Abstract
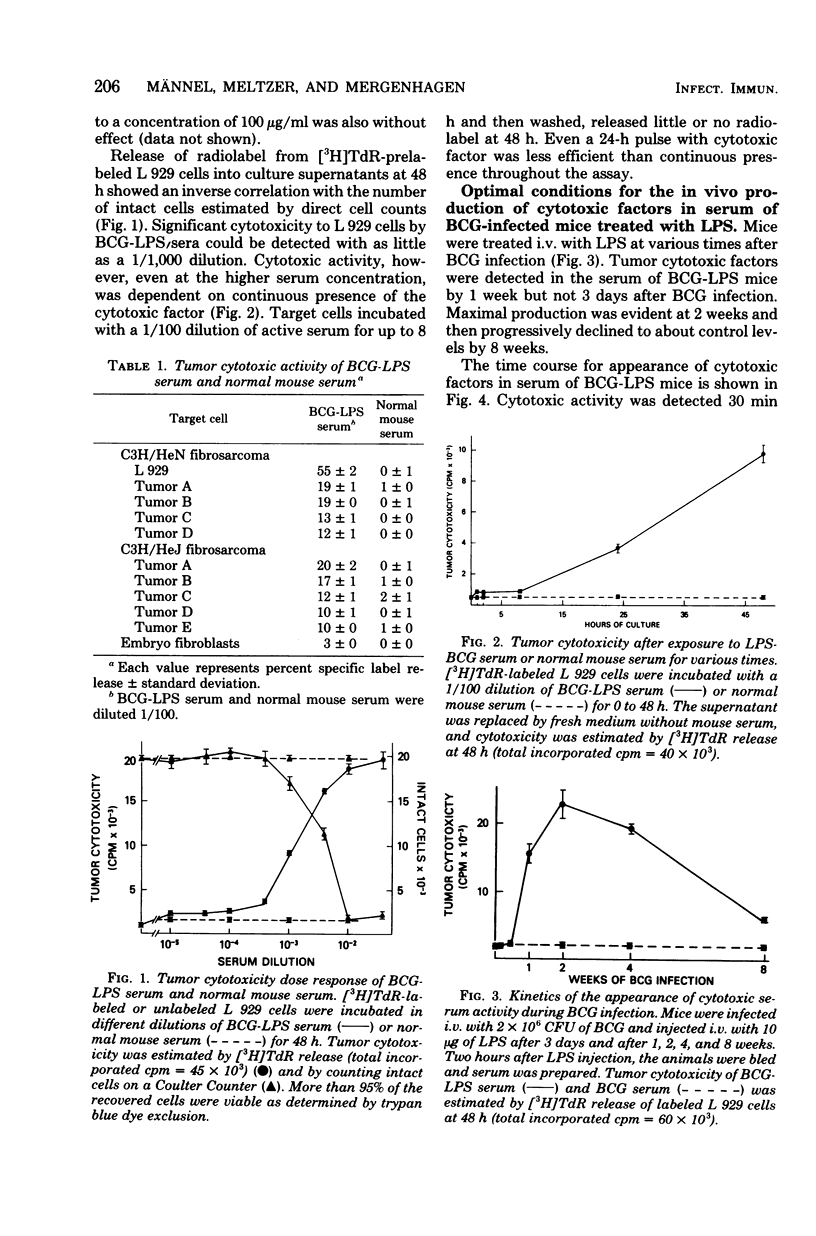
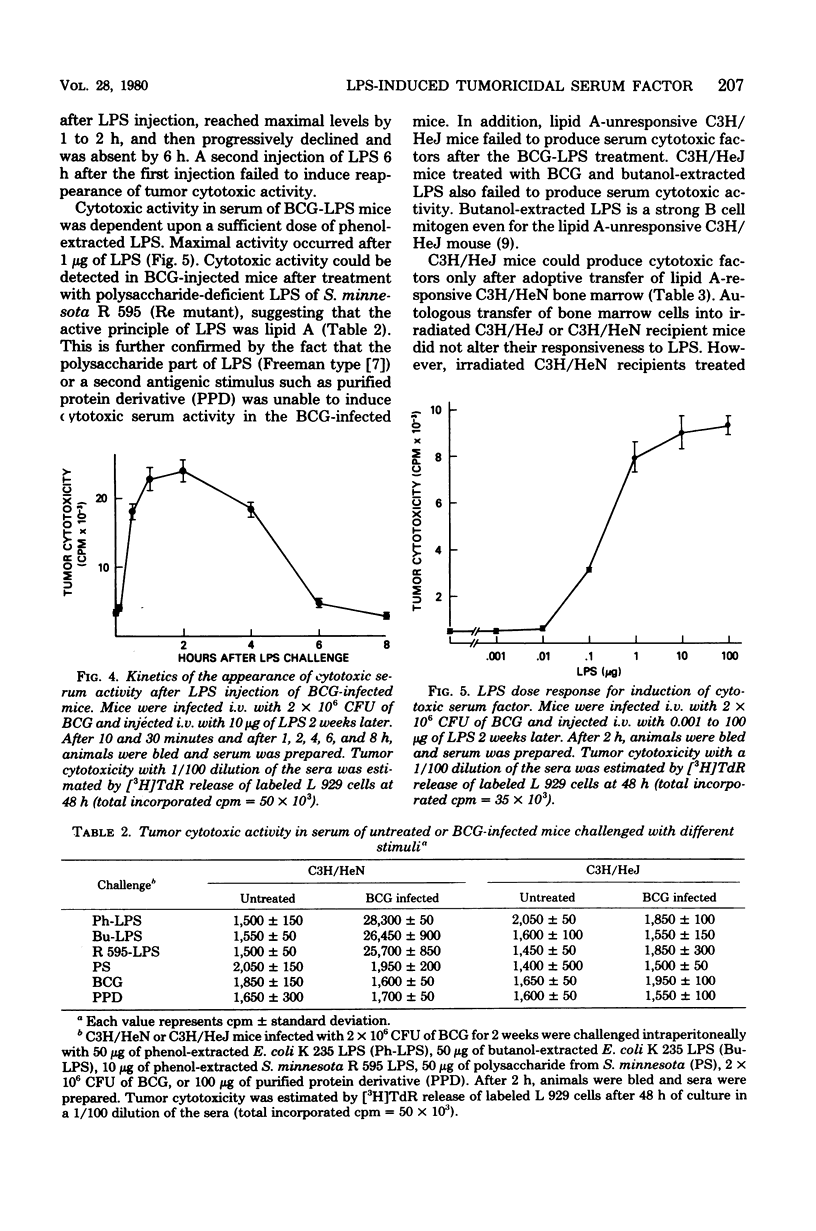
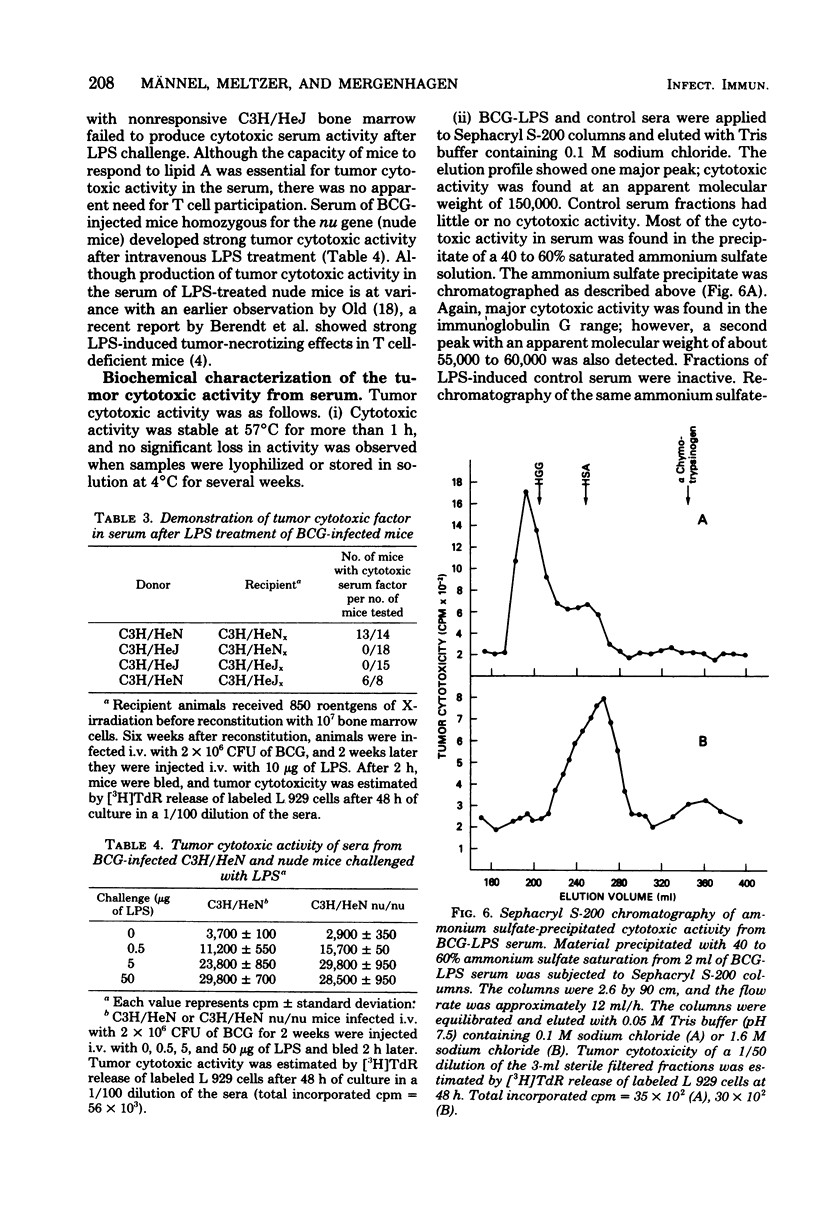
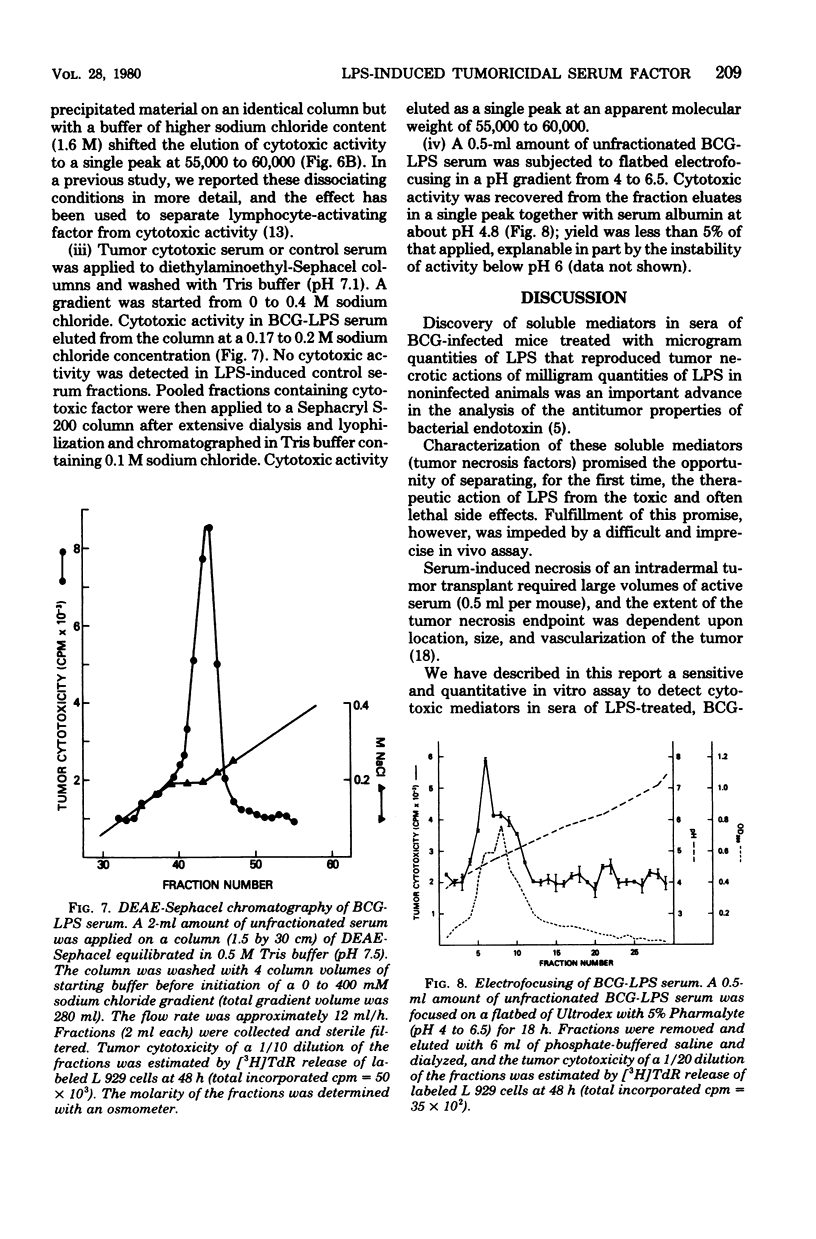
Serum from Mycobacterium bovis BCG-infected mice treated with lipopolysaccharide was cytotoxic to tumor cells in vitro. Serum-induced cytotoxicity was estimated by measuring release of [3H]thymidine into culture supernatants of prelabeled tumor target cells. Serum from BCG-infected mice not treated with lipopolysaccharide or from uninfected mice treated with lipopolysaccharide was inactive. Moreover, although serum cytotoxic activity was evident with 10 syngeneic or allogeneic tumor cell lines, little or no effect was observed with normal embryonic fibroblast target cells. Maximal titers of serum cytotoxic activity were detected 14 days after BCG infection and 2 h after LPS treatment. Serum of BCT-infected, T-cell-deficient nude mice developed strong cytotoxic activity after LPS treatment; however, lipopolysaccharide-insensitive C3H/HeJ mice could produce this cytotoxic activity only after adoptive transfer with lipopolysaccharide-responsive C3H/HeN bone marrow. Physicochemical characterization of the serum cytotoxic activity revealed a heat-stable (56 degrees C, 30 min) entity with a molecular weight of about 60,000 and an isoelectric point at pH 4.8. Biological and physicochemical characteristics of this serum cytotoxic activity as defined by an in vitro assay were very similar to characteristics of tumor necrosis factor and suggest that this molecule may be a major effector mechanism for the antitumor actions of lipopolysaccharide.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksamit R. R., Kim K. J. Macrophage cell lines produce a cytotoxin. J Immunol. 1979 May;122(5):1785–1790. [PubMed] [Google Scholar]
- Bartlett G. L., Zbar B., Rapp H. J. Suppression of murine tumor growth by immune reaction to the Bacillus Calmette-Guérin strain of Mycobacterium bovis. J Natl Cancer Inst. 1972 Jan;48(1):245–257. [PubMed] [Google Scholar]
- Berendt M. J., North R. J., Kirstein D. P. The immunological basis of endotoxin-induced tumor regression. Requirement for T-cell-mediated immunity. J Exp Med. 1978 Dec 1;148(6):1550–1559. doi: 10.1084/jem.148.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman G. G. The preparation and properties of a specific polysaccharide from Bact. typhosum Ty(2): With an addendum by J. St L. Philpot, From the Department of Biochemistry, Oxford. Biochem J. 1942 Apr;36(3-4):340–356. doi: 10.1042/bj0360340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Goodman M. G., Parks D. E., Weigle W. O. Immunologic responsiveness of the C3H/HeJ mouse: differential ability of butanol-extracted lipopolysaccharide (LPS) to evoke LPS-mediated effects. J Exp Med. 1978 Mar 1;147(3):800–813. doi: 10.1084/jem.147.3.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Dobrjansky A., Carswell E. A., Kassel R. L., Old L. J., Fiore N., Schwartz M. K. Partial purification of a serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1976 Feb;73(2):381–385. doi: 10.1073/pnas.73.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helson L., Green S., Carswell E., Old L. J. Effect of tumour necrosis factor on cultured human melanoma cells. Nature. 1975 Dec 25;258(5537):731–732. doi: 10.1038/258731a0. [DOI] [PubMed] [Google Scholar]
- Kramer J. J., Granger G. A. The in vitro induction and release of a cell toxin by immune C57B1-6 mouse peritoneal macrophages. Cell Immunol. 1972 Jan;3(1):88–100. doi: 10.1016/0008-8749(72)90229-8. [DOI] [PubMed] [Google Scholar]
- Matthews N. Tumour-necrosis factor from the rabbit. II. Production by monocytes. Br J Cancer. 1978 Aug;38(2):310–315. doi: 10.1038/bjc.1978.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews N., Watkins J. F. Tumour-necrosis factor from the rabbit. I. Mode of action, specificity and physicochemical properties. Br J Cancer. 1978 Aug;38(2):302–309. doi: 10.1038/bjc.1978.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Sievert H. W., Barlow G. H., Finley R. A., Lee A. Y. Chemical, physical, biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry. 1967 Aug;6(8):2363–2372. doi: 10.1021/bi00860a011. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Betz S. J., Jacobs D. M. Isolation of a lipid A bound polypeptide responsible for "LPS-initiated" mitogenesis of C3H/HeJ spleen cells. J Exp Med. 1976 Sep 1;144(3):840–846. doi: 10.1084/jem.144.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männel D. N., Farrar J. J., Mergenhagen S. E. Separation of a serum-derived tumoricidal factor from a helper factor for plaque-forming cells. J Immunol. 1980 Mar;124(3):1106–1110. [PubMed] [Google Scholar]
- Männel D. N., Rosenstreich D. L., Mergenhagen S. E. Mechanism of lipopolysaccharide-induced tumor necrosis: requirement for lipopolysaccharide-sensitive lymphoreticular cells. Infect Immun. 1979 May;24(2):573–576. doi: 10.1128/iai.24.2.573-576.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor. Clin Bull. 1976;6(3):118–120. [PubMed] [Google Scholar]
- Ostrove J. M., Gifford G. E. Stimulation of RNA synthesis in L-929 cells by rabbit tumor necrosis factor. Proc Soc Exp Biol Med. 1979 Mar;160(3):354–358. doi: 10.3181/00379727-160-40449. [DOI] [PubMed] [Google Scholar]
- Parr I., Wheeler E., Alexander P. Similarities of the anti-tumour actions of endotoxin, lipid A and double-stranded RNA. Br J Cancer. 1973 May;27(5):370–389. doi: 10.1038/bjc.1973.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed W. P., Lucas Z. J. Cytotoxic activity of lymphocytes. V. Role of soluble toxin in macrophage-inhibited cultures of tumor cells. J Immunol. 1975 Aug;115(2):395–404. [PubMed] [Google Scholar]
- Russell S. W., Doe W. F., McIntosh A. T. Functional characterization of a stable, noncytolytic stage of macrophage activation in tumors. J Exp Med. 1977 Dec 1;146(6):1511–1520. doi: 10.1084/jem.146.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers G., Braungart D., Leonard E. J. Mouse lymphotoxin. J Immunol. 1976 Jul;117(1):130–135. [PubMed] [Google Scholar]


