Abstract
Dopamine (DA) modulates neuronal activity in the prefrontal cortex (PFC) and is necessary for optimal cognitive function. Dopamine transmission in the PFC is also important for the behavioral adaptations produced by repeated exposure to cocaine. Therefore, we investigated the effects of repeated cocaine treatment followed by withdrawal (2–4 weeks) on the responsivity of cortical cells to electrical stimulation of the ventral tegmental area (VTA) and to systemic administration of DA D1 or D2 receptor antagonists. Cortical cells in cocaine- and saline-treated animals exhibited a similar decrease in excitability after the administration of D1 receptor antagonists. In contrast, cortical neurons from cocaine-treated rats exhibited a lack of D2-mediated regulation relative to saline rats. Furthermore, in contrast to saline-treated animals, VTA stimulation did not increase cortical excitability in the cocaine group. These data suggest that withdrawal from repeated cocaine administration elicits some long-term neuroadaptations in the PFC, including (1) reduced D2-mediated regulation of cortical excitability, (2) reduced responsivity of cortical cells to phasic increases in DA, and (3) a trend toward an overall decrease in excitability of PFC neurons.
Keywords: cocaine, prefrontal cortex, electrophysiology, excitability, dopaminergic receptors, in vivo
Introduction
The ventral tegmental area (VTA) provides the primary dopaminergic input to the prefrontal cortex (PFC), and VTA firing evokes tonic and phasic dopamine (DA) release (Grace, 2000). The short- and long-term actions of DA in the PFC of drug-naive animals have been well documented (for review, see Seamans and Yang, 2004). However, the long-term neuroadaptations produced in cortical activity and in the DAergic modulation of cortical activity by withdrawal from psychostimulants are not well explored.
Repeated treatment with cocaine alters various aspects of dopamine transmission in the PFC and subcortical structures. In vivo electrophysiological studies have reported that, after 2–3 weeks of cocaine withdrawal, membrane bistability normally observed in pyramidal cells is reduced (Trantham et al., 2002). Peterson et al. (2000) have shown that cortical neurons recorded from amphetamine-pretreated rats and withdrawn for 3 d show increased responsiveness to glutamate and decreased responsiveness to dopamine, suggesting that the medial PFC (mPFC) is transiently hyperexcitable during amphetamine withdrawal. Recently, Goto and Grace (2005) reported that repeated cocaine administration disrupted the synaptic plasticity at hippocampal and prefrontal cortical inputs to the nucleus accumbens, and Brady et al. (2005) have shown that repeated methamphetamine administration can disrupt excitatory synaptic interactions in the nucleus accumbens. These studies, performed in intact, anesthetized preparations, indicate that, independently of the psychostimulant used, repeated treatment elicits long-term changes in cortical and subcortical activity.
In the present study, we assessed the effects of DA D1 and D2 receptor antagonists on the current-evoked and spontaneous excitability of pyramidal cells recorded in rats treated repeatedly with cocaine or saline and withdrawn for 2–4 weeks. Moreover, we evaluated the cortical responses to electrical stimulation delivered into the VTA. We hypothesized that repeated cocaine treatment followed by a relatively longer withdrawal period produces a long-term reduction in cortical excitability and will disrupt DAergic modulation of PFC. This hypothesis was evaluated using in vivo intracellular recordings from PFC pyramidal neurons in anesthetized rats.
Materials and Methods
Animal preparation.
All animals were handled in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals published by the US Public Health Service, and the Medical University of South Carolina Animal Care and Use Committees approved the specific protocol. Subjects were male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA), weighing 200–250 g at the start of the experiment. Animals were housed in pairs in a temperature-controlled colony room on a 12 h light/dark cycle (lights on at 7:00 A.M.), and food and water were available ad libitum. Animals were allowed to acclimatize to the colony room for 3–5 d after their arrival. Cocaine hydrochloride was donated by National Institute on Drug Abuse (Baltimore, MD). Two groups of rats were used in the present study: a saline group, that received daily intraperitoneal injections of saline for 7 d, and a group that was injected daily for 7 d with cocaine using a dosing regimen described previously to produce locomotor sensitization (Pierce et al., 1996; Bowers et al., 2004). On the first and last days of cocaine administration, the rats were injected intraperitoneally with 15 mg/kg cocaine. On the intervening days, the animals were injected intraperitoneally with 30 mg/kg cocaine.
Intracellular recordings in vivo.
Rats were anesthetized with a mixture of ketamine/xylazine (30 and 25 mg/kg, i.p., respectively) and placed in a stereotaxic apparatus. The cisterna magna was drained, and a hole was drilled over the PFC [coordinates from bregma: anteroposterior (AP), 3.2 mm; lateral (L), ±0.6 mm; ventral (V), 4–5.0 mm at 10° inclination] (Paxinos and Watson, 1998) and VTA (from bregma: AP, −4.9 mm; L, ±0.5 mm; V, 7.7 mm). Intracellular microelectrodes were pulled from Omegadot tubing (outer diameter, 1.5 mm; World Precision Instruments, Sarasota, FL). The electrodes were filled with 3 m potassium acetate (electrode resistance, 25–40 MΩ in situ). Impalements were defined as stable if the resting membrane potential was more negative than −55 mV and the action potential amplitude was at least 50 mV. A head-stage amplifier connected to a preamplifier (NEURODATA IR-283; Cygnus Technology, Delaware Water Gap, PA) amplified signals. Current was injected across a bridge circuit, with electrode potentials and current injection amplitude monitored on a personal computer screen using a National Instruments (Austin, TX) board as an interface to a computer running custom Labview software. Electrical stimulation of the VTA was delivered through bipolar concentric electrodes (model SNE-10; David Kopf Instruments, Tujunga, CA) delivering a train of pulses at 20 Hz/2 s (2–6 mA). Previously, we reported the effects of the same stimulation protocol in naive rats (Lavin et al., 2005).
After successful impalement of a cell, 10 min of stable baseline recordings were obtained before experimental recordings began. The spontaneous activity of the cells was recorded in bins of 10 s, every 30 s throughout the experiment. The current-evoked excitability was recorded in bins of 1 s every 30 s throughout the experiment by passing a constant-current pulse (1 s duration) that evoked at least three action potentials during baseline recordings and maintaining that current through the rest of the experiment. Through all of the experiments, the membrane potential was monitored closely, and changes in Vm was corrected by addition of current via the recording electrode. The adjustment of membrane potential was performed only after VTA stimulation or drug administration, not during baseline.
Drugs were administered either intraperitoneally (sulpiride, 1.5 or 15 mg/kg), or intravenously (SCH 23390 [R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride], 1 or 0.1 mg/kg). The temperature of the animal was maintained at 36 ± 0.5°C through a thermostatically controlled heating pad.
Histology and statistics.
At the end of the experiments, the animals were given an overdose of anesthetic and perfused transcardially with saline, followed by 10% buffered Formalin. The brain was removed and placed in a solution of 15% sucrose at 4°C. Coronal slices, 60 μm thick, were cut using a freezing microtome and collected in phosphate buffer. The slices were stained with cresyl violet to aid in the localization of the electrode track and the stimulating electrode. The spontaneous and current-evoked firing of neurons recorded in the PFC was compared using a two-tailed, two-sample Student's t test or ANOVA with repeated measures and post hoc Fisher's test. Statistical significance was set at p < 0.05, and all results are presented as mean ± SEM.
Results
Recordings were performed in the deep layers (V–VI) of the prelimbic and infralimbic PFC in 10 rats treated with saline and in 12 rats treated with cocaine. All neurons included in data analysis exhibited stable membrane properties for at least 25 min and fulfilled criteria of resting membrane potential, amplitude, and duration of action potentials. Thirteen neurons were recorded in the saline-treated group and 23 in the cocaine-treated group. Of the 13 cells recorded in saline-treated animals, five were silent (38%) and six exhibited membrane bistability (46%), and two neurons were firing in a tonic, nonbistable manner; the average rheobase current for evoked excitability was 100 ± 18 pA. In the cocaine-treated group, 14 of 23 neurons were silent (61%), 5 of 23 (22%) exhibited membrane bistability, and 4 of 23 neurons were firing in a tonic, nonbistable manner. The average rheobase current for evoked excitability was 120 ± 15 pA. Repeated cocaine administration did not significantly affect spike threshold (saline, −49.1 ± 3.2 mV; cocaine, −50.9 ± 3.4 mV), spike amplitude (saline, 61.1 ± 4.3 mV; cocaine, 58 ± 3.3 mV), or input resistance (saline, 71.3 ± 11 MΩ; cocaine, 60 ± 14.1 mV)
Effect of DA receptor antagonists on cortical excitability
In the absence of VTA stimulation, previous microdialysis studies reveal an in vivo level of extracellular DA in the 0.5–5.0 nm range (Hedou et al., 2001; Nakamura et al., 2001; Marsteller et al., 2002; Phillips et al., 2004). Modulation of the excitability of PFC neurons by this tonic background DA was revealed by recording the basal current-evoked excitability of the cell every 30 s for at least 10 min, followed by systemic administration of either D1 (SCH 23390) or D2 (sulpiride) receptor antagonists and subsequent measurement of current-evoked excitability every 30 s for at least another 10 min.
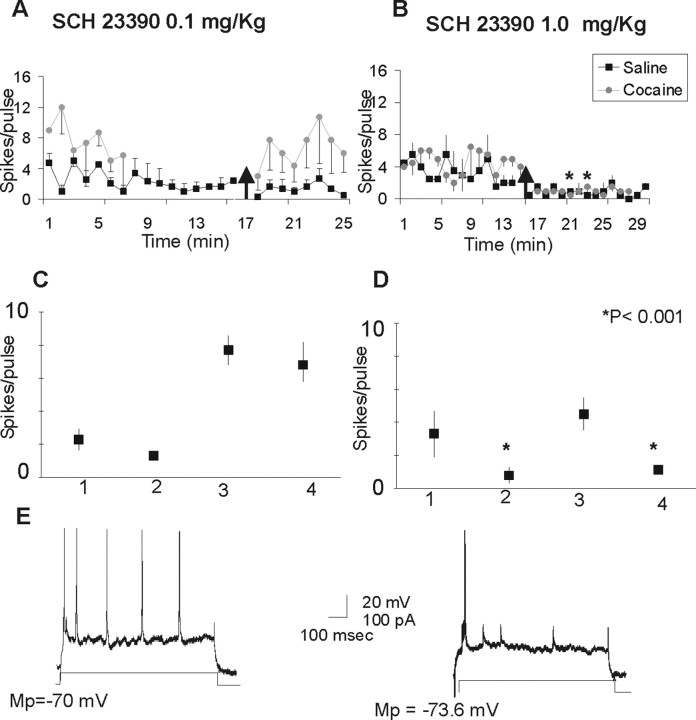
Administration of low doses of SCH 23390 (0.1 mg/kg) elicited a nonsignificant decrease in the current-evoked excitability of cortical cells recorded in saline- and cocaine-treated animals (F(1,13) = 3.1; p < 0.100) (Fig. 1A). There was a difference in the baseline firing between cocaine- and saline-treated animals for this particular group, but the results in both sets of animals showed the same trend.
Figure 1.
Effects of the D1 receptor antagonist SCH 23390 in the current-evoked excitability of PFC neurons. A, Systemic administration of a low dose of SCH 23390 (0.1 mg/kg) produces a nonsignificant decrease in the current-evoked excitability of cortical cells recorded in saline-treated (n = 4) and cocaine-treated (n = 5) animals. Data are shown as mean ± SEM per pulse. The arrow indicates administration of the drug. B, Systemic administration of SCH 23390 at 1.0 mg/kg produces a significant decrease in the current-evoked excitability of cortical cells recorded in saline-treated (n = 5) and cocaine-treated (n = 8) animals. Black squares, Saline animals; gray circles, cocaine animals. C, Graph summarizing the changes in current-evoked excitability elicited by SCH 23390 (0.1 mg/kg) in the saline- and cocaine-treated groups. D, Graph summarizing the changes in current-evoked excitability elicited by SCH 23390(1.0 mg/kg) in the saline- and cocaine-treated groups. 1, Saline baseline; 2, saline plus SCH23390; 3, cocaine baseline; 4, cocaine plus SCH 23390. E, Representative traces of current-evoked excitability obtained in a cocaine-treated animal. The left trace illustrates the current-evoked spikes per pulse during a baseline recording; the right trace illustrates the current-evoked spikes after SCH 23390 (1 mg/kg). Mp, Membrane potential.
Systemic administration of the selective D1 receptor antagonist SCH 23390 (1 mg/kg) produced a significant decrease in the current-evoked excitability of cortical cells recorded in saline- and cocaine-treated animals. Data were evaluated using an ANOVA with repeated measures; the between-group factor was group (saline vs cocaine), and the repeated measure was treatment (baseline vs SCH 23390). The ANOVA revealed a main effects of group (F(1,24) = 4.5; p = 0.04) and SCH 23390 treatment (F(1,24) = 126.9; p < 0.0001) on intrinsic excitability; the group × treatment interaction reached the trend level (F(1,24) = 3.2; p = 0.08). The main effect of treatment indicated that SCH 23390 application reduced intrinsic excitability in both the control and cocaine groups (Fig. 1B).
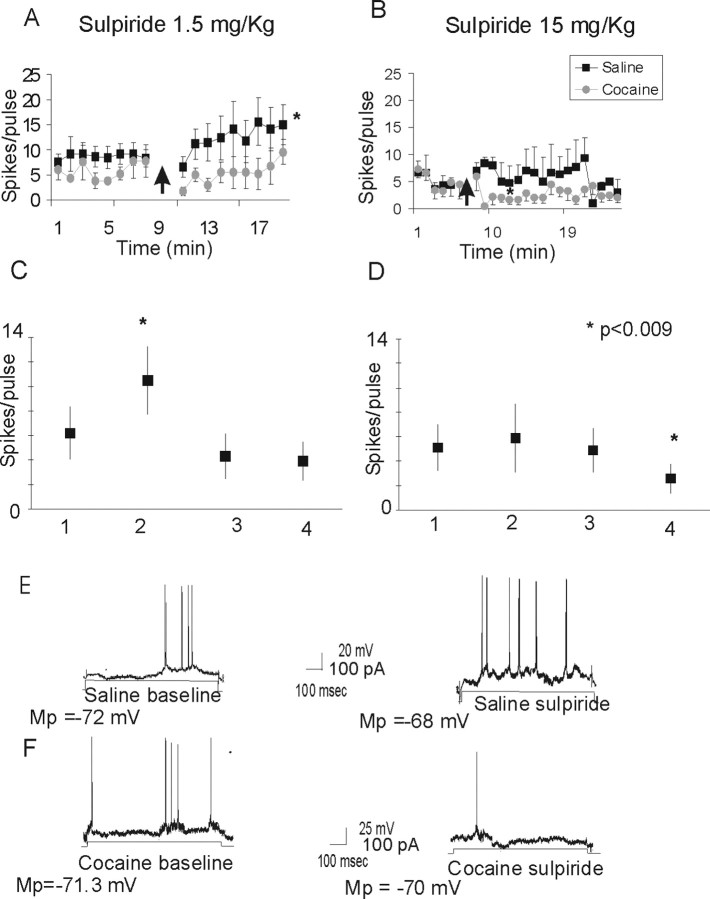
Administration of the D2 receptor antagonist sulpiride (1.5 mg/kg) elicited a significant increase in the current-evoked excitability in the saline-treated group and no effect in the cocaine-treated group (Fig. 2A). A repeated-measures ANOVA revealed main effects of group (F(1,22) = 24.7; p < 0.0001), sulpiride treatment (F(1,22) = 5.7; p = 0.03), and a group × treatment interaction (F(1,22) = 3.9; p = 0.05). Post hoc analyses designed to decompose the interaction revealed a significant difference between cocaine and saline subjects in response to sulpiride (p < 0.001). The different results obtained with sulpiride in the saline- and cocaine-treated groups were not the product of recording some unusually excitable cells in the saline group, because sulpiride increased the average number of spikes in five of seven neurons by 86.6% and slightly decreased excitability in two of seven neurons by 35.6%. Higher doses of sulpiride (15 mg/kg) elicited an increase in the current-evoked excitability in the saline-treated group. In contrast, the excitability decreased in response to sulpiride in the cocaine group (F(1,21) = 8.1; p < 0.009) (Fig. 2B).
Figure 2.
Effects of the D2 receptor antagonist sulpiride in the current-evoked excitability of PFC neurons. A, Systemic administration of sulpiride (1.5 mg/kg) produce a significant increase in the current-evoked excitability of PFC neurons recorded in saline-treated animals (n = 4), but it did not affect the excitability of cortical cells in cocaine-treated animals (n = 5). The arrow indicates administration of the drug. B, High doses of sulpiride (15 mg/kg) produce an increase in the current-evoked excitability of PFC neurons recorded in saline animals (n = 3). However, in cocaine animals (n = 5), sulpiride (15 mg/kg) elicited a significant decrease in current-evoked excitability. C, Graph summarizing the changes in current-evoked excitability elicited by sulpiride (1.5 mg/kg) in the saline- and cocaine-treated groups. D, Graph summarizing the changes in current-evoked excitability elicited by systemic administration of higher doses of sulpiride (15 mg/kg) in the saline- and cocaine-treated groups. 1, Saline baseline; 2, saline plus sulpiride; 3, cocaine baseline; 4, cocaine plus sulpiride. E, Representative trace of the current-evoked excitability in a saline-treated animal during baseline recording (left trace) and after administration of sulpiride (right trace). F, Representative trace of the current-evoked excitability elicited in a cocaine-treated animal during baseline recording (left trace) and after sulpiride administration (right trace). Mp, Membrane potential.
Effects of VTA stimulation on current-evoked excitability
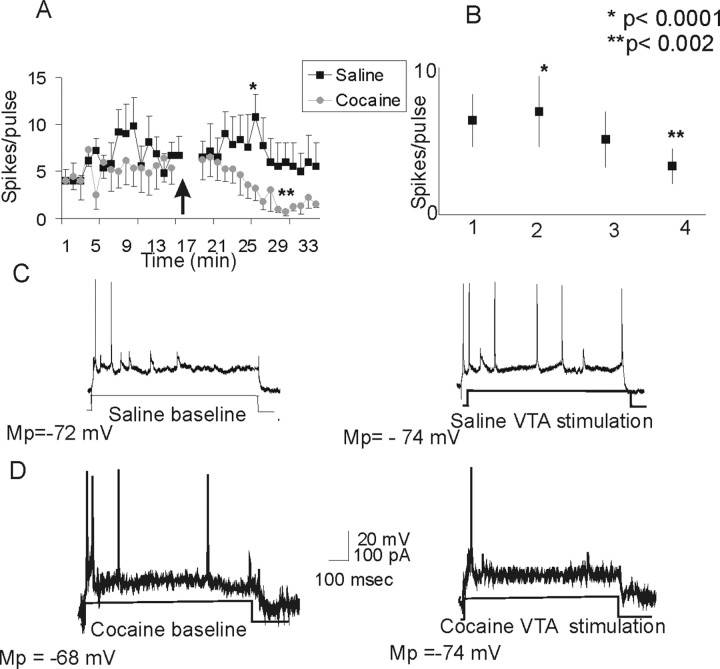
Similar to reports in drug-naive animals (Lavin et al., 2005), a train of stimulation delivered into the VTA induced a long-lasting increase in the current-evoked excitability of PFC neurons in the saline-treated group (Fig. 3). Whereas saline-exposed rats showed an increase in current-evoked excitability after VTA stimulation, cocaine-treated animals showed a significant decrease in excitability after stimulation (p < 0.002) (Fig. 3). A repeated-measures ANOVA revealed a significant effect of group (F(1,30) = 11.2; p < 0.0022) and an interaction between group and treatment (F(1,30) = 23.8; p < 0.0001).
Figure 3.
Electrical stimulation of the VTA differentially affects current-evoked cortical excitability in saline- and cocaine-treated animals. A, Stimulation of the VTA with a train of pulses (20 Hz/2 s) induced a significant long-lasting increase in cortical excitability in saline-treated rats (p < 0.0001; n = 4). However, the same protocol induced a significant decrease in cortical excitability in the cocaine-treated group (p < 0.002; n = 8). The arrow indicates stimulation of the VTA. B, Graph depicting the average changes in excitability elicited by VTA stimulation in the saline- and cocaine-treated groups. 1, Saline baseline; 2, saline plus VTA stimulation; 3, cocaine baseline; 4, cocaine plus VTA stimulation. C, Representative traces from a saline-treated animal illustrating the increase in the number of current-evoked spikes after VTA stimulation. D, Representative traces from a cocaine-treated animal illustrating the decrease in the number of current-evoked spikes after VTA stimulation. Mp, Membrane potential.
Effects of VTA stimulation on spontaneous activity
Akin to reports in the literature using extracellular unit recordings in naive animals (Bunney and Aghajanian, 1979; Sesack and Bunney, 1989), VTA stimulation reduced the spontaneous firing frequency of PFC neurons in both saline- and cocaine-treated animals. An ANOVA revealed a significant effect of VTA stimulation (F(1,26) = 5.7; p < 0.002) (Fig. 4), but there was no significant effect of cocaine or interaction between cocaine and VTA stimulation.
Figure 4.
Electrical stimulation of the VTA elicited similar effects in spontaneous activity in saline- and cocaine-treated rats. A, Stimulation of the VTA with a train of pulses (20 Hz/2 s) induced a decrease in the spontaneous activity of cortical cells recorded in saline-treated (n = 8) and cocaine-treated (n = 9) animals. The arrow indicates stimulation of the VTA. B, Graph summarizing the changes in spontaneous excitability in the saline- and cocaine-treated groups after VTA stimulation. 1, Saline baseline; 2, saline plus VTA stimulation; 3, cocaine baseline; 4, cocaine plus VTA stimulation. C, Representative trace of spontaneous activity in a saline-treated rat during baseline recordings (left trace) and after VTA stimulation (right trace). D, Representative trace of spontaneous activity in a cocaine-treated rat during baseline recordings (left trace) and after VTA stimulation (right trace). Mp, Membrane potential.
Discussion
Our results indicate a lack of responsivity to D2 modulation of current-evoked activity in cortical pyramidal cells after 2–4 weeks of withdrawal from repeated treatment with cocaine. Furthermore, contrary to what has been found in naive and in saline-treated animals, stimulation of the VTA decreased current-evoked excitability in cocaine-treated rats. These data suggest that repeated cocaine administration alters intrinsic currents in pyramidal neurons and also alters dopaminergic signaling in the PFC.
Effects of D1 and D2 receptor antagonists on cortical cell activity
In saline- and cocaine-treated rats, administration of the selective D1 receptor antagonist SCH 23390 decreased current-evoked excitability. This effect of the D1 receptor antagonist on PFC activity has been demonstrated previously in naive animals (Yang and Seamans, 1996; Shi et al., 1997; Henze et al., 2000; Lavin and Grace, 2001; Wang and O'Donnell, 2001; Lavin et al., 2005). Our results show that saline- and cocaine-treated animals exhibit a reduction in current-evoked firing after systemic administration of D1 receptor antagonists, suggesting that neither D1 receptor responsivity nor DA action on D1 receptors is affected by withdrawal from chronic cocaine. It is important to note that, in a group of experiments, we used intravenous administration of SCH 23390 at a dose of 1.0 mg/kg. Although this dose has been widely used in electrophysiological and behavioral experiments assessing D1 receptor activity, it has also been reported to interact with serotonin receptors (Lewis and O'Donnell, 2000; Brady and O'Donnell, 2004; Daniela et al., 2004). Therefore, we also tested SCH 23390 at doses of 0.1 mg/kg intravenously. It was found that, in saline- and cocaine-treated animals, lower doses of SCH 23390 decrease current-evoked excitability.
Our experiments show that cocaine pretreatment abolished the effects of the D2 antagonist sulpiride. In saline-treated rats as well as in naive animals (Lavin et al., 2005), systemic administration of the selective D2 receptor sulpiride increased the current-evoked firing of cortical cells. In contrast, sulpiride failed to increase cortical excitability in cocaine-withdrawn animals. Indeed, even when higher doses of sulpiride (15 mg/kg) were tested, the D2 antagonist failed to induce an increase in the current-evoked excitability of cortical neurons. The relatively pronounced effect of cocaine on D2 versus D1 antagonists is consistent with a recent report showing that withdrawal from cocaine markedly upregulates the activator of G-protein signaling AGS3 (Bowers et al., 2004). The upregulation of AGS3 selectively limits D2 signaling via Gi-coupled receptors without affecting D1 Gs signaling (Blumer et al., 2002; Bowers et al., 2004), and, after withdrawal from chronic cocaine, the upregulation of AGS3 in the PFC inhibited D2 agonist-induced GTPγS binding (Bowers et al., 2004).
A sizable body of evidence describes the fundamental role of PFC D2 receptors in cocaine addiction. Beyer and Steketee (2002) demonstrated that intra-mPFC injections of the D2 receptor agonist quinpirole blocked cocaine-induced sensitization. Moreover, in studies with human addicts, Volkow et al. (2001) reported lower levels of D2 receptor availability in the orbitofrontal cortex, suggesting that dysregulation of this type of receptor could underlie the loss of control and compulsive drug intake in drug addicts.
Although we have interpreted our findings in terms of direct effects by DA antagonists in the PFC, it is important to be mindful that, in these experiments, the DAergic antagonists were injected systemically. Thus, DA receptors in other parts of the corticolimbic circuit in which the PFC is embedded were also blocked, and a definitive assertion that DA receptors in PFC were solely responsible for the observed effects cannot be made.
Repeated treatment with cocaine depresses evoked cortical excitability
A train of stimuli delivered into the VTA induced a long-lasting increase in current-evoked excitability coupled to a decrease in spontaneous activity in the PFC of saline-treated or drug-naive rats (Lavin et al., 2005). This is consistent with both in vitro intracellular/patch-clamp data showing a DA-mediated increase in current-evoked excitability and in vivo extracellular recordings revealing a DA-dependent reduction in spontaneous firing (for review, see Seamans and Yang, 2004). This opposing action on spontaneous versus current-evoked firing is presumably attributable to the modulation of multiple PFC currents by DA and was directly predicted by network models incorporating such complex modulations (Durstewitz et al., 2000). Our results show that cocaine treatment abolished the VTA-mediated increase in current-evoked excitability but did not affect the VTA-mediated decrease in spontaneous activity. These results suggest that, besides affecting responsivity to D2 receptor antagonists, cocaine treatment is affecting intrinsic currents in cortical cells. Arguing against this possibility, recent studies in dissociated PFC pyramidal cells after withdrawal from cocaine demonstrate increased excitability as a result of a decreased voltage-gated and inwardly rectifying Ik and enhanced L-type ICa2+ (Dong et al., 2005; Nasif et al., 2005a). Moreover, Nasif et al. (2005b) showed that repeated cocaine administration increased the membrane excitability of PFC neurons in slices. The apparent contradictory findings on excitability between recordings in vitro and in vivo suggest that cocaine-induced neuroadaptations in the circuit in which the PFC pyramidal cells are embedded are critically affecting excitability. Moreover, our experiments were performed after 2 weeks of withdrawal, and the recordings were performed in adult animals (3 months old) versus the young animals used in brain slices or dissociated cell preparations (4–6 weeks old). As indicated herein, altered signaling through D1 versus D2 constitutes one cocaine-induced adaptation that could alter excitability in pyramidal cells.
Conclusions
The balance between D1 and D2 receptors contributes to optimal cortical function. The reduction in responsiveness to D2 receptor antagonists in cocaine-withdrawn animals is consistent with previously reported increases in AGS3 content in the PFC of cocaine withdrawn rats that selectively reduce GTPγS binding induced by D2 but not D1 receptors (Bowers et al., 2004). Also, these findings are consistent with the reduction in cortical D2 receptors and the overall prefrontal cortical hypoactivity reported to occur in cocaine addicts (Grant et al., 2000; Goldstein and Volkow, 2002). Together, these data indicate that the dysfunction of the PFC implicated in psychostimulant addiction (Jentsch and Taylor, 1999; Berke and Hyman, 2000; Kalivas et al., 2005) may in part result from reduced D2 signaling and reduced excitability of pyramidal cells. Future studies will have to be performed in rats trained to self-administer cocaine to investigate the contributions of contingent versus noncontingent drug administration, as well as in animals undergoing a broader range of withdrawal periods.
Footnotes
This work was supported by National Institute on Drug Abuse Grants 14698 (A.L.) and 1 P50-DA15369 (P.W.K.) and National Institutes of Health Grant C06 RR015455. We thank Laurence Neely and Joe Vuthiganon for technical assistance.
References
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Beyer CE, Steketee JD. Cocaine sensitization: modulation by dopamine D2 receptors. Cereb Cortex. 2002;12:526–535. doi: 10.1093/cercor/12.5.526. [DOI] [PubMed] [Google Scholar]
- Blumer JB, Chandler LJ, Lanier SM. Expression analysis and subcellular distribution of the two G-protein regulators AGS3 and LGN indicate distinct functionality. Localization of LGN to the midbody during cytokinesis. J Biol Chem. 2002;277:15897–15903. doi: 10.1074/jbc.M112185200. [DOI] [PubMed] [Google Scholar]
- Bowers MS, McFarland K, Lake RW, Peterson YK, Lapish CC, Gregory ML, Lanier SM, Kalivas PW. Activator of G protein signaling 3: a gatekeeper of cocaine sensitization and drug seeking. Neuron. 2004;42:269–281. doi: 10.1016/s0896-6273(04)00159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady AM, O'Donnell P. Dopaminergic modulation of prefrontal cortical input to nucleus accumbens neurons in vivo. J Neurosci. 2004;24:1040–1049. doi: 10.1523/JNEUROSCI.4178-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady AM, Glick SD, O'Donnell P. Selective disruption of nucleus accumbens gating mechanisms in rats behaviorally sensitized to methamphetamine. J Neurosci. 2005;25:6687–6695. doi: 10.1523/JNEUROSCI.0643-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney BS, Aghajanian GK. Electrophysiological studies of dopamine-innervated cells in the frontal cortex. Adv Biochem Psychopharmacol. 1977;16:65–70. [PubMed] [Google Scholar]
- Daniela E, Brennan K, Gittings D, Hely L, Schenk S. Effect of SCH 23390 on (±)-3,4-methylenedioxymethamphetamine hyperactivity and self-administration in rats. Pharmacol Biochem Behav. 2004;77:745–750. doi: 10.1016/j.pbb.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Dong Y, Nasif FJ, Tsui JJ, Ju WY, Cooper DC, Hu XT, Malenka RC, White FJ. Cocaine-induced plasticity of intrinsic membrane properties in prefrontal cortex pyramidal neurons: adaptations in potassium currents. J Neurosci. 2005;25:936–940. doi: 10.1523/JNEUROSCI.4715-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. J Neurophysiol. 2000;83:1733–1750. doi: 10.1152/jn.2000.83.3.1733. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. Neuron. 2005;47:255–266. doi: 10.1016/j.neuron.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Grace AA. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction. 2000;95(Suppl 2):S119–S128. doi: 10.1080/09652140050111690. [DOI] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38:1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Hedou G, Homberg J, Feldon J, Heidbreder CA. Expression of sensitization to amphetamine and dynamics of dopamine neurotransmission in different laminae of the rat medial prefrontal cortex. Neuropharmacology. 2001;40:366–382. doi: 10.1016/s0028-3908(00)00174-x. [DOI] [PubMed] [Google Scholar]
- Henze DA, Gonzalez-Burgos GR, Urban NN, Lewis DA, Barrionuevo G. Dopamine increases excitability of pyramidal neurons in primate prefrontal cortex. J Neurophysiol. 2000;84:2799–2809. doi: 10.1152/jn.2000.84.6.2799. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Lavin A, Grace AA. Stimulation of D1-type dopamine receptors enhances excitability in prefrontal cortical pyramidal neurons in state-dependent manner. J Neurosci. 2001;104:335–346. doi: 10.1016/s0306-4522(01)00096-3. [DOI] [PubMed] [Google Scholar]
- Lavin A, Nogueira L, Lapisch C, Wightman M, Phillips P, Seamans JK. Mesocortical dopamine neurons operate in distinct temporal domains using multimodal signaling. J Neurosci. 2005;25:5013–5023. doi: 10.1523/JNEUROSCI.0557-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BL, O'Donnell P. Ventral tegmental area afferents to the prefrontal cortex maintain membrane potential “up” states in pyramidal neurons via D(1) dopamine receptors. Cereb Cortex. 2000;10:1168–1175. doi: 10.1093/cercor/10.12.1168. [DOI] [PubMed] [Google Scholar]
- Marsteller DA, Gerasimov MR, Schiffer WK, Geiger JM, Barnett CR, Borg JS, Scott S, Ceccarelli J, Volkow ND, Molina PE, Alexoff DL, Dewey SL. Related acute handling stress modulates methylphenidate-induced catecholamine overflow in the medial prefrontal cortex. Neuropsychopharmacology. 2002;27:163–170. doi: 10.1016/S0893-133X(02)00288-9. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Shirane M, Koshikawa N. Site-specific activation of dopamine and serotonin transmission by aniracetam in the mesocorticolimbic pathway of rats. Brain Res. 2001;897:82–92. doi: 10.1016/s0006-8993(01)02096-0. [DOI] [PubMed] [Google Scholar]
- Nasif FJ, Hu XT, White FJ. Repeated cocaine administration increases voltage-sensitive calcium currents in response to membrane depolarization in medial prefrontal cortex pyramidal neurons. J Neurosci. 2005a;25:3674–3679. doi: 10.1523/JNEUROSCI.0010-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasif FJ, Sidiropoulou K, Hu XT, White FJ. Repeated cocaine administration increases membrane excitability of pyramidal neurons in the rat medial prefrontal cortex. J Pharmacol Exp Ther. 2005b;312:1305–1313. doi: 10.1124/jpet.104.075184. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 4. San Diego: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Peterson JD, Wolf ME, White FJ. Altered responsiveness of medial prefrontal cortex neurons to glutamate and dopamine after withdrawal from repeated amphetamine treatment. Synapse. 2000;36:342–344. doi: 10.1002/(SICI)1098-2396(20000615)36:4<342::AID-SYN11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Ahn S, Floresco SB. Magnitude of dopamine release in medial prefrontal cortex predicts accuracy of memory on a delayed response task. J Neurosci. 2004;24:547–553. doi: 10.1523/JNEUROSCI.4653-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Bunney BS. Pharmacological characterization of the receptor mediating electrophysiological responses to dopamine in the rat medial prefrontal cortex: a microiontophoretic study. J Pharmacol Exp Ther. 1989;248:1323–1333. [PubMed] [Google Scholar]
- Shi WX, Zheng P, Liang XF, Bunney BS. Characterization of dopamine-induced depolarization of prefrontal cortical neurons. Synapse. 1997;26:415–422. doi: 10.1002/(SICI)1098-2396(199708)26:4<415::AID-SYN9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Trantham H, Szumlinski KK, McFarland K, Kalivas PW, Lavin A. Repeated cocaine administration alters the electrophysiological properties of prefrontal cortical neurons. Neuroscience. 2002;113:749–753. doi: 10.1016/s0306-4522(02)00246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Wang J, O'Donnell P. D1 dopamine receptors potentiate NMDA-mediated excitability increase in layer V prefrontal cortical pyramidal neurons. Cereb Cortex. 2001;11:452–462. doi: 10.1093/cercor/11.5.452. [DOI] [PubMed] [Google Scholar]
- Yang CR, Seamans JS. Dopamine D1 receptor actions in layer V–VI rat prefrontal cortex neurons in vitro: modulation of dendritic–somatic signal integration. J Neurosci. 1996;16:1922–1935. doi: 10.1523/JNEUROSCI.16-05-01922.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]