gomez RA. Aberrant renal vascular morphology and renin expression in mutant mice lacking angiotensin-converting enzyme. Hypertension. 1997;29:216–221. doi: 10.1161/01.hyp.29.1.216. [DOI] [PubMed] [Google Scholar]
- 26.Hilgers KF, Norwood VF, Gomez RA. Angiotensin’s role in renal development. Semin Nephrol. 1997;17:492–501. [PubMed] [Google Scholar]
- 27.Tufro-McReddie A, Romano LM, Harris JM, Ferder L, Gomez RA. Angiotensin II regulates nephrogenesis and renal vascular development. Am J Physiol. 1995;269:F110–F115. doi: 10.1152/ajprenal.1995.269.1.F110. [DOI] [PubMed] [Google Scholar]
- 28.Tsuchida S, Matsusaka T, Chen X, Okubo S, Niimura F, Nishimura H, Fogo A, Utsunomiya H, Inagami T, Ichikawa I. Murine double nullizygotes of the angiotensin type 1A and 1B receptor genes duplicate severe abnormal phenotypes of angiotensinogen nullizygotes. J Clin Invest. 1998;101:755–760. doi: 10.1172/JCI1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi N, Chernavvsky DR, Gomez RA, Igarashi P, Gitelman HJ, Smithies O. Uncompensated polyuria in a mouse model of Bartter’s syndrome. Proc Natl Acad Sci U S A. 2000;97:5434–5439. doi: 10.1073/pnas.090091297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gribouval O, Moriniere V, Pawtowski A, et al. Spectrum of mutations in the renin-angiotensin system genes in autosomal recessive renal tubular dysgenesis. Hum Mutat. 2012;33:316–326. doi: 10.1002/humu.21661. [DOI] [PubMed] [Google Scholar]
- 31.Pentz ES, Moyano MA, Thornhill BA, Sequeira Lopez ML, Gomez RA. Ablation of renin-expressing juxtaglomerular cells results in a distinct kidney phenotype. Am J Physiol Regul Integr Comp Physiol. 2014;286:R474–R483. doi: 10.1152/ajpregu.00426.2003. [DOI] [PubMed] [Google Scholar]
- 32.Brunskill EW, Sequeira-Lopez ML, Pentz ES, Lin E, Yu J, Aronow BJ, Potter SS, Gomez RA. Genes that confer the identity of the renin cell. J Am Soc Nephrol. 2011;22:2213–2225. doi: 10.1681/ASN.2011040401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sequeira Lopez ML, Gomez RA. Novel mechanisms for the control of renin synthesis and release. Curr Hypertens Rep. 2010;12:26–32. doi: 10.1007/s11906-009-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pentz ES, Sequeira Lopez ML, Cordaillat M, Gomez RA. Identity of the renin cell is mediated by cAMP and chromatin remodeling: an in vitro model for studying cell recruitment and plasticity. Am J Physiol Heart Circ Physiol. 2008;294:H699–H707. doi: 10.1152/ajpheart.01152.2007. [DOI] [PubMed] [Google Scholar]
- 35.Chevalier RL, Gomez RA, Jones CE. Developmental determinants of recovery after relief of partial ureteral obstruction. Kidney Int. 1988;33:775–781. doi: 10.1038/ki.1988.66. [DOI] [PubMed] [Google Scholar]
- 36.Gomez RA. Molecular biology of components of the renin-angiotensin system during development. Pediatr Nephrol. 1990;4:421–423. doi: 10.1007/BF00862529. [DOI] [PubMed] [Google Scholar]
- 37.Lin EE, Pentz ES, Sequeira-Lopez ML, Gomez RA. Aldo-keto reductase 1b7, a novel marker for renin cells, is regulated by cyclic AMP signaling. Am J Physiol Regul Integr Comp Physiol. 2015;309:R576–R584. doi: 10.1152/ajpregu.00222.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Medrano S, Monteagudo MC, Sequeira-Lopez MLS, Pentz ES, Gomez RA. Two microRNAs -miR-330 and miR-125b-5p- mark the juxtaglomerular cell and balance its smooth muscle phenotype. Am J Physiol Renal Physiol. 2012;302:F29–F37. doi: 10.1152/ajprenal.00460.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lattion AL, Michel JB, Arnauld E, Corvol P, Soubrier F. Myocardial recruitment during ANF mRNA increase with volume overload in the rat. Am J Physiol. 1986;251:H890–H896. doi: 10.1152/ajpheart.1986.251.5.H890. [DOI] [PubMed] [Google Scholar]
- 40.Gerber H, Peter HJ, Bachmeier C, Kaempf J, Studer H. Progressive recruitment of follicular cells with graded secretory responsiveness during stimulation of the thyroid gland by thyrotropin. Endocrinology. 1987;120:91–96. doi: 10.1210/endo-120-1-91. [DOI] [PubMed] [Google Scholar]
- 41.Lin CT, Palmer W, Wu JY, Chan L. Estrogen induction of very low density apolipoprotein II synthesis, a major avian liver yolk protein, involves the recruitment of hepatocytes. Endocrinology. 1986;118:538–544. doi: 10.1210/endo-118-2-538. [DOI] [PubMed] [Google Scholar]
- 42.Schuit FC, In’t Veld PA, Pipeleers DG. Glucose stimulates proinsulin biosynthesis by a dose-dependent recruitment of pancreatic beta cells. Proc Natl Acad Sci U S A. 1988;85:3865–3869. doi: 10.1073/pnas.85.11.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borensztein P, Germain S, Fuchs S, Philippe J, Corvol P, Pinet F. cis-regulatory elements and trans-acting factors directing basal and cAMP-stimulated human renin gene expression in chorionic cells. Circ Res. 1994;74:764–773. doi: 10.1161/01.res.74.5.764. [DOI] [PubMed] [Google Scholar]
- 44.Horiuchi M, Nakamura N, Tang SS, Barrett G, Dzau VJ. Molecular mechanism of tissue-specific regulation of mouse renin gene expression by cAMP. Identification of an inhibitory protein that binds nuclear transcriptional factor. J Biol Chem. 1991;266:16247–16254. [PubMed] [Google Scholar]
- 45.Nakamura N, Burt DW, Paul M, Dzau VJ. Negative control elements and cAMP responsive sequences in the tissue-specific expression of mouse renin genes. Proc Natl Acad Sci U S A. 1989;86:56–59. doi: 10.1073/pnas.86.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Everett AD, Carey RM, Chevalier RL, Peach MJ, Gomez RA. Renin release and gene expression in intact rat kidney microvessels and single cells. J Clin Invest. 1990;86:169–175. doi: 10.1172/JCI114680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klar J, Sandner P, Muller MW, Kurtz A. Cyclic AMP stimulates renin gene transcription in juxtaglomerular cells. Pflugers Arch. 2002;444:335–344. doi: 10.1007/s00424-002-0818-9. [DOI] [PubMed] [Google Scholar]
- 48.Pan L, Black TA, Shi Q, Jones CA, Petrovic N, Loudon J, Kane C, Sigmund CD, Gross KW. Critical roles of a cyclic AMP responsive element and an E-box in regulation of mouse renin gene expression. J Biol Chem. 2001;276:45530–45538. doi: 10.1074/jbc.M103010200. [DOI] [PubMed] [Google Scholar]
- 49.Todorov VT, Volkl S, Friedrich J, Kunz-Schughart LA, Hehlgans T, Vermeulen L, Haegeman G, Schmitz ML, Kurtz A. Role of CREB1 and NF{kappa}B-p65 in the down-regulation of renin gene expression by tumor necrosis factor {alpha} J Biol Chem. 2005;280:24356–24362. doi: 10.1074/jbc.M502968200. [DOI] [PubMed] [Google Scholar]
- 50.Gomez RA, Pentz ES, Jin X, Cordaillat M, Sequeira Lopez ML. CBP and p300 are essential for renin cell identity and morphological integrity of the kidney. Am J Physiol Heart Circ Physiol. 2009;296:H1255–H1262. doi: 10.1152/ajpheart.01266.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen L, Kim SM, Oppermann M, Faulhaber-Walter R, Huang Y, Mizel D, Chen M, Lopez ML, Weinstein LS, Gomez RA, Briggs JP, Schnermann J. Regulation of renin in mice with Cre recombinase-mediated deletion of G protein Gsalpha in juxtaglomerular cells. Am J Physiol Renal Physiol. 2007;292:F27–F37. doi: 10.1152/ajprenal.00193.2006. [DOI] [PubMed] [Google Scholar]
- 52.Neubauer B, Machura K, Chen M, Weinstein LS, Oppermann M, Sequeira-Lopez ML, Gomez RA, Schnermann J, Castrop H, Kurtz A, Wagner C. Development of vascular renin expression in the kidney critically depends on the cyclic AMP pathway. Am J Physiol Renal Physiol. 2009;296:F1006–F1012. doi: 10.1152/ajprenal.90448.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen L, Faulhaber-Walter R, Wen Y, Huang Y, Mizel D, Chen M, Sequeira Lopez ML, Weinstein LS, Gomez RA, Briggs JP, Schnermann J. Renal failure in mice with Gsalpha deletion in juxtaglomerular cells. Am J Nephrol. 2010;32:83–94. doi: 10.1159/000314635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268:225–232. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- 55.Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- 56.Castellanos Rivera RM, Monteagudo MC, Pentz ES, Glenn ST, Gross KW, Carretero O, Sequeira-Lopez ML, Gomez RA. Transcriptional regulator RBP-J regulates the number and plasticity of renin cells. Physiol Genomics. 2011;43:1021–1028. doi: 10.1152/physiolgenomics.00061.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castellanos-Rivera RM, Pentz ES, Lin E, Gross KW, Medrano S, Yu J, Sequeira-Lopez ML, Gomez RA. Recombination Signal Binding Protein for Ig-kappaJ Region Regulates Juxtaglomerular Cell Phenotype by Activating the Myo-Endocrine Program and Suppressing Ectopic Gene Expression. J Am Soc Nephrol. 2015;26:67–80. doi: 10.1681/ASN.2013101045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pippin JW, Sparks MA, Glenn ST, Buitrago S, Coffman TM, Duffield JS, Gross KW, Shankland SJ. Cells of renin lineage are progenitors of podocytes and parietal epithelial cells in experimental glomerular disease. Am J Pathol. 2013;183:542–557. doi: 10.1016/j.ajpath.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Starke C, Betz H, Hickmann L, Lachmann P, Neubauer B, Kopp JB, Sequeira-Lopez ML, Gomez RA, Hohenstein B, Todorov VT, Hugo CP. Renin lineage cells repopulate the glomerular mesangium after injury. J Am Soc Nephrol. 2015;26:48–54. doi: 10.1681/ASN.2014030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Belyea BC, Xu F, Pentz ES, Medrano S, Li M, Hu Y, Turner S, Legallo R, Jones CA, Tario JD, Liang P, Gross KW, Sequeira-Lopez ML, Gomez RA. Identification of renin progenitors in the mouse bone marrow that give rise to B-cell leukaemia. Nat Commun. 2014;5:3273. doi: 10.1038/ncomms4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fournier D, Luft FC, Bader M, Ganten D, Andrade-Navarro MA. Emergence and evolution of the renin-angiotensin-aldosterone system. J Mol Med (Berl) 2012;90:495–508. doi: 10.1007/s00109-012-0894-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berg AC, Chernavvsky-Sequeira C, Lindsey J, Gomez RA, Sequeira-Lopez ML. Pericytes synthesize renin. World J Nephrol. 2013;2:11–16. doi: 10.5527/wjn.v2.i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 64.Prieto-Carrasquero MC, Botros FT, Pagan J, Kobori H, Seth DM, Casarini DE, Navar LG. Collecting duct renin is upregulated in both kidneys of 2-kidney, 1-clip goldblatt hypertensive rats. Hypertension. 2008;51:1590–1596. doi: 10.1161/HYPERTENSIONAHA.108.110916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sequeira-Lopez ML, Weatherford ET, Borges GR, Monteagudo MC, Pentz ES, Harfe BD, Carretero O, Sigmund CD, Gomez RA. The microRNA-processing enzyme dicer maintains juxtaglomerular cells. J Am Soc Nephrol. 2010;21:460–467. doi: 10.1681/ASN.2009090964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumagai H, Katoh S, Hirosawa K, Kimura M, Hishida A, Ikegaya N. Renal tubulointerstitial injury in weanling rats with hyperhomocysteinemia. Kidney Int. 2002;62:1219–1228. doi: 10.1111/j.1523-1755.2002.kid558.x. [DOI] [PubMed] [Google Scholar]
- 67.Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, Lan HY, Kivlighn S, Johnson RJ. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 68.Neubauer B, Machura K, Schnermann J, Wagner C. Renin expression in large renal vessels during fetal development depends on functional {beta}1/{beta}2-adrenergic receptors. Am J Physiol Renal Physiol. 2011;301:F71–F77. doi: 10.1152/ajprenal.00443.2010. [DOI] [PubMed] [Google Scholar]
- 69.Kim SM, Chen L, Faulhaber-Walter R, Oppermann M, Huang Y, Mizel D, Briggs JP, Schnermann J. Regulation of renin secretion and expression in mice deficient in beta1- and beta2-adrenergic receptors. Hypertension. 2007;50:103–109. doi: 10.1161/HYPERTENSIONAHA.107.087577. [DOI] [PubMed] [Google Scholar]
- 70.Kurtz L, Schweda F, de WC, Kriz W, Witzgall R, Warth R, Sauter A, Kurtz A, Wagner C. Lack of connexin 40 causes displacement of renin-producing cells from afferent arterioles to the extraglomerular mesangium. J Am Soc Nephrol. 2007;18:1103–1111. doi: 10.1681/ASN.2006090953. [DOI] [PubMed] [Google Scholar]
- 71.Kurt B, Paliege A, Willam C, Schwarzensteiner I, Schucht K, Neymeyer H, Sequeira-Lopez ML, Bachmann S, Gomez RA, Eckardt KU, Kurtz A. Deletion of von Hippel-Lindau protein converts renin-producing cells into erythropoietin-producing cells. J Am Soc Nephrol. 2013;24:433–444. doi: 10.1681/ASN.2012080791. [DOI] [PMC free article] [PubMed] [Google Scholar]