Abstract
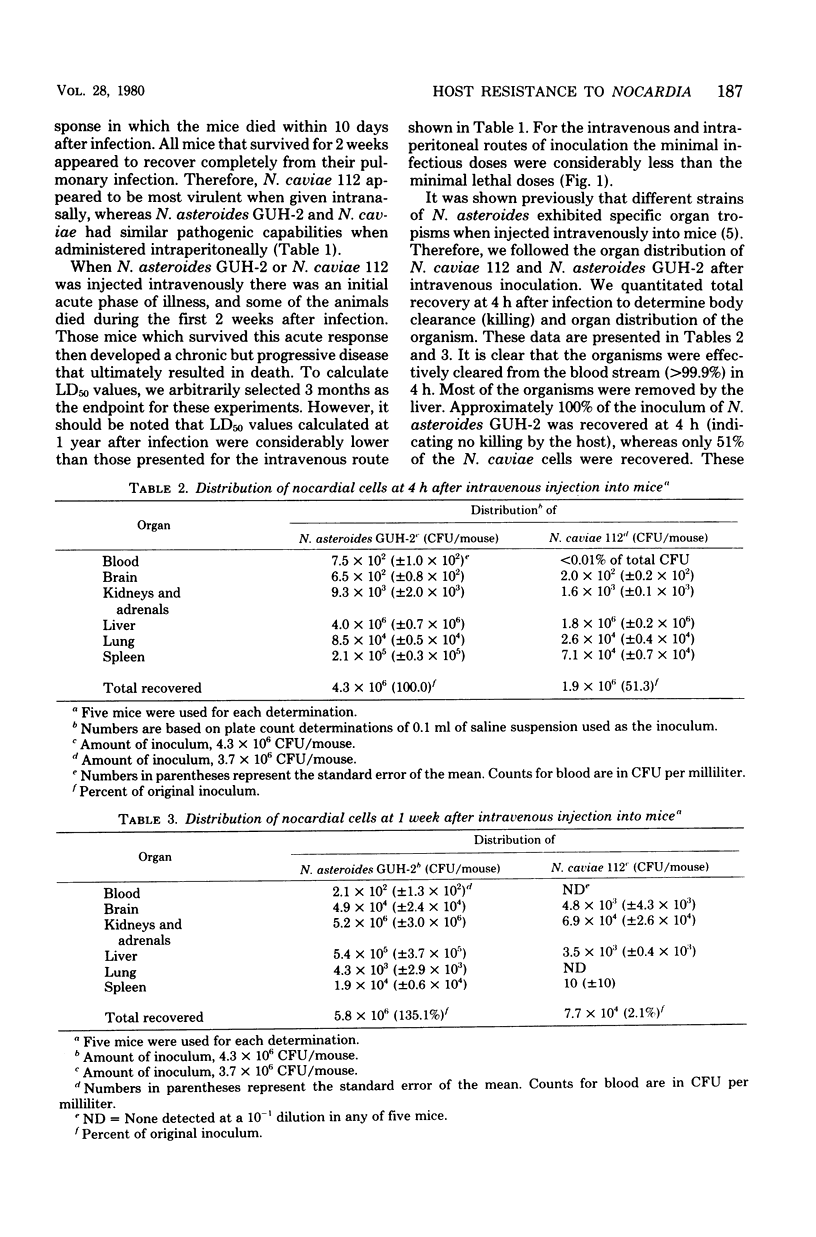
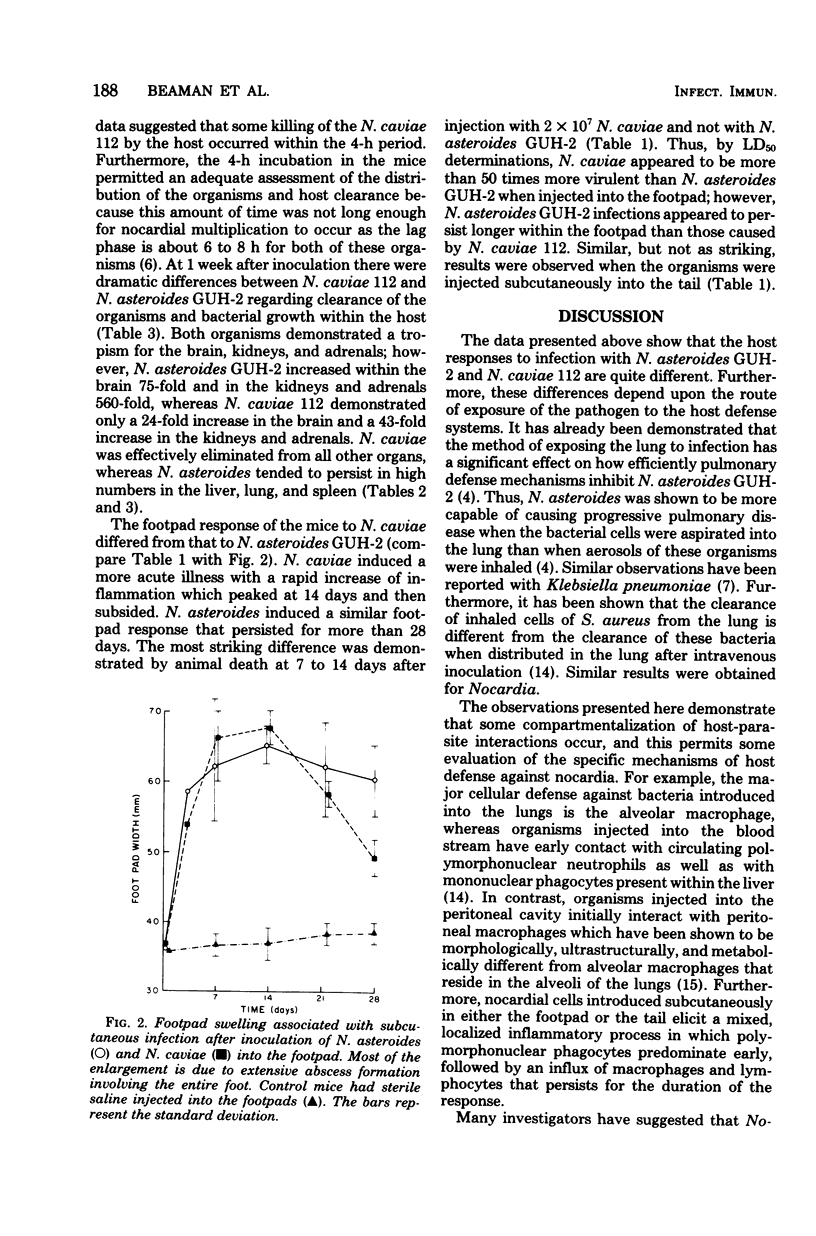
Virulent strains of Nocardia asteroides and Nocardia caviae were injected into mice by five different routes. When these organisms were grown to the same stage of growth in the same medium and otherwise prepared identically, it was found that they differed significantly in their ability to infect and kill the host, depending entirely upon the route of inoculation. Thus, N. caviae 112 was 30 times more virulent than N. asteroides GUH-2 when administered intranasally, whereas N. asteroides was at least 10 times more pathogenic than N. caviae when injected intravenously. They had similar degrees of virulence when given intraperitoneally. N. asteroides GUH-2 induced a more persistent and progressive infection than N. caviae 112 when injected into the footpads of mice; however, the latter strain was more lethal for the animals when given by this route. Different routes of infecting mice indicate a compartmentalization of the host response to different strains of nocardia. Therefore, the use of different strains of nocardia under carefully controlled and defined conditions should make it possible to dissect the nocardia-host interactions at the cellular levels.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. C., Gold O., Finland M. Activity of minocycline against Nocardia asteroides: comparison with tetracycline in agar-dilution and standard disc-diffusion tests and with sulfadiazine in an experimental infection of mice. J Lab Clin Med. 1973 May;81(5):787–793. [PubMed] [Google Scholar]
- Beaman B. L. An ultrastructural analysis of Nocardia during experimental infections in mice. Infect Immun. 1973 Nov;8(5):828–840. doi: 10.1128/iai.8.5.828-840.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Gershwin M. E., Maslan S. Infectious agents in immunodeficient murine models: pathogenicity of Nocardia asteroides in congenitally athymic (nude) and hereditarily asplenic (Dh/+) mice. Infect Immun. 1978 May;20(2):381–387. doi: 10.1128/iai.20.2.381-387.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Goldstein E., Gershwin M. E., Maslan S., Lippert W. Lung response to congenitally athymic (nude), heterozygous, and Swiss Webster mice to aerogenic and intranasal infection by Nocardia asteroides. Infect Immun. 1978 Dec;22(3):867–877. doi: 10.1128/iai.22.3.867-877.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Maslan S. Effect of cyclophosphamide on experimental Nocardia asteroides infection in mice. Infect Immun. 1977 Jun;16(3):995–1004. doi: 10.1128/iai.16.3.995-1004.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Maslan S. Virulence of Nocardia asteroides during its growth cycle. Infect Immun. 1978 Apr;20(1):290–295. doi: 10.1128/iai.20.1.290-295.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendt R. F. Relationship of method of administration to respiratory virulence of Klebsiella pneumoniae for mice and squirrel monkeys. Infect Immun. 1978 May;20(2):581–583. doi: 10.1128/iai.20.2.581-583.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folb P. I., Jaffe R., Altmann G. Nocardia asteroides and Nocardia brasiliensis infections in mice. Infect Immun. 1976 May;13(5):1490–1496. doi: 10.1128/iai.13.5.1490-1496.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez Ochoa A. Virulence of nocardiae. Can J Microbiol. 1973 Aug;19(8):901–904. doi: 10.1139/m73-144. [DOI] [PubMed] [Google Scholar]
- González-Ochoa A., Sandoval-Cuellar A. Different degrees of morbidity, in the white mouse, induced by Nocardia brasiliensis, Nocardia asteroides and Nocardia caviae. Sabouraudia. 1976 Nov;14(3):255–259. doi: 10.1080/00362177685190381. [DOI] [PubMed] [Google Scholar]
- Harrow E. M., Jakab G. J., Brody A. R., Green G. M. The pulmonary response to a bacteremic challenge. Am Rev Respir Dis. 1975 Jul;112(1):7–16. doi: 10.1164/arrd.1975.112.1.7. [DOI] [PubMed] [Google Scholar]
- Hocking W. G., Golde D. W. The pulmonary-alveolar macrophage (first of two parts). N Engl J Med. 1979 Sep 13;301(11):580–587. doi: 10.1056/NEJM197909133011104. [DOI] [PubMed] [Google Scholar]
- Krick J. A., Remington J. S. Resistance to infection with Nocardia asteroides. J Infect Dis. 1975 Jun;131(6):665–672. doi: 10.1093/infdis/131.6.665. [DOI] [PubMed] [Google Scholar]
- Kurup P. V., Randhawa H. S., Sandhu R. S., Abraham S. Pathogenicity of Nocardia caviae, N. asteroies and N. brasiliensis. Mycopathol Mycol Appl. 1970;40(2):113–130. doi: 10.1007/BF02051989. [DOI] [PubMed] [Google Scholar]
- Mason K. N., Hathaway B. M. A study of Nocardia asteroides. White mice used as test animals. Arch Pathol. 1969 Apr;87(4):389–392. [PubMed] [Google Scholar]
- Mishra S. K., Sandhu R. S., Randhawa H. S., Damodaran V. N., Abraham S. Effect of cortisone administration on experimental nocardiosis. Infect Immun. 1973 Feb;7(2):123–129. doi: 10.1128/iai.7.2.123-129.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUNYON E. H. Nocardia asteroides; studies of its pathogenicity and drug sensitivities. J Lab Clin Med. 1951 May;37(5):713–720. [PubMed] [Google Scholar]
- Smith I. M., Hayward A. H. Nocardia caviae and Nocardia asteroides: comparative bacteriological and mouse pathogenicity studies. J Comp Pathol. 1971 Jan;81(1):79–87. doi: 10.1016/0021-9975(71)90058-2. [DOI] [PubMed] [Google Scholar]
- Uesaka I., Oiwa K., Yasuhira K., Kobara Y., McClung N. M. Studies on the pathogenicity of Nocardia isolates for mice. Jpn J Exp Med. 1971 Oct;41(5):443–457. [PubMed] [Google Scholar]




