Abstract
Background
Leishmaniasis is a rapidly expanding zoonosis that shows increasing urbanization. Concern exists regarding the role of wildlife in visceral leishmaniasis (VL) transmission, due to frequent natural or anthropogenic environmental changes that facilitate contact between wildlife, humans and their pets. The municipality of Campinas, in southeastern Brazil, initially recorded VL in 2009, when the first autochthonous case was confirmed in a dog living in an upscale residential condominium, located inside an environmentally protected area (EPA). Since then, disease transmission remains restricted to dogs inhabiting two geographically contiguous condominiums within the EPA.
Methodology/Principal findings
We conducted a cross-sectional study of the VL focus to investigate Leishmania spp. infection in domestic dogs, wild mammals and sand flies using molecular tools and recommended serological techniques. Canine seroprevalences of 1.5% and 1.2% were observed in 2013 and 2015, respectively. Six insect species, confirmed or suspected vectors or potential transmitters of Leishmania, were identified. Two specimens of the main L. (L.) infantum vector in Brazil, Lutzomyia longipalpis, were captured in the EPA. Natural infection by L. (L.) infantum was recorded in one Expapillata firmatoi specimen and two Pintomyia monticola. Natural infection by L. (L.) infantum and Leishmania subgenus Viannia was also detected in two white-eared opossums (Didelphis albiventris), a known reservoir of VL. Geographical coordinates of each sampling of infected animals were plotted on a map of the EPA, demonstrating proximity between these animals, human residences, including the dogs positive for VL, and forest areas.
Conclusions/Significance
The EPA, which is inhabited by humans, has an active VL focus. The risk of establishing and maintaining disease transmission foci in similar scenarios, i.e. wild areas that undergo environmental modifications, is evident. Moreover, different epidemiological profiles of VL must be included to elaborate prevention and control measures that consider the particularities of each transmission area.
Author summary
Leishmaniasis are neglected tropical diseases that represent a major public health problem in the world. New outbreaks have been recorded in the outskirts of some cities and phlebotomine vectors show geographic expansion, potentially associated with environmental and climate changes. We investigated a recent focus of visceral leishmaniasis (VL) in dogs from two residential condominiums located in an environmentally protected area in the municipality of Campinas, São Paulo, southeastern Brazil. The purpose of the study was to investigate Leishmania (L.) infantum infection, the etiological agent of the disease, in vector insects (phlebotominae), domestic (dog) and wild hosts. Antibody prevalence was determined in 1.5% and 1.2% of dogs examined in 2013 and 2015, respectively. In addition, natural infection by the VL agent was confirmed in two species of sand flies and two white-eared opossums. All positive animals were captured near human residences and in forest areas. Scenarios like these, with changes in ecosystems and the proximity of wild and domestic animals and humans, can promote the occurrence of zoonoses. The participation of wildlife in the transmission cycle of zoonoses has assumed greater importance in recent years as a result of the consolidation of the One Health concept.
Introduction
In the last 35 years, visceral leishmaniasis (VL) has reemerged as an important public health problem in different parts of the world, with the expansion of transmission and the occurrence of new cases [1].
In the Americas, the disease occurs within a complex network of relationships involving the etiological agent, Leishmania (Leishmania) infantum (Synonym: L. chagasi) and its different populations: sand flies, mainly of the species Lutzomyia longipalpis [2]; domestic or wild vertebrates, sources of vector infection; and susceptible hosts. All of these can be present in a certain time and space, interacting with the particularities of the environment.
Although the dog is considered the main reservoir in the urban environment, it is necessary to consider the possibility of other non-human hosts participating in the maintenance of L. (L.) infantum in the endemic environment. These include several species belonging to different orders of wild and/or synanthropic animals, which are naturally infected in several regions and ecotypes [3].
In Brazil, until the mid-1980s VL was considered a disease of primarily wild and rural environments. From the late 1980s onward, epidemics have occurred in the urban environment, together with rapid expansion of transmission foci in hundreds of municipalities, including populous urban centers and several capitals [4–6]. The rural epidemiological pattern has been modified by increasing urbanization, while the geographic expansion of the disease has been observed in previously safe municipalities [7–10]. Expansion of the spaces occupied by Lu. longipalpis has also been verified in endemic urban environments in several regions of the country [11,12].
In the state of São Paulo, southeastern Brazil, VL transmission is also in full expansion. Over a period of 17 years, from 1997 to 2014, the presence of the vector Lu. longipalpis has been confirmed in 177 municipalities in the state, with 2,467 autochthonous human cases, 214 deaths and a mortality rate of 8.7% [13,14].
It is currently possible to recognize at least three different transmission patterns and epidemiological profiles of the disease in the state of São Paulo: the first occurs in 76 municipalities and involves both human and canine VL cases, besides the presence of the vector Lu. Longipalpis. The second occurs in 47 municipalities, seven of which without records of Lu. longipalpis, and involving only canine cases, with no record of human autochthony. Finally, a third pattern involves only human cases, which occurs in nine municipalities, three of which without reports of the vector [13].
Little is known of the dynamics of the expansion of new outbreaks, the environmental and epidemiological determinants of the disease, or the dispersion and behavior of vectors and hosts potentially involved in the different VL transmission cycles. What is known, however, is that increasing environmental degradation of natural and/or anthropogenic origin is a concern in several parts of the world.
The destruction or replacement of the original vegetal cover of a region can alter the composition and interaction of its fauna [15], which can lead to modifications and adaptations in the biology of vectors and hosts of diseases like leishmaniasis.
The initial VL focus in Campinas was identified in 2009, based on an autochthonous canine case reported in an environmentally protected area (EPA) within the municipality [16,17]. This area has undergone intense environmental changes since the 1970s, with deforestation for road and residential condominiums construction.
The epidemiological investigation conducted by the Municipal and State Health Department for the first case of canine VL (CVL) in 2009 determined an anti-Leishmania seroprevalence of 2.0% (4/198) in dogs and none of the 40 wild mammals captured in this occasion was positive for L. (L.) infantum by polymerase chain reaction (PCR). Sand flies were present in 16/85 (18.8%) residences and Lu. longipalpis was verified in three of the 16 (18.8%) houses with the presence of sand flies, being 22.2% of the females positive for L. (L.) infantum by PCR [17].
Since it was the first suspected case of autochthony for VL and in order to confirm the occurrence of L. (L.) infantum for the first time in this geographic area, at that occasion biological samples collected from dogs diagnosed positive in serological tests were sent to a reference laboratory, the Adolfo Lutz Institute (IAL), which confirms the occurrence of the parasite through isolation in culture medium and molecular tools.
From 2009 to 2016, no human cases have yet been diagnosed in Campinas and notification of canine cases remains geographically restricted to the two contiguous residential condominiums located inside the EPA.
In order to identify the different components involved in the transmission cycle of this CVL focus in Campinas, we conducted a broad epidemiological investigation involving the capture of sand flies and free-living wild animals in other areas of the EPA and integrated this research with the results of canine serological surveys.
Methods
Ethics statement
This research was approved by the Ethics Committee on Animal Use (ECAU) of Campinas State University (protocol no. 3296–1), the ECAU of the Adolfo Lutz Institute and the Pasteur Institute (protocol no. 01/2013) and by the Brazilian Institute of Environment and Renewable Natural Resources, through the Biodiversity Authorization and Information System (IBAMA, SISBIO, no. 42926-1/2).
Study area
The municipality of Campinas (22°53’20” S, 47°04’40” W) has approximately one million inhabitants and is located in the southeast region of São Paulo, 100 km from the state capital. In 1993, the Mayor’s Office of the municipality of Campinas delimited an area of 223 Km2 in the east of the city as an EPA, located between latitudes 22°45’00” and 22°56’00” S and longitudes 46°52’30” and 47°00’00” W [15,18]. This area contains the largest number of forest fragments in the municipality, including several Atlantic Forest remnants, as well as fauna diversity of 68 species of wild mammals, including several endangered species [15,19].
Despite the cultural and biological richness, over the last few decades, the EPA has undergone significant environmental changes, with the implantation of upscale horizontal condominiums, which opened up new roads, established new inhabitants and altered the delimitation of the urban perimeters [15,17,20].
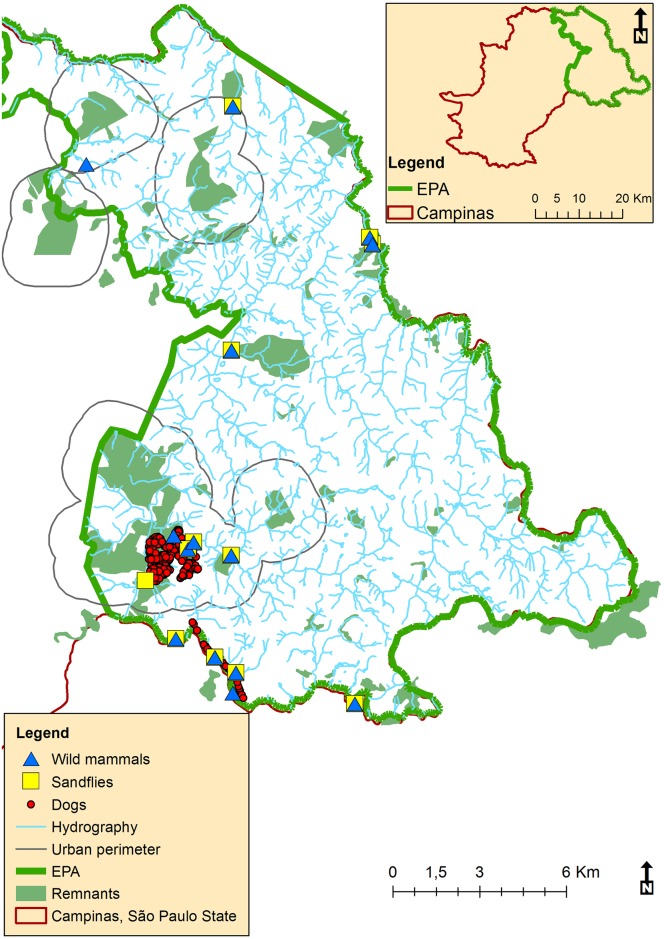
In 2009, the first autochthonous case of CVL in the municipality of Campinas was reported in a dog that lived in one of these residential condominiums inside the EPA, close to residual forest fragments, where contact between the wild fauna, humans and their pets can occur [16,17]. Since then, new canine cases have been reported in this same condominium and in another that is geographically contiguous and shares the same environmental characteristics. The proximity of human residences to these green areas is shown in Fig 1, together with the area of CVL focus in the EPA.
Fig 1. Study area.
Localization of residences in the condominium where the first autochthonous case of canine visceral leishmaniasis was detected in the municipality of Campinas, São Paulo, Brazil, in 2009.
We set traps at 18 different points throughout the EPA to capture vectors and wild animals. These capture points were defined based on criteria that included easy of access by roads, proximity to water courses, size of the forest area, forest connections with urban perimeters and proximity to the CVL focus. They were defined with the help of Google Earth software (Google, version 7.1.2.2041, compiled in 2013).
A serological survey was conducted among domestic dogs in the condominium residences where the CVL focus was located. The sampling points, obtained by GPS (Global Position System), are shown in Fig 2.
Fig 2. Location of the Campinas environmentally protected area (EPA), São Paulo, Brazil, indicating the sampling points of wild mammals and sand flies in the years of 2014 and 2015 and of dogs in 2013 and 2015.
The forest fragments and water resources of the EPA were mapped by the Municipal Health Department of Campinas using a geographic information system (GIS) with the MapInfo software, using a matrix layer of an aerial photograph of the region, obtained in 2007. A vector layer was superimposed, which showed the remnants of Atlantic Forest in the state of São Paulo, produced by the project “SOS Mata Atlântica”, obtained from the National Institute of Space Research website (INPE, http://www.inpe.br). ArcGIS software, v. 10.1 (Environmental System Research Institute, CA), was used to plot the geographic coordinates and draw the maps with all geographic objects and the cartographic base of the municipality available from the Brazilian Institute of Geography and Statistics (IBGE).
Serological survey of domestic dogs
The survey area of canine cases in the EPA was defined based on the VL Surveillance Program of the state of São Paulo, which establishes a radius of 200 m from the confirmed canine case for conducting surveillance and control actions, and is expanded, whenever necessary, until samples from at least 100 dogs are obtained [21]. In this study, we included blood serum samples collected from all dogs resident in the two condominiums where the CVL focus was located, in addition to areas adjacent to the same (Fig 2).
The serological survey was conducted as part of the planned monitoring and control actions of the Municipal Health Department of Campinas and the IAL, performed in the dog population residing inside the EPA.
After authorization from the owners, data pertaining to the dogs were recorded in a database and serum samples were collected. The serological tests used were those recommended by the National Program for Surveillance and Control of Leishmaniasis: the Dual Path Platform rapid immunochromatographic test (TR DPP, Bio-Manguinhos, Brazil) for screening and the enzyme-linked immunosorbent assay (ELISA, Bio-Manguinhos, Brazil) to confirm the diagnosis, following the manufacturers’ protocols.
In this study, we included the surveys conducted in 2013 and 2015, considering that in 2014, there was no supply of kit for canine serological diagnosis from Brazilian Ministry of Health to the Municipal Health Department of Campinas, making it impossible to conduct the serological survey in this year, since this is the official technique for dog survey in Brazil.
Sand fly and wild mammal collection
Sand flies and mammal captures were made monthly from April 2014 to March 2015, for three consecutive nights per month, simultaneously in three different points selected in EPA: one inside the condominiums where there is the CVL focus, another in the forest fragments with no contact with urban areas and a third randomly selected point.
For the collection of sand flies we used two or three modified CDC light traps (Horst, Brazil) per point of collection for two or three consecutive nights. The light traps were set up about one meter from the forest ground [22]. Species were classified as proposed by Galati [22] and the abbreviation scheme of genus names was that described by Marcondes [23].
Due to their high morphological resemblance, taxonomic identification of Brumptomyia females ended at the genus level. Evandromyia sallesi and Ev. cortelezzii were considered as Ev. cortelezzii-sallesi, based on the intermediate morphological forms observed and the morphological similarity of their females.
Wild mammals were captured for three consecutive nights per month, also from April 2014 to March 2015, using sixty tomahawk traps (Gabrisa, Brazil) with dimensions of 30 x 21 x 20 cm and 55 x 20 x 20 cm, baited with banana and chicken simultaneously at three different points in the EPA. A total of 20 traps per point (totally 60 traps) were distributed along the soil of forest region in linear transects with 10 to 20 meters between the traps [24].
The captured mammals were identified with microchip implants (Microchips Brasil, Brazil), and blood samples were collected by venipuncture during manual containment. Samples were stored at -20°C until DNA extraction.
The capture, containment, handling and sampling of the captured mammals were all carried out according to guidelines for the use of wild mammals in research, as recommended by the American Society of Mammologists [25].
Genomic DNA extraction from samples of sand flies and wild mammals
DNA extraction of total blood samples from wild mammals was performed within 48 h of the conclusion of each capture, using a QIAamp DNA mini kit in a QIAcube DNA extractor (Qiagen, Netherlands).
To measure the quantity and purity of DNA from the samples, we used the NanoDrop ND-1000 (Thermo Fisher Scientific, EUA) equipment. To evaluate the endogenous quality, DNA integrity and the presence of inhibitors in blood samples, we performed PCR reactions using primers for the conserved gene of the interphotoreceptor retinoid-binding protein (IRBP), IRBP-CF-FWD (5'-TCCAACACCACCACTGAGATCTGGAC-3') and IRBP-CF-REV (5'-GTGAGGAAGAAATCGGACTGGCC-3') [26], or for the β1 (5’-ACCACCAACTTCATCCACGTTCACC-3’) and β2 genes (5’-CTTCTGACACAACTGTGTTCACTAGC-3’) [27].
The female sand flies were grouped in pools (1–6 specimens) according to species and location. DNA was extracted using a DNeasy Blood and Tissue kit (Qiagen, Netherlands). The quantity and purity of the DNA samples was measured using an Epoch spectrophotometer (BioTek, Winooski, Vermont).
Detection of Leishmania DNA
PCR was performed using the primers LITSR (5’ CTGGATCATTTTCCGATG 3’) and L5.8S (5’ TGATACCACTTATCGCACTT 3’) to amplify a 300–350 base pair (bp) fragment from the intergenic region of the Leishmania DNA, internal transcribed spacer-1 (ITS-1) [28] and primers Lch14 and Lch15, which amplify a 167 bp of the kinetoplast minicircle DNA of L. (L.) infantum [29].
As reaction controls, we used ultrapure water and extracted DNA from in vitro cultures of standard strains of L. (L.) infantum (MHOM/BR/2002/LPC-RPV), L. (V.) braziliensis (MHOM/BR/1975/M2903) and L. (L.) major (MHOM/IL/1980/FRIEDLIN).
The reaction mixture contained 1.3 μL of buffer (50mM KCl, 20mM Tris-HCl pH 8.4), 0.4 μL MgCl2 (1.6 μM), 0.25 μL of each oligonucleotide (0.2 μM), 0.25 μL dNTP (0.2 mM), 0.25 μL of Platinum Taq DNA polymerase (Invitrogen, Brazil) and 8.3 μL of ultrapure water, with 1 μL of extracted DNA with a minimal concentration of 10 ng/μL. Thermal cycling conditions followed those of El Tai et al. (2000) [28] and Silva et al. [29]. The amplified products were identified by 1.5% agarose gel electrophoresis containing 1.0 μL/10 mL of SYBR safe DNA gel stain (Invitrogen, Life Technologies, USA).
Genetic sequencing
Amplified products were purified with the Illustra GFX PCR DNA and Gel Band Purification kit (GE Healthcare, UK). Genetic sequencing by the Sanger method was performed in a Genetic Analyzer 3500 automated sequencer using a BigDye Terminator v 3.1 Cycle Sequencing kit (Applied Biosystems, Life Technologies, USA).
The sense and antisense sequences were visualized using Chromas v 2.1.1 software (Technelysium Pty Ltd, Australia), and were submitted to global alignment using MEGA5 software [30] and compared with sequences deposited in the GenBank, using the nucleotide basic local alignment search tool (BLASTn, http://www.ncbi.nlm.nih.gov/BLAST).
Data analysis
The annual anti-Leishmania canine seroprevalences in the study area and the respective 95% confidence intervals (95%CI) were calculated. The frequency and percentages of sand flies and wild mammals infected, with their respective 95%CI, were also described. Statistical analysis was performed in Stata software, v. 11.0 (StataCorp LP, USA).
Results
Human residences were observed in close proximity to the forest fragments, where the CVL focus is located inside the Campinas EPA (Fig 1). The distribution of trap sites for capturing sand flies and wild animals is shown in Fig 2, besides domestic dogs sampled.
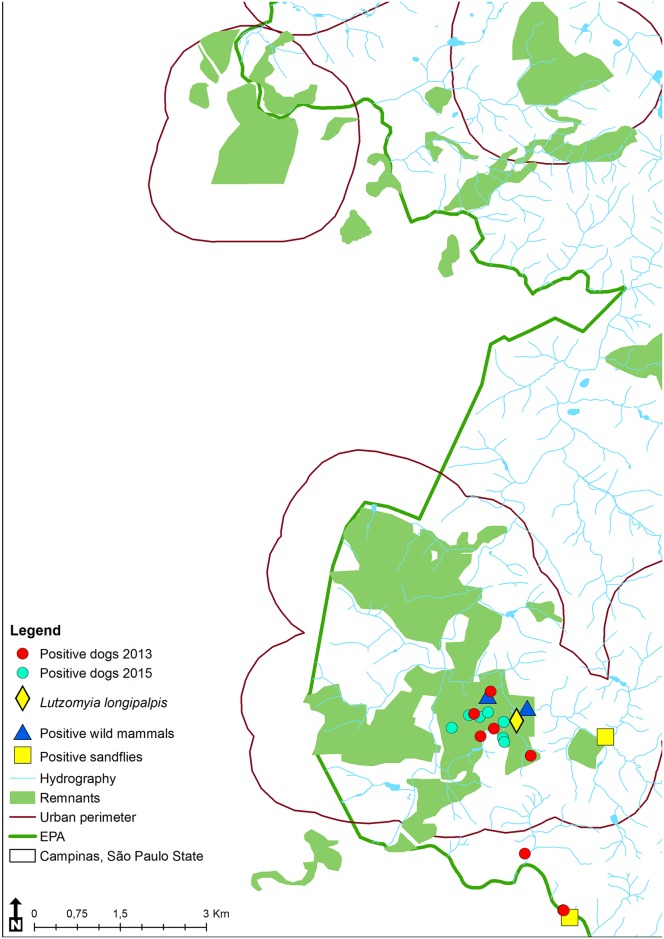
Fig 3 shows the spatial distribution of seropositive dogs, sand flies and wild mammals naturally infected by species of Leishmania, confirmed by molecular techniques.
Fig 3. Campinas environmentally protected area (EPA), São Paulo, Brazil, showing the distribution of dogs positive for visceral leishmaniasis in 2013 and 2015 and of wild mammals and sand flies captured in 2014 and 2015 and naturally infected by Leishmania spp.
Serological surveys in dogs
In the canine surveys of 2013 and 2015 were evaluated, respectively, 590 and 571 blood samples from domestic dogs, performing 1,161 examinations (S1 Table). The seroprevalence determined and the respective confidence interval are shown in Table 1.
Table 1. Prevalence of anti-Leishmania antibodies determined in two canine serological surveys in the Campinas environmentally protected area, 2013 and 2015.
| Year | No. of dogs tested | Reactive dogsa |
Prevalence (%) |
Confidence interval (95%) |
|---|---|---|---|---|
| 2013 | 590 | 9 | 1.5 | 0.5–2.5 |
| 2015 | 571 | 7 | 1.2 | 0.3–2.1 |
| Total | 1,161 | 16 | 1.4 | 0.7–2.0 |
aDogs that tested positive in screening with the Dual Path Platform rapid immunochromatographic test (TR DPP, Bio-Manguinhos, Brazil) and confirmation by enzyme-linked immunosorbent assay (ELISA, Bio-Manguinhos, Brazil). Data source: Zoonosis surveillance unit (UVZ), Municipal Secretariat of Health, Campinas, SP, Brazil.
Phlebotomine sand flies
Four hundred and seventy-seven specimens of sand flies were collected, 291 of which were male and 186 female (S2 Table). Six species known or suspected of being vectors were identified as follows: Nyssomyia whitmani (107 males, 47 females), Migonemyia migonei (81 males, 34 females), Pintomia fischeri (21 males, 12 females), Nyssomyia neivai (12 males, 13 females), Pintomyia pessoai (3 males, 1 female) and Lu. longipalpis (2 males) (Table 2).
Table 2. Phlebotomine sand flies captured monthly using CDC light traps in the Campinas environmentally protected area, São Paulo, Brazil, from April 2014 to March 2015.
| Species | Number of specimens | Relative % M/F | |||
|---|---|---|---|---|---|
| Males | Females | N total | |||
| Brumptomyia sp. | 52 | 43 | 95 | 19.92 | |
| Evandromyia cortelezzii-sallesi | 3 | 2 | 5 | 1.05 | |
| Evandromyia edwardsi | 0 | 1 | 1 | 0.21 | |
| Evandromyia lenti | 0 | 1 | 1 | 0.21 | |
| Expapillata firmatoi | 2 | 8 | 10 | 2.10 | |
| Lutzomyia longipalpis | 2 | 0 | 2 | 0.42 | |
| Migonemyia migonei | 81 | 34 | 115 | 24.11 | |
| Nyssomyia neivai | 12 | 13 | 25 | 5.24 | |
| Nyssomyia whitmani | 107 | 45 | 152 | 31.87 | |
| Pintomyia fischeri | 21 | 12 | 33 | 6.92 | |
| Pintomyia monticola | 2 | 23 | 25 | 5.24 | |
| Pintomyia pessoai | 3 | 1 | 4 | 0.84 | |
| Psathyromyia aragaoi | 0 | 1 | 1 | 0.21 | |
| Psathyromyia pascalei | 5 | 2 | 7 | 1.47 | |
| Psychodopygus ayrozai | 1 | 0 | 1 | 0.21 | |
| Total | Number | 291 | 186 | 477 | 1.56 |
| % | 61.01 | 38.99 | - | 100 | |
PCR tests were performed on all 186 sand fly females, grouped in 74 pools (14 Brumptomyia sp., 2 Evandromyia cortelezzii-sallesi, 1 Ev. edwardsi, 1 Ev. lenti, 6 Expapillata firmatoi, 11 Mg. migonei, 3 Ny. neivai, 13 Ny. whitmani, 6 Pi. fischeri, 13 Pi. monticola, 1 Pi. pessoai, 1 Psathyromyia aragaoi, 2 Pa. pascalei), aimed at investigating the rate of natural infection.
The ITS-1 PCR detected a 300–350 bp fragment in three samples (1.6%; 95%CI 0.3–4.6%) belonging to Ex. firmatoi (1/10; 10.0%; 95%CI 0.2–44.5%) and Pi. monticola (2/25; 8.0%; 95%CI 1.0–26.0%) that characterized the samples as positive for Leishmania genus.
These three samples, one of Ex. firmatoi and two of Pi. monticola, were also amplified with Lch14/15 primers, producing the expected 167 bp products, later confirmed as L. chagasi (synonymous L. (L.) infantum) by genetic sequencing that showed 96 to 100% similarity with kinetoplast minicircle sequences deposited in the GenBank (Accession numbers AF308682.1, E values = 2e-37; 2e-50 and 2e-49).
Wild mammals
Eighty-two wild mammals from six species were sampled: 1 Mazama gouazoubira (brocket deer; 1.2%), 1 Sciurus (Guerlinguetus) aestuans (squirrel; 1.2%), 8 Callithrix jacchus (white-tufted-ear marmoset; 9.8%), 11 Didelphis aurita (black-eared opossum; 13.4%), 18 Callithrix penicillata (black-tufted-ear marmoset; 22.0%) and 43 Didelphis albiventris (white-eared opossum; 52.4%). Six (6/82; 7.3%) D. albiventris were identified by a microchip reading in a second capture and sampled again (S3 Table).
Amplification of an expected 300–350 bp product was observed in 2/88 (2.3%; 95%CI 0.3–8.0%) samples from D. albiventris (2/43; 4.7%; 95%CI 0.6–15.8%). The first amplicon was confirmed as L. (L.) infantum and the second as Leishmania subgenus Viannia, making both the first records of infection by Leishmania species in wild mammals in this region [31].
In a third D. albiventris sample, a 500 bp product was confirmed as Trypanosoma rangeli by genetic sequencing (98% similarity; E value = 0.0, GenBank accession number AY230237.1 and others). No sample was positive when using the primers Lch14/15.
Discussion
Participation of wild fauna in transmission cycles of VL
The presence of natural infection by L. (L.) infantum in female sand flies and natural infection by two Leishmania species in a mammal species considered to be a potential reservoir (D. albiventris) provides strong evidence that sylvatic Leishmania transmission cycle is occurring in the EPA. However, it was not possible to confirm whether VL in Campinas occurred due to a wild enzootic cycle of transmission. Since dogs cohabit with infected wild animals, the hypothesis of the infection of wild mammals by infected dogs cannot be ruled out.
In the investigation conducted into the first case of CVL in the EPA in 2009, 40 wild mammals belonging to the species Nectomys squamipes, D. albiventris, C. penicillata and Gracilianus agilis were captured in wooded areas of the EPA and samples were examined by PCR, but none presented positive for VL [17]. In our investigation, infection among wild fauna by different species of Leishmania [31] was confirmed for the first time in this region, together with another species of trypanosomatid, T. rangeli. In addition, at the time of the first investigation, proximity of 100 m between human residences and forest areas where sand flies and wild mammals were captured was demonstrated [17].
In our study, although the capture of wild animals and phlebotominae was conducted in forest fragments in several locations within the EPA, the occurrence of L. (L.) infantum in these animals was observed only in locations close to seropositive domestic dogs. This spatial distribution suggests the involvement of these animals in the CVL focus, even though low Lu. longipalpis density and a small number of infected wild animals was observed.
Some authors suggest that the public health impact of VL infected dogs in urban areas is greater than in rural and wild regions, and that attention should be given to individuals living or frequenting these locations. Restricted contact between wildlife and domestic dogs has also been proposed to reduce the probability of VL transmission to humans and wild animals from dogs [32,33].
Discussion concerning the participation of wild species in the transmission of zoonotic parasites has become particularly important in recent years with the consolidation of the One Health concept [34,35]. Anthropogenic changes in ecosystems are particularly important in this context, resulting in greater proximity between wild and domestic animals and humans [36].
Environmental degradation and proximity between wildlife and humans and their domestic animals
Proximity to forest areas and wildlife with human residences is a worrying scenario. In the state of Rio de Janeiro, dogs living within 100 m of forests presented a 3.5-fold higher risk of acquiring VL. The presence of opossums in the peridomicile increased the chances of infection in dogs 2.6-fold, with a 30.0% prevalence in these wild animals [37].
Another study detected a 20% prevalence of VL in dogs from rural areas in the state of Minas Gerais, located up to 2 Km from five EPAs that contained fragments of Atlantic Forest. The risk factors for these dogs were different from those living in urban areas [33].
In the Chaco region of Argentina, Barroso et al. [38] surveyed 77 dogs living in a forest area where two cases of human VL had occurred and determined a 13% prevalence. The authors suggested that the emergence of cases in dogs and humans in a wild region that was endemic for tegumentary leishmaniasis, with a relatively low canine prevalence, is clearly compatible with the involvement of wild mammals as reservoirs and that parasite transmission occurs from these animals. This scenario is very similar to the CVL focus located in the Campinas EPA.
Sand fly fauna in the Campinas EPA
In the EPA, the presence of 15 phlebotomine sand flies species, even at low frequencies, shows a diversity of fauna, as previously reported in forest environments [39,40].
One interesting factor is the low density of Lu. longipalpis in the study area. Based on analysis of sexual pheromones secreted by males, it is accepted that Lu. longipalpis is a species complex [41]. The chemotype population of Lu. longipalpis found in Campinas, cembrene-1, is different to the chemotype population found in the western region of the state of São Paulo, (S)-9-methylgermacrene-B [42]. The remarkable differences between the epidemiological situations, population size and sibling complex of Lu. longipalpis in Campinas, corroborates the findings of Casanova et al. [43], who suggested there are different vectorial capacities and competence between siblings.
In this study, two species of phlebotomine sand flies, Pi. monticola and Ex. firmatoi, were found naturally infected with L. (L.) infantum. These species are essentially sylvatic and highly anthropophilic [44,45]. The participation of these species in the transmission of L. (V.) braziliensis and L. (L.) infantum has been suggested in the municipalities of Divinópolis, Minas Gerais, and in Rio de Janeiro, respectively, with Pi. monticola and Ex. firmatoi [46,47].
Females of different species were also found naturally infected by L. (L.) infantum in other endemic areas, such as specimens belonging to the cortelezzii complex in the Brazilian States of Minas Gerais and Mato Grosso do Sul—which are not amongst the incriminated leishmaniasis vector—and may be involved in a wild or rural cycle of L. (L.) infantum transmission in these areas [48,49]. Mg. migonei–a known vector of cutaneous leishmaniasis—was also found naturally infected in the Brazilian States of Pernambuco and Ceará. Some authors suggested that this species could act as a potential vector in VL transmission, particularly in areas where Lu. longipalpis is absent [50,51]. It is important to note that recent studies have demonstrated the high susceptibility of Mg. migonei to infection with L. (L.) infantum, reinforcing this hypothesis [52,53].
The detection of Leishmania DNA in a sand fly species does not prove vector competence [54]. We cannot exclude the persistence of DNA without any infective role, particularly because there are infected dogs and wild animals in the vicinity of these infected sand flies [55]. However, these results reinforce the need for further studies to investigate the vectorial capacity of these species, especially experimental studies.
Particularities of VL focus in the study area
Although canine VL cases usually precede the occurrence of the disease in humans, no cases of human VL have been recorded in Campinas city to date. This fact could be associated with the local transmission characteristics related to the diversity and competence of the vectors, the presence of wild reservoirs and hosts, the characteristics of the parasite (not investigated), as well as the pattern of human contact, with transmission in sparsely populated area and in luxury condominiums.
The hypothesis that in some new VL foci in the state of São Paulo, subpopulations of Lu. longipalpis could be present and account for the existence and perpetuation of L. (L.) infantum enzootic wild cycles should be considered, particularly in areas around preserved forest environments or in recent residential projects.
On the other hand, Motoie et al. [56] identified the presence of genetically distinct populations of L. (L.) infantum in São Paulo, indicating that the inherent characteristics of the parasite itself could also be responsible for different epidemiological patterns of VL observed in new foci in the state.
In addition to factors directly associated with the transmission cycle, the occurrence of CVL cases in the Campinas EPA seems to be related to the occupation process, in which anthropic activities changed the natural landscapes, which has forest fragments containing wild fauna. In these regions, the exposure of humans and their pets to parasites and vectors of diseases to which they have not been previously exposed can result in disease outbreaks from natural enzootic foci.
The process of urban expansion within the EPA in the 1970s and 1980s was associated with an outbreak of cutaneous leishmaniasis in human inhabitants of the EPA in 1993 and 1994 [20]. In our study, an opossum was infected with a species of the subgenus Viannia, known to be responsible for tegumentary leishmaniasis in Brazil, which confirms the current risk of infection in this area and, again, the possible involvement of the wild fauna in the maintenance of the transmission cycle.
In addition, environmental modifications seem to be related to the adaptation of phlebotomine vectors to urban environments due to a decrease in the availability of wild animals as a food source, making dogs and humans more accessible alternatives to the vector [21,57].
Particularities of different areas of VL transmission versus prophylaxis and disease control
The VL Surveillance and Control Program in Brazil supervises the main actions to reduce morbidity and lethality, aimed at early diagnosis and treatment of human cases, vector control and identification and elimination of seropositive domestic dogs [57]. Although there is good theoretical support for these measures, there is no evidence of their effectiveness in reducing the prevalence in endemic regions nor in the expansion of new outbreaks [58–60].
In Europe, although the domestic dog is considered the main reservoir in endemic areas, wild reservoirs have been proposed as potentially responsible for the lack of success in VL control [61]. In Brazil, the zoonotic transmission cycle is concentrated in areas where human habitation is located close to the wild cycle of the disease [2].
Although serological tests may be subject to false positive and/or negative results, due to its sensitivity and specificity, their use is valued for epidemiological surveillance purposes [22, 53].
Despite the serological tests used in this study, Grimaldi Jr. et al. [62]reported that DPP displayed high specificity (96%) but low sensitivity (47%) in identify asymptomatic dogs, but the sensitivity was significantly higher (98%) in diseased cases, indicating that this convenient test may be useful to identify the most infectious dogs.
In another study, Laurenti et al. [63] report that DPP detected both asymptomatic and symptomatic dogs in equal proportions. Using this test, 42/47 (89.4%) symptomatic dogs were detected positive, besides 35/38 (92.1%) asymptomatic dogs, with good accuracy of 92.7%. The ELISA BioManguinhos was positive in 43/47 (91.5%) symptomatic dogs and 34/38 (89.5%) asymptomatic dogs, with accuracy of 84.3%. Although, the combination of the two tests, as used in our study and in Brazilian surveillance activities, results in 99.1% sensitivity and 73.9% specificity, showing that it is a useful serology protocol for surveys.
In our study, the proximity of seropositive dogs housing sites with capture sites of wild mammals and sandflies infected with Leishmania spp. reinforces the assumption that they may be related. However, as shown in Fig 1, the human residences were constructed in the middle of the vegetation, being difficult to delimit the occurrence of independent transmission cycles in peridomiciliar or wild environment.
The need to elaborate distinct control and prophylaxis strategies, according to the characteristics of each transmission area, including the rural and wild areas in the control programs is now becoming evident. Studies that clarify the patterns of infection in different locations are useful for the success of such actions. Thus, investigating a new VL focus in all its distinct aspects contributes to our understanding of the key elements of the transmission dynamics and disease control. Actions that do not consider these particularities are less likely to succeed.
Supporting information
Campinas Environmentally Protected Area, 2013 and 2015.
(XLSX)
Campinas Environmentally Protected Area, April 2014 to March 2015.
(XLS)
Campinas Environmentally Protected Area, April 2014 to March 2015.
(XLS)
(PDF)
Acknowledgments
The authors gratefully acknowledge the staff of the Surveillance Unit of Zoonosis of the Campinas Municipal Health Secretariat and of the Adolfo Lutz Institute for their technical support, especially in the fieldwork.
Data Availability
All relevant data are within the paper and its Supporting Information files. The data of the Leishmania genetic sequences detected in wild animals are available from the GenBank database (accession numbers KX580706 and KX580707).
Funding Statement
This research received financial support of São Paulo Research Foundation (FAPESP, http://www.fapesp.br), grant nos. 2014/27212-0, 2014/13049-0 and 2016/02572-0, besides the financial support of the Research Program for the Unified Health System (PPSUS, FAPESP, CNPq—National Council for Scientific and Technological Development No. 12/51267-4. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7: e35671 doi: 10.1371/journal.pone.0035671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Control of the leishmaniases: report of a meeting of the WHO Expert Committee on the control of leishmaniasis, Geneva, 22–26 March, 2010. WHO Tech Rep Ser. 2010.
- 3.Roque ALR, Jansen AM. Wild and synanthropic reservoirs of Leishmania species in the Americas. Int J Parasitol Parasites Wildl. 2014;3(3): 251–262. doi: 10.1016/j.ijppaw.2014.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa CHN, Pereira HF, Araújo M V. Epidemia de leishmaniose visceral no Estado do Piauí, Brasil, 1980–1986. Rev Saude Publica. 1990;24(5): 361–372. doi: 10.1590/S0034-89101990000500003 [DOI] [PubMed] [Google Scholar]
- 5.Costa JML, Viana GMC, Saldanha ACR, Nascimento MDSB, Alvim AC, Burattini MN, et al. Leishmaniose visceral no estado do Maranhão, Brasil. A evolução de uma epidemia. Cad Saude Publica. 1995;11(2): 321–324. [DOI] [PubMed] [Google Scholar]
- 6.de Araújo VEM, Morais MHF, Reis IA, Rabello A, Carneiro M. Early clinical manifestations associated with death from visceral leishmaniasis. PLoS Negl Trop Dis. 2012;6 doi: 10.1371/journal.pntd.0001511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Marzochi MCA, Marzochi KBF. Tegumentary and visceral leishmaniases in Brazil: emerging anthropozoonosis and possibilities for their control. Cad Saude Publica. 1994;10(Suppl.2): 359–375. doi: 10.1590/S0102-311X1994000800014 [DOI] [PubMed] [Google Scholar]
- 8.Desjeux P. The increase in risk factors for leishmaniasis worldwide. Trans R Soc Trop Med Hyg. 2001;95(3): 239–243. [DOI] [PubMed] [Google Scholar]
- 9.Bevilacqua PD, Paixão HH, Modena CM, Castro MCPS. Urbanização da leishmaniose visceral em Belo Horizonte. Arq Bras Med Vet Zootec. 2001;53(1): 1–8. doi: 10.1590/S0102-09352001000100001 [Google Scholar]
- 10.Gontijo CMF, Melo MN. Leishmaniose visceral no Brasil: quadro atual, desafios e perspectivas. Rev Bras Epidemiol. 2004;7(3): 338–349. doi: 10.1590/S1415-790X2004000300011 [Google Scholar]
- 11.Souza GD, dos Santos E, Andrade Filho JD. The first report of the main vector of visceral leishmaniasis in America, Lutzomyia longipalpis (Lutz & Neiva) (Diptera: Psychodidae: Phlebotominae), in the state of Rio Grande do Sul, Brazil. Mem Inst Oswaldo Cruz. 2009;104(8): 1181–1182. doi: 10.1590/S0074-02762009000800017 [DOI] [PubMed] [Google Scholar]
- 12.Rangel EF, Vilela ML. Lutzomyia longipalpis (Diptera, Psychodidae, Phlebotominae) and urbanization of visceral leishmaniasis in Brazil. Cad Saude Publica. 2008;24(12): 2948–2952. doi: 10.1590/S0102-311X2008001200025 [DOI] [PubMed] [Google Scholar]
- 13.de Ciaravolo RMC, de Oliveira SS, Hiramoto RM, Henriques LF, Taniguchi HH, Viviani A Junior, et al. Classificação epidemiológica dos municípios segundo o programa de vigilância e controle da leishmaniose visceral no estado de São Paulo, dezembro de 2014. Bol Epidemiológico Paul. 2015;12(143): 9–22. [Google Scholar]
- 14.Tolezano JE, Luvizotto MCR, Uliana SRB, Araújo MFL, Taniguchi HH, Barbosa JAR, et al. Leishmaniose visceral americana (LVA) em Araçatuba, região oeste do estado de São Paulo. Investigações laboratoriais e diagnóstico de uma doença emergente em terras paulistas. Rev Soc Bras Med Trop. 1999;32 (supl.): 218. [Google Scholar]
- 15.Seplama. Secretaria de Planejamento e Meio Ambiente. Plano de gestão da área de proteção ambiental da região de Sousas e Joaquim Egídio, APA municipal. 1996. 177p. http://www.campinas.sp.gov.br/governo/seplama/planos-locais-de-gestao/doc/plgapa.pdf
- 16.Savani ESMM, Presotto D, Roberto T, de Camargo MCGO, Nicoletti DSR, Sacramento DV. First occurrence of an authochthonous canine case of Leishmania (Leishmania) infantum chagasi in the municipality of Campinas, State of São Paulo, Brazil. Rev Inst Med Trop Sao Paulo. 2011;53(4): 227–229. doi: 10.1590/S0036-46652011000400010 [DOI] [PubMed] [Google Scholar]
- 17.von Zuben APB, Angerami RN, Castagna C, Baldini MBD, Donalisio MR. The first canine visceral leishmaniasis outbreak in Campinas, State of São Paulo Southeastern Brazil. Rev Soc Bras Med Trop. 2014;47(3): 385–388. doi: 10.1590/0037-8682-0126-2013 [DOI] [PubMed] [Google Scholar]
- 18.Mattos C de O. Contribuição ao planejamento e gestão da Área de Proteção Ambiental de Sousas e Joaqum Egídio, Campinas, SP. M.Sc. Thesis, Universidade de São Paulo. 1996. http://www.bibliotecadigital.unicamp.br/document/?code=vtls000183299
- 19.PMC. Prefeitura Municipal de Campinas. Fundação Instituto de Pesquisas Econômicas. 2009. http://www.apacampinas.cnpm.embrapa.br. Cited 3 Sep 2013.
- 20.Corte AA, Nozawa MR, do Ferreira MC, Pignatti MG, Rangel O, Lacerra SS. Aspectos eco-epidemiológicos da leishmaniose tegumentar americana no Município de Campinas. Cad Saude Publica. 1996;12(4): 465–472. doi: 10.1590/S0102-311X1996000400004 [DOI] [PubMed] [Google Scholar]
- 21.São Paulo. Secretaria de Estado da Saúde. Superintendência de Controle de Endemias. Coordenadoria de Controle de Doenças. Manual de vigilância e controle da leishmaniose visceral americana do estado de São Paulo. São Paulo: A Secretaria; 2006.
- 22.Galati EAB. Classificação de Phlebotominae In: Rangel EF, Lainson R, editors. Flebotomíneos do Brasil. Rio de Janeiro: Ed. Fiocruz; 2003. [Google Scholar]
- 23.Marcondes CB. A Proposal of generic and subgeneric abbreviations for Phlebotomine sandflies (Diptera: Psychodidae: Phlebotominae) of the World. Entomol News. 2007;118(4): 351–356. doi: 10.3157/0013-872X(2007)118[351:APOGAS]2.0.CO;2 [Google Scholar]
- 24.Cardoso RM, de Nadjar NSLA, Romero GAS, Souza TTCM, Dietrich AG, Mender JD, et al. Expanding the knowledge about Leishmania species in wild mammals and dogs in the Brazilian savannah. Parasit Vectors. 2015;8: 171 doi: 10.1186/s13071-015-0780-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sikes RS, Gannon WL. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J Mammal. 2011;92(1): 235–253. doi: 10.1644/10-MAMM-F-355.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferreira EC, Gontijo CM, Cruz I, Melo MN, Silva AM. Alternative PCR protocol using a single primer set for assessing DNA quality in several tissues from a large variety of mammalian species living in areas endemic for leishmaniasis. Mem Inst Oswaldo Cruz. 2010;105(7): 895–898. doi: 10.1590/S0074-02762010000700009 [DOI] [PubMed] [Google Scholar]
- 27.Lee CN, Cavanagh HM, Lo ST, Ng CS. Human papillomavirus infection in non-neoplastic uterine cervical disease in Hong Kong. Br J Biomed Sci. 2001;58(2): 85–91. [PubMed] [Google Scholar]
- 28.el Tai NO, Osman OF, el Fari M, Presber W, Schönian G. Genetic heterogeneity of ribosomal internal transcribed spacer in clinical samples of Leishmania donovani spotted on filter paper as revealed by single-strand conformation polymorphisms and sequencing. Trans R Soc Trop Med Hyg. 2000;94(5): 575–579. [DOI] [PubMed] [Google Scholar]
- 29.Silva RC, Richini-Pereira VB, Kikuti M, Marson PM, Langoni H. Detection of Leishmania (L.) infantum in stray dogs by molecular techniques with sensitive species-specific primers. Vet Q. 2017;37(1): 23–30. doi: 10.1080/01652176.2016.1252073 [DOI] [PubMed] [Google Scholar]
- 30.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10): 2731–2739. doi: 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paiz LM, Donalisio MR, Richini-Pereira VB, Motoie G, Castagna CL, Tolezano JE. Infection by Leishmania spp. in free-ranging opossums (Didelphis albiventris) in an environmentally protected area inhabited by humans in Southeastern Brazil. Vector-Borne Zoonotic Dis. 2016;16(11): 728–730. doi: 10.1089/vbz.2016.2001 [DOI] [PubMed] [Google Scholar]
- 32.de Curi NHA, Miranda I, Talamoni SA. Serologic evidence of Leishmania infection in free-ranging wild and domestic canids around a Brazilian National Park. Mem Inst Oswaldo Cruz. 2006;101(1): 99–101. [DOI] [PubMed] [Google Scholar]
- 33.de Curi NHA, de Paschoal AMO, Massara RL, Marcelino AP, Ribeiro AA, Passamani M, et al. Factors associated with the seroprevalence of leishmaniasis in dogs living around Atlantic Forest fragments. PLoS One. 2014;9(8): e104003 doi: 10.1371/journal.pone.0104003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palatnik-de-Sousa CB, Day MJ. One Health: The global challenge of epidemic and endemic leishmaniasis. Parasit Vectors. 2011;4: 197 doi: 10.1186/1756-3305-4-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bidaisee S, Macpherson CNL. Zoonoses and one health: a review of the literature. J Parasitol Res. 2014;2014: 874345 doi: 10.1155/2014/874345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson RC. Parasite zoonoses and wildlife: One health, spillover and human activity. Int J Parasitol. 2013;43(12–13): 1079–1088. doi: 10.1016/j.ijpara.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cabrera MA, Paula AA, Camacho LA, Marzochi MC, Xavier SC, da Silva AV, et al. Canine visceral leishmaniasis in Barra de Guaratiba, Rio de Janeiro, Brazil: assessment of risk factors. Rev Inst Med trop Sao Paulo. 2003;45(2): 79–83. [DOI] [PubMed] [Google Scholar]
- 38.Barroso PA, Marco JD, Locatelli FM, Cardozo RM, Hoyos CL, Mora MC, et al. Visceral leishmaniasis caused by Leishmania infantum in Salta, Argentina: possible reservoirs and vectors. Am J Trop Med Hyg. 2015;93(2): 334–339. doi: 10.4269/ajtmh.14-0267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pereira Jr. AM. Fauna de flebotomíneos (Diptera: Psychodidae) e taxa de infecção natural por Leishmania Ross (Kinetoplastida: Trypanosomatidae) em ambientes de várzea e de terra firme no município de Tefé, Amazonas, Brasil. M.Sc. Thesis, Instituto Nacional de Pesquisas da Amazônia. 2014. http://bdtd.inpa.gov.br/handle/tede/1535
- 40.Andrade Filho JD, Carneiro APS, Lima MLN, Santiago RM, Gama MA, Santos CA, et al. Flebotomíneos de Timóteo, Estado de Minas Gerais, Brasil (Diptera: Psychodidae). Cad Saude Publica. 1997;13(4): 767–770. [PubMed] [Google Scholar]
- 41.Watts PC, Hamilton JG, Ward RD, Noyes HA, Souza NA, Kemp SJ, et al. Male sex pheromones and the phylogeographic structure of the Lutzomyia longipalpis species complex (Diptera: Psychodidae) from Brazil and Venezuela. Am J Trop Med Hyg. 2005;73(4): 734–743. 73/4/734 [PubMed] [Google Scholar]
- 42.Casanova C, Colla-jacques FE, Hamilton JG, Brazil RP, Shaw JJ. Distribution of Lutzomyia longipalpis chemotype populations in São Paulo State, Brazil. PLoS Negl Trop Dis. 2015;9(3): e0003620 doi: 10.1371/journal.pntd.0003620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casanova C, Hamilton JGC, Trigo JR, Costa AIP. Identification of sex pheromones of Lutzomyia longipalpis (Lutz & Neiva, 1912) populations from the state of São Paulo, Brazil. Mem Inst Oswaldo Cruz. 2006;101(1): 113–115. [DOI] [PubMed] [Google Scholar]
- 44.Galati EAB, Marassá AM, Gonçalves-Andrade RM, Consales CA, Bueno EFM. Phlebotomines (Diptera, Psychodidae) in the Speleological Province of the Ribeira Valley: 2. Parque Estadual do Alto Ribeira (PETAR), São Paulo State, Brazil. Rev Bras Entomol. 2010;54(3): 477–487. [Google Scholar]
- 45.Gomes AC, Barata MS, Rocha e Silva EO, Galati EAB. Aspectos ecológicos da leishmaniose tegumentar americana. 6. Fauna flebotomínica antropofílica de matas residuais situadas na região centro-nordeste do Estado de São Paulo. Rev Inst Med Trop Sao Paulo. 1989;31: 32–39. [DOI] [PubMed] [Google Scholar]
- 46.de Souza MB, de Marzochi MCA, de Carvalho RW, Ribeiro PC, dos Pontes CS, Caetano JM, et al. Ausência da Lutzomyia longipalpis em algumas áreas de ocorrência de leishmaniose visceral no Município do Rio de Janeiro. Cad Saude Publica. 2003;19(6): 1881–1885. doi: 10.1590/S0102-311X2003000600033 [DOI] [PubMed] [Google Scholar]
- 47.Margonari C, Soares RP, Andrade-Filho JD, Xavier DC, Saraiva L, Fonseca AL, et al. Phlebotomine sand flies (Diptera: Psychodidae) and Leishmania infection in Gafanhoto Park, Divinópolis, Brazil. J Med Entomol. 2010;47(6): 1212–1219. doi: 10.1603/ME09248 [DOI] [PubMed] [Google Scholar]
- 48.Carvalho GML, Andrade Filho JD, Falcão AL, Rocha Lima ACVM, Gontijo CMF. Naturally infected Lutzomyia sand flies in a Leishmania-Endemic area of Brazil. Vector-Borne Zoonotic Dis. 2008;8(3): 407–414. doi: 10.1089/vbz.2007.0180 [DOI] [PubMed] [Google Scholar]
- 49.Saraiva L, Carvalho GML, Gontijo CMF, Quaresma PF, Lima ACVMR, Falcão AL, et al. Natural infection of Lutzomyia neivai and Lutzomyia sallesi (Diptera: Psychodidae) by Leishmania infantum chagasi in Brazil. J Med Entomol. 2009;46(5): 1159–1163. doi: 10.1603/033.046.0525 [DOI] [PubMed] [Google Scholar]
- 50.de Carvalho MR, Valença HF, da Silva FJ, de Pita-Pereira D, de Pereira TA, Britto C, et al. Natural Leishmania infantum infection in Migonemyia migonei (França, 1920) (Diptera:Psychodidae:Phlebotominae) the putative vector of visceral leishmaniasis in Pernambuco State, Brazil. Acta Trop. 2010;116: 108–110. doi: 10.1016/j.actatropica.2010.03.009 [DOI] [PubMed] [Google Scholar]
- 51.Rodrigues ACM, Melo LM, Magalhães RD, de Moraes NB, de Souza AD, Bevilaqua CML. Molecular identification of Lutzomyia migonei (Diptera: Psychodidae) as a potential vector for Leishmania infantum (Kinetoplastida: Trypanosomatidae). Vet Parasitol. 2016;220: 28–32. doi: 10.1016/j.vetpar.2016.02.018 [DOI] [PubMed] [Google Scholar]
- 52.Guimarães VCFV, Pruzinova K, Sadlova J, Volfova V, Myskova J, Brandão Filho SPB, et al. Lutzomyia migonei is a permissive vector competent for Leishmania infantum. Parasit Vectors. 2016;9: 159 doi: 10.1186/s13071-016-1444-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galvis-Ovallos F, da Silva MD, da Bispo GBS, de Oliveira AG, Neto JRG, dos Malafronte RS, et al. Canine visceral leishmaniasis in the metropolitan area of São Paulo: Pintomyia fischeri as potential vector of Leishmania infantum. Parasite. 2017;24: 2 doi: 10.1051/parasite/2017002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seblova V, Sadlova J, Carpenter S, Volf P. Speculations on biting midges and other bloodsucking arthropods as alternative vectors of Leishmania. Parasit Vectors. 2014;7: 222 doi: 10.1186/1756-3305-7-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Senghor MW, Niang AA, Depaquit J, Ferté H, Faye MN, Elguero E, et al. Transmission of Leishmania infantum in the canine leishmaniasis focus of Mont-Rolland, Senegal: ecological, parasitological and molecular evidence for a possible role of Sergentomyia sand flies. PLoS Negl Trop Dis. 2016;10(11): e0004940 doi: 10.1371/journal.pntd.0004940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Motoie G, Ferreira GE, Cupolillo E, Canavez F, Pereira-Chioccola VL. Spatial distribution and population genetics of Leishmania infantum genotypes in São Paulo State, Brazil, employing multilocus microsatellite typing directly in dog infected tissues. Infect Genet Evol. 2013;18: 48–59. doi: 10.1016/j.meegid.2013.04.031 [DOI] [PubMed] [Google Scholar]
- 57.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Manual de vigilância e controle da leishmaniose visceral. Brasília: Editora do Ministério da Saúde; 2014.
- 58.Werneck GL, de Pereira TJCF, de Carvalho FAA, Costa CHN, Gouvêa MV, Chaves FC, et al. Avaliação da efetividade das estratégias de controle da leishmaniose visceral na cidade de Teresina, Estado do Piauí, Brasil: resultados do inquérito inicial 2004. Epidemiol Serv Saúde. 2008;17(2): 87–96. [Google Scholar]
- 59.A Costa FAL. The dog as a risk factor in transmission of visceral leishmaniasis: a review. Adv Infect Dis. 2012;2: 37–47. doi: 10.4236/aid.2012.22006 [Google Scholar]
- 60.von Zuben APB, Donalísio MR. Dificuldades na execução das diretrizes do Programa de Vigilância e Controle da Leishmaniose Visceral em grandes municípios brasileiros. Cad Saude Publica. 2016;32(6): e00087415 doi: 10.1590/0102-311X00087415 [DOI] [PubMed] [Google Scholar]
- 61.Millán J, López-roig M, Cabezón O. Absence of Leishmania infantum in cave bats in an endemic area in Spain. Parasitol Res. 2014;113(5): 1993–1995. doi: 10.1007/s00436-014-3855-3 [DOI] [PubMed] [Google Scholar]
- 62.Grimaldi G Jr, Teva A, Ferreira AL, dos Santos CB, Pinto I de-S, de-Azevedo CT, et al. Evaluation of a novel chromatographic immunoassay based on Dual-Path Platform technology (DPP® CVL rapid test) for the serodiagnosis of canine visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2012;106: 54–59. doi: 10.1016/j.trstmh.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 63.Laurenti MD, de Leandro MVS Jr, Tomokane TY, De Lucca HRL, Aschar M, Souza CSF, et al. Comparative evaluation of the DPP® CVL rapid test for canine serodiagnosis in area of visceral leishmaniasis. Vet Parasitol 2014;205: 444–450. doi: 10.1016/j.vetpar.2014.09.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Campinas Environmentally Protected Area, 2013 and 2015.
(XLSX)
Campinas Environmentally Protected Area, April 2014 to March 2015.
(XLS)
Campinas Environmentally Protected Area, April 2014 to March 2015.
(XLS)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. The data of the Leishmania genetic sequences detected in wild animals are available from the GenBank database (accession numbers KX580706 and KX580707).