Abstract
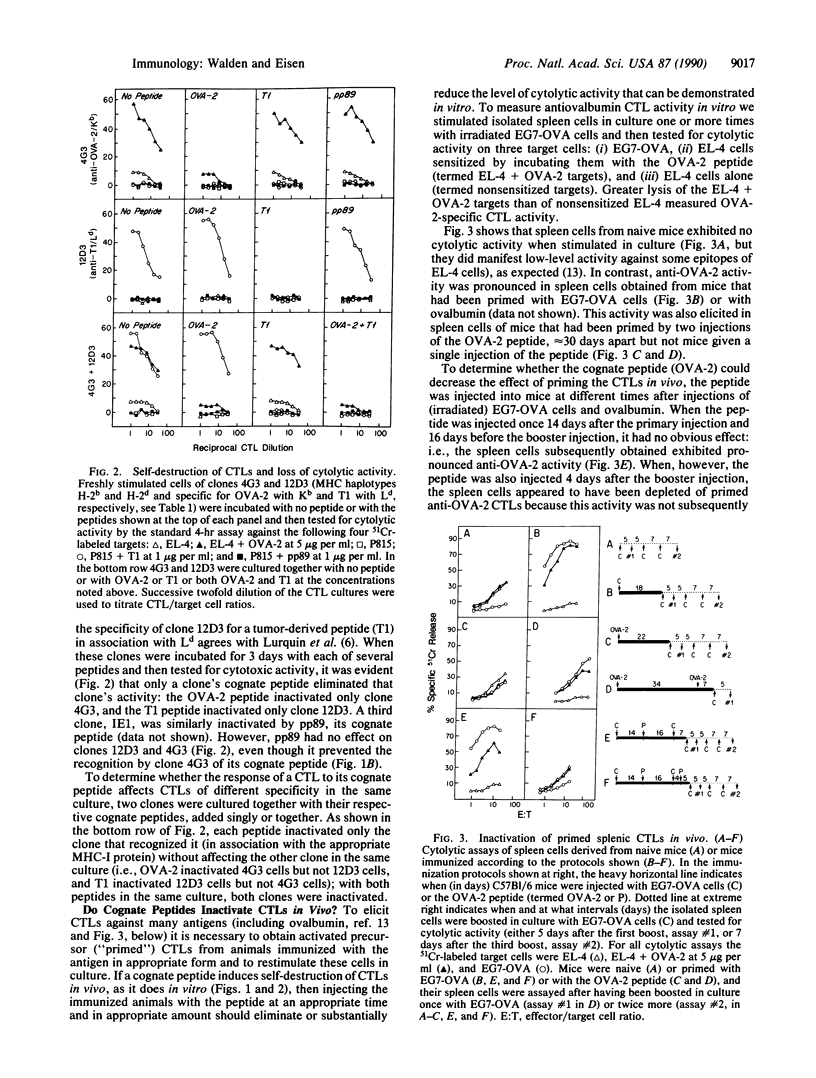
Cytotoxic T lymphocytes (CTLs) have been shown to be relatively resistant to cytolytic attack by other CTLs. We show here, however, that cloned CTLs, in the absence of other cells, are destroyed by exposure to their cognate peptides (defined as those that in association with major histocompatibility complex proteins are recognized by the antigen-specific receptor of the T cell). Destruction is proportional to peptide concentration and can be prevented by a second peptide that competes with the cognate peptide for presentation by the class I major histocompatibility complex proteins of the CTLs. The speed and extent of peptide-induced changes in the appearance of CTLs suggest that the destruction may be due primarily to self-recognition and self-destruction of individual CTLs (suicide) rather than to the destruction of some CTLs by others of the same clone in the same culture (fratricide). This effect may also take place in vivo because the appropriately timed injection of a cognate peptide into ovalbumin-immunized mice appeared to deplete their spleens of primed anti-ovalbumin CTLs. The results point to a possible physiologic mechanism for postthymic elimination of cytolytic T cells that recognize their own peptides in association with their own major histocompatibility complex protein. The results also raise the possibility that cognate peptides might eventually prove therapeutically useful for eliminating CTL clones that cause pathological cell destruction, as in some autoimmune diseases and some viral infections.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aichele P., Hengartner H., Zinkernagel R. M., Schulz M. Antiviral cytotoxic T cell response induced by in vivo priming with a free synthetic peptide. J Exp Med. 1990 May 1;171(5):1815–1820. doi: 10.1084/jem.171.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allbritton N. L., Verret C. R., Wolley R. C., Eisen H. N. Calcium ion concentrations and DNA fragmentation in target cell destruction by murine cloned cytotoxic T lymphocytes. J Exp Med. 1988 Feb 1;167(2):514–527. doi: 10.1084/jem.167.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. J. Immunology. Stimulating killer cells. Nature. 1989 Nov 30;342(6249):478–479. doi: 10.1038/342478a0. [DOI] [PubMed] [Google Scholar]
- Blakely A., Gorman K., Ostergaard H., Svoboda K., Liu C. C., Young J. D., Clark W. R. Resistance of cloned cytotoxic T lymphocytes to cell-mediated cytotoxicity. J Exp Med. 1987 Oct 1;166(4):1070–1083. doi: 10.1084/jem.166.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer H. C., Bastin J. M., Askonas B. A., Townsend A. R. Influenza-specific cytotoxic T-cell recognition is inhibited by peptides unrelated in both sequence and MHC restriction. Immunology. 1989 Feb;66(2):163–169. [PMC free article] [PubMed] [Google Scholar]
- Carbone F. R., Bevan M. J. Induction of ovalbumin-specific cytotoxic T cells by in vivo peptide immunization. J Exp Med. 1989 Mar 1;169(3):603–612. doi: 10.1084/jem.169.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deres K., Schild H., Wiesmüller K. H., Jung G., Rammensee H. G. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature. 1989 Nov 30;342(6249):561–564. doi: 10.1038/342561a0. [DOI] [PubMed] [Google Scholar]
- Golstein P. Sensitivity of cytotoxic T cells to T-cell mediated cytotoxicity. Nature. 1974 Nov 1;252(5478):81–83. doi: 10.1038/252081a0. [DOI] [PubMed] [Google Scholar]
- Henkart P. A. Mechanism of lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 1985;3:31–58. doi: 10.1146/annurev.iy.03.040185.000335. [DOI] [PubMed] [Google Scholar]
- Kranz D. M., Eisen H. N. Resistance of cytotoxic T lymphocytes to lysis by a clone of cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1987 May;84(10):3375–3379. doi: 10.1073/pnas.84.10.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppers R. C., Henney C. S. Evidence for direct linkage between antigen recognition and lytic expression in effector T cells. J Exp Med. 1976 Mar 1;143(3):684–689. doi: 10.1084/jem.143.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Skidmore B. J., Green N., Chiller J. M., Feldmann M. Induction of tolerance in influenza virus-immune T lymphocyte clones with synthetic peptides of influenza hemagglutinin. J Exp Med. 1983 May 1;157(5):1434–1447. doi: 10.1084/jem.157.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurquin C., Van Pel A., Mariamé B., De Plaen E., Szikora J. P., Janssens C., Reddehase M. J., Lejeune J., Boon T. Structure of the gene of tum- transplantation antigen P91A: the mutated exon encodes a peptide recognized with Ld by cytolytic T cells. Cell. 1989 Jul 28;58(2):293–303. doi: 10.1016/0092-8674(89)90844-1. [DOI] [PubMed] [Google Scholar]
- Maryanski J. L., Boon T. Immunogenic variants obtained by mutagenesis of mouse mastocytoma P815. IV. Analysis of variant-specific antigens by selection of antigen-loss variants with cytolytic T cell clones. Eur J Immunol. 1982 May;12(5):406–412. doi: 10.1002/eji.1830120509. [DOI] [PubMed] [Google Scholar]
- Moore M. W., Carbone F. R., Bevan M. J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988 Sep 9;54(6):777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- Ottenhoff T. H., Mutis T. Specific killing of cytotoxic T cells and antigen-presenting cells by CD4+ cytotoxic T cell clones. A novel potentially immunoregulatory T-T cell interaction in man. J Exp Med. 1990 Jun 1;171(6):2011–2024. doi: 10.1084/jem.171.6.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton R. M., Wraith D. C., Askonas B. A. Influenza peptide-induced self-lysis and down-regulation of cloned cytotoxic T cells. Immunology. 1990 Jun;70(2):223–229. [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Konigsberg P. J. Cytolytic T cell granules. Isolation, structural, biochemical, and functional characterization. J Exp Med. 1984 Sep 1;160(3):695–710. doi: 10.1084/jem.160.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poenie M., Tsien R. Y., Schmitt-Verhulst A. M. Sequential activation and lethal hit measured by [Ca2+]i in individual cytolytic T cells and targets. EMBO J. 1987 Aug;6(8):2223–2232. doi: 10.1002/j.1460-2075.1987.tb02494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase M. J., Rothbard J. B., Koszinowski U. H. A pentapeptide as minimal antigenic determinant for MHC class I-restricted T lymphocytes. Nature. 1989 Feb 16;337(6208):651–653. doi: 10.1038/337651a0. [DOI] [PubMed] [Google Scholar]
- Reddehase M. J., Zawatzky R., Weiland F., Bühring H. J., Mutter W., Koszinowski U. H. Stable expression of clonal specificity in murine cytomegalovirus-specific large granular lymphoblast lines propagated long-term in recombinant interleukin 2. Immunobiology. 1987 Aug;174(4-5):420–431. doi: 10.1016/s0171-2985(87)80015-3. [DOI] [PubMed] [Google Scholar]
- Shi Y. F., Szalay M. G., Paskar L., Sahai B. M., Boyer M., Singh B., Green D. R. Activation-induced cell death in T cell hybridomas is due to apoptosis. Morphologic aspects and DNA fragmentation. J Immunol. 1990 May 1;144(9):3326–3333. [PubMed] [Google Scholar]
- Shimojo N., Maloy W. L., Anderson R. W., Biddison W. E., Coligan J. E. Specificity of peptide binding by the HLA-A2.1 molecule. J Immunol. 1989 Nov 1;143(9):2939–2947. [PubMed] [Google Scholar]
- Skinner M., Marbrook J. The most efficient cytotoxic T lymphocytes are the least susceptible to lysis. J Immunol. 1987 Aug 15;139(4):985–987. [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Ucker D. S., Ashwell J. D., Nickas G. Activation-driven T cell death. I. Requirements for de novo transcription and translation and association with genome fragmentation. J Immunol. 1989 Dec 1;143(11):3461–3469. [PubMed] [Google Scholar]
- Verret C. R., Firmenich A. A., Kranz D. M., Eisen H. N. Resistance of cytotoxic T lymphocytes to the lytic effects of their toxic granules. J Exp Med. 1987 Nov 1;166(5):1536–1547. doi: 10.1084/jem.166.5.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello A., Heath W. R., Sherman L. A. Consequences of self-presentation of peptide antigen by cytolytic T lymphocytes. J Immunol. 1989 Sep 1;143(5):1512–1517. [PubMed] [Google Scholar]
- Wölfel T., Van Pel A., De Plaen E., Lurquin C., Maryanski J. L., Boon T. Immunogenic (tum-) variants obtained by mutagenesis of mouse mastocytoma P815. VIII. Detection of stable transfectants expressing a tum- antigen with a cytolytic T cell stimulation assay. Immunogenetics. 1987;26(3):178–187. doi: 10.1007/BF00365909. [DOI] [PubMed] [Google Scholar]