Abstract
Over the past decade, human brain imaging investigations have reported altered regional cerebral blood flow (rCBF) in the interictal phase of migraine. However, there have been conflicting findings across different investigations, making the use of perfusion imaging in migraine pathophysiology more difficult to define. These inconsistencies may reflect technical constraints with traditional perfusion imaging methods such as single‐photon emission computed tomography and positron emission tomography. Comparatively, pseudocontinuous arterial spin labeling (pCASL) is a recently developed magnetic resonance imaging technique that is noninvasive and offers superior spatial resolution and increased sensitivity. Using pCASL, we have previously shown increased rCBF within the primary somatosensory cortex (S1) in adult migraineurs, where blood flow was positively associated with migraine frequency. Whether these observations are present in pediatric and young adult populations remains unknown. This is an important question given the age‐related variants of migraine prevalence, symptomology, and treatments. In this investigation, we used pCASL to quantitatively compare and contrast blood flow within S1 in pediatric and young adult migraineurs as compared with healthy controls. In migraine patients, we found significant resting rCBF increases within bilateral S1 as compared with healthy controls. Furthermore, within the right S1, we report a positive correlation between blood flow value with migraine attack frequency and cutaneous allodynia symptom profile. Our results reveal that pediatric and young adult migraineurs exhibit analogous rCBF changes with adult migraineurs, further supporting the possibility that these alterations within S1 are a consequence of repeated migraine attacks. Hum Brain Mapp 38:4078–4087, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: migraine, magnetic resonance imaging, cerebral blood flow, pseudocontinuous arterial spin labeling, primary somatosensory cortex
INTRODUCTION
Neuroimaging investigations have shown alterations in cerebral perfusion [Hodkinson et al., 2015], volume [DaSilva et al., 2007a], structure [DaSilva et al., 2007b], chemical composition [Prescot et al., 2009], and low‐frequency oscillations [Hodkinson et al., 2016] in the interictal phase of the migraine brain. However, there have been inconsistent findings across studies making the use of imaging as a potential brain biomarker for migraine more difficult to define. One cortical region that appears to show consistency of migraine‐induced changes across multiple adult studies is the primary somatosensory cortex [DaSilva et al., 2007a; Hodkinson et al., 2015; Maleki et al., 2012]. One question related to this is whether these changes are also present in the pediatric and young adult migraine population. This is an important question given the variants of migraine prevalence, symptoms, and treatments from pediatric through to adult populations with episodic migraine [Sonal Sekhar et al., 2012], a population that is less affected by disease duration.
Blood flow in the brain is coupled to regional metabolism that is regulated by neurons and astrocytes [Attwell et al., 2010], and it may be that resting cerebral blood flow reflects the underlying pathophysiology of migraine. Certainly, abnormal cerebral blood flow has been reported in the interictal phase of the migraine brain. For example, single‐photon emission tomography (SPECT) investigations have reported hyperperfusion in widespread cortical regions [Cheng et al., 2013; De Benedittis et al., 1999], whereas others have reported primarily normal cerebral blood flow in migraine patients [Bartolini et al., 2005; Soriani et al., 1997]. Moreover, positron emission tomography (PET) investigations in migraine patients also report conflicting findings, with both widespread hypoperfusion [Kim et al., 2010] and hyperperfusion [Kassab et al., 2009] reported in cortical regions such as the primary somatosensory cortex. These inconsistencies may reflect technical constraints such as the relatively poor spatial resolution and sensitivity to traditional nuclear functional imaging techniques. Furthermore, these methods are invasive and are reliant upon radioactive tracers or contrasts, limiting its repeatability and application which is particularly problematic in pediatric and adolescent populations.
We have previously employed a recently developed magnetic resonance imaging technique, psuedocontinuous arterial spin labeling (pCASL), to investigate changes in regional cerebral blood flow in the interictal phase of migraine within an adult population [Hodkinson et al., 2015]. PCASL is noninvasive, offers superior spatial resolution, and allows for the absolute quantification of cerebral blood flow. Specifically, migraine patients displayed bilateral hyperperfusion within the primary somatosensory cortex, where the degree of blood flow was positively correlated to migraine frequency [Hodkinson et al., 2015]. Here, we used pCASL to extend our previous findings and determine whether these correspond with developing brains of pediatric and young adult populations. Although there are variants in prevalence, symptomology, and treatments, these differences are not substantial to prompt differential diagnosis. Given this, we hypothesize that pediatric and young adult migraine patients will display greater regional blood flow within the primary somatosensory cortex as compared to healthy controls, and that these values will be positively correlated with migraine frequency.
MATERIALS AND METHODS
Subjects
Twenty‐six migraine patients (13 males, 13 females; mean ± SEM age: 15.7 ± 0.95 years; range 8–24 years) and 26 healthy age‐ and gender‐matched controls (13 males, 13 females; mean ± SEM age: 15.5 ± 0.89 years; range 9–24 years) were recruited for the study. All migraine patients were recruited by the Neurology and Headache Clinics at Boston Children's Hospital and advertisements within the general community (Longwood Medical and Greater Boston Area). Nine patients were recruited from Boston Children's Hospital Clinics (either the Neurology or Headache Clinic) with the remainder recruited from the Greater Boston Area (craigslist, flyers around community, word‐of‐mouth). Migraine patients were diagnosed in accordance with the International Classification for Headache Disorders, second edition (ICHD‐II) (2004). Patients were excluded from the study if they reported symptoms of developing a migraine attack 72 h prior to the study, or 24 h following the study session. Migraine patients were not on any daily preventative medications at the time of the study. Healthy controls were recruited from the following sources: (1) flyers posted on bulletin boards in the Longwood Medical Area and Greater Boston Area; (2) online list servers (e.g., craigslist, college job boards); (3) previous participants in the group's studies; and (4) word of mouth. Healthy controls were excluded if they reported symptoms consistent with any type of migraine, or if they had any ongoing pain condition. All subjects were instructed not to take other medications and to avoid caffeine within 4 h of the study scanning session. Four controls and two patients were taking daily vitamins. Two controls and two patients were taking allergy medications. One patient was taking asthma medications. On the day of the study session, all migraine patients were required to report, on average, their migraine frequency, duration, quality, and their pain distribution [Barmettler et al., 2015]. In addition, all patients completed items derived from the Allodynia Symptom Questionnaire [Ashkenazi et al., 2007; Jakubowski et al., 2005; Lipton et al., 2008]. All procedures for the study were approved by the Institutional Review Board at Boston Children's Hospital, Harvard Medical School.
MRI Acquisition
All subjects were positioned supine within a Siemens Magnetom Trio 3 T MRI scanner (Siemens Healthcare Inc., USA), and data acquired using a 32‐channel head coil. For image registration, a high‐resolution T1‐weighted anatomical image was collected (repetition time = 2,520 ms, echo time = 1.7 ms, raw voxel size = 1.0 × 1.0 × 1.0 mm thick). With the subject still at rest, a sequence of pseudocontinuous arterial spin labeling (pCASL) [Wu et al., 2007] covering the whole brain was collected (40 label/control image sets, 24 axial slices in ascending order, TR/TE = 3,890/12 ms, matrix = 64 × 64, raw voxel size = 3.4 × 3.4 × 6.0 mm thick, labeling time = 1,480 ms, post label delay time = 1,300 ms, slice acquisition time = 46.25 ms). The total pCASL acquisition time corresponded to 5.2 min.
MRI Analysis
Image preprocessing
Using SPM12 [Friston et al., 1994], image preprocessing and quantification of cerebral blood flow (CBF) maps were undertaken with the ASL toolbox [Wang et al., 2008]. For each subject, label and control image series were independently motion corrected to remove artificially induced motion [Wang, 2012]. The difference between the control and label acquisitions were then estimated by surround subtraction [Lu et al., 2006] and CBF was quantified according to the following equation:
where ΔM is the difference between the label and control images (i.e., perfusion difference), λ is the blood/tissue water partition coefficient, R 1a is the longitudinal relaxation rate of blood, α is the tagging efficiency, ω is the postlabeling delay time, τ is the duration of the labeling RF pulse train, and M 0 is estimated by the control image intensity [Wang et al., 2008]. The parameters set in this investigation were λ = 0.9 g/mL [Herscovitch and Raichle, 1985], α = 0.85 [Dai et al., 2008], ω = 1,300 ms, and τ = 1,480 ms. Given that the T1 relaxation time of blood varies considerably with age and gender in pediatric populations, we set this parameter in accordance with a population‐based estimate of blood T1 as predicted by Wu et al. [2010]. The transformation of pCASL image series to absolute units of CBF (mL/100 g/min) was computated in native space.
The T1‐weighted image was then spatially normalized to the Montreal Neurological Institute (MNI) template using SPM12's unified segmentation and these parameters applied to the T1‐coregistered mean CBF map. The resultant spatially normalized (2 mm isotropic) CBF maps were then spatially smoothed using an 8 mm full‐width at half‐maximum (FWHM) Gaussian filter.
Resting CBF analysis
Significant differences in regional CBF between controls and migraineurs were determined within a general linear model using independent two‐sample t tests. To account for the nonlinear trajectories of age on regional CBF observed in pediatric populations [Satterthwaite et al., 2014], we applied a quadratic transformation to each subjects age and included these values as a nuisance variable. Gender and global CBF were also factored out by modeling them as nuisance variables. The primary somatosensory cortex, derived from the Harvard–Oxford cortical atlas [Smith et al., 2004], was set at a probabilistic threshold of 25%, binarized and included as an explicit mask so that only voxels within this region were explored. Following an initial uncorrected threshold of P < 0.001, small volume corrections of 12‐mm‐radius spheres were performed using the local activation peak coordinates from the group comparison. For each cluster, absolute blood flow values were extracted and significant differences in resting blood flow between controls and migraine patients were identified using two‐sample t test (P < 0.05). Significant linear relationships between blood flow values and age, disease duration, and frequency were determined using Pearson's partial correlation analyses, while controlling for global CBF influences (P < 0.05).
RESULTS
Clinical Demographics
Following the scanning session, four migraine patients were excluded from the study due to reports of a migraine attack within 24 hrs, with the remaining 22 patients (10 males, 12 females; mean ± SEM age: 15.1 ± 1.1 years; range 8–24 years) included in the analysis. Subsequently, the 4 age‐ and gender‐matched controls were excluded, with the remaining 22 controls (10 males, 12 females; mean ± SEM age: 14.9 ± 0.9 years; range 9–24 years) included in the analysis. Patients reported, on average, a migraine duration of 5 years (±SEM: 0.86, range: 0.5–14 years) and an average migraine attack frequency of 3/month (±SEM: 0.71, range: 0.5–15 episodes). Of the 22 patients, 13 reported bilateral pain (59%), 5 with unilateral pain occurring on the right or left side (23%), and 4 with unilateral pain confined to the left side (18%) during a migraine attack. Individual migraine patient characteristics, including pain duration, frequency, laterality, and analgesic medications are displayed in Table 1. Finally, analogous with our previous migraine investigation [Hodkinson et al., 2015], we found a significant correlation between migraine duration and the patients' age (r = 0.84, P = 0.0001).
Table 1.
Clinical characteristics of migraine patients
| Patient code | Age (years) | Gender | Age of onset | Duration (years) | Frequency (/month) | Laterality | Analgesic medication |
|---|---|---|---|---|---|---|---|
| M01 | 15 | F | 7 | 8 | 3 | U (L & R) | Sumatriptan, ibuprofen |
| M02 | 13 | F | 10 | 3 | 6 | U (L & R) | None |
| M03 | 13 | M | 9 | 4 | 2 | B | Naproxen, sumatriptan, ondansetron |
| M04 | 23 | F | 13 | 10 | 7 | B | None |
| M05 | 16 | M | 13 | 3 | 2 | B | Imitrex, ibuprofen |
| M06 | 23 | M | 9 | 14 | 2 | B | Acetaminophen |
| M07 | 13 | M | 11 | 2 | 1 | B | Naproxen, imitrex |
| M08 | 16 | F | 10 | 6 | 4 | B | Imitrex, motrin |
| M09 | 9 | F | 7 | 2 | 1 | U (L & R) | Tyenol, ibuprofen |
| M10 | 15 | F | 11 | 4 | 0.5 | U (L) | Imitrex |
| M11 | 15 | M | 12 | 3 | 1 | B | Imitrex, naproxen |
| M12 | 8 | F | 8 | 0.5 | 5 | B | None |
| M13 | 16 | M | 13 | 3 | 0.3 | U (L) | Almotriptan |
| M14 | 13 | F | 11 | 2 | 1 | U (L) | Ibuprofen, imitrex |
| M15 | 9 | M | 5 | 4 | 5 | B | Imitrex |
| M16 | 16 | F | 6 | 10 | 3 | B | Ibuprofen, riboflavin, sumatriptan |
| M17 | 24 | M | 14 | 10 | 0.2 | B | Advil |
| M18 | 21 | F | 14 | 7 | 1.5 | B | Ibuprofen |
| M19 | 10 | M | 7 | 3 | 2 | U (L & R) | Zomatriptan, ondansetron |
| M20 | 8 | M | 7 | 1 | 15 | B | Ibuprofen |
| M21 | 22 | F | 9 | 13 | 0.2 | U (L) | Excedrin migraine |
| M22 | 13 | F | 10 | 3 | 1 | U (L & R) | Imitrex |
| Mean ± SEM | 15.1 ± 1.1 | 9.8 ± 0.6 | 5.2 ± 0.9 | 2.9 ± 0.7 |
F, female; M, male; B, bilateral; U, unilateral; L, left; R, right.
Migraine patients were also required to complete the Allodynia Symptom Questionnaire to determine if, during a migraine attack, they typically developed cutaneous allodynia (Fig. 1A). Of the 22 patients, 18 reported at least one symptom of cutaneous allodynia (82%), with 11 of these patients reporting at least three symptoms (61%). Given these reports, it is pertinent to note that some descriptors were gender specific, including “shaving” and “necklace” and that not all items were applicable to all patients, such as “eyeglasses.” In contrast, patients reported their pain quality during a migraine attack, with the greatest proportion of patients describing their pain as “throbbing” (77%) and “pounding” (73%) (Fig. 1B). Finally, 19 of the 22 patients illustrated their typical pain distribution during a migraine attack, revealing a predominate spread to the frontotemporal region (Fig. 1C).
Figure 1.
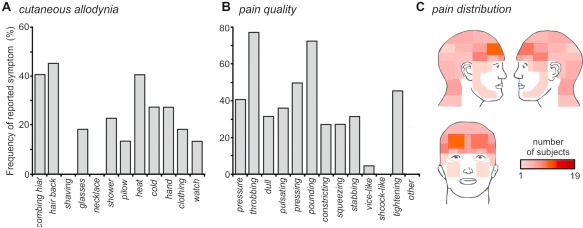
(A) Cutaneous allodynia symptom profile; frequency (percentage of patients) responses to Allodynia Symptom Questionnaire items of skin hypersensitivity. (B) Pain quality; frequency (percentage of patients) of descriptors chosen. (C) Pain distribution; overlap of individual pain referral patterns, where the pink–orange–red scale bar corresponds to degree of overlap. [Color figure can be viewed at http://wileyonlinelibrary.com]
Resting Cerebral Blood Flow
Within the primary somatosensory cortex, migraine patients displayed significantly greater rCBF as compared with healthy controls (Fig. 2A). Significant increases in rCBF were observed in both the left (mean [±SEM] mL/100g/min; controls: 52.4 ± 1.9, migraineurs: 64.2 ± 3.0; P = 0.0023) and the right (controls: 55.1 ± 2.0, migraineurs: 67.1 ± 2.6; P = 0.0009) primary somatosensory cortex (Fig. 2B and Table 2). There were no significant rCBF decreases in migraine patients as compared with controls. Furthermore, there was no significant difference in global CBF between these groups (controls: 48.2 ± 1.7, migraineurs: 47.0 ± 2.0; P = 0.66).
Figure 2.
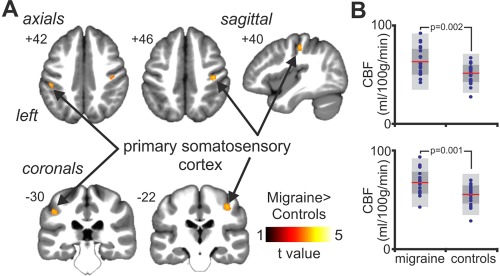
(A) Regional blood flow increases (hot scale bar) in migraine subjects as compared with healthy controls, overlaid onto axials, coronals and a sagittal T1‐weighted anatomical image set. Slice locations are located at the top left of each image and are in Montreal Neurological Institute space. Note that voxel‐wise comparison was restricted to the primary somatosensory cortex, derived from Harvard–Oxford probabilistic cortical atlas. (B) Plots of cerebral blood flow (CBF) values for the left (top) and right (bottom) primary somatosensory cortex. Individual data plots are shown in blue, with the mean (red line), 1 standard deviation (dark‐grey region) and with the 95% confidence interval (light‐grey region). Significant differences between migraine patients and healthy controls were determined using an independent two‐sample t test, with the P value above the plots. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Brain regions in which cerebral blood flow (CBF) were significantly greater in migraine subjects as compared with healthy controls
| X | Y | Z | t value | Cluster size | Mean ± SEM CBF (ml/100 g/min) | ||
|---|---|---|---|---|---|---|---|
| Controls | Migraineurs | ||||||
| Migraineurs > Controls | |||||||
| Left primary somatosensory cortex | −46 | −32 | 40 | 3.55 | 39 | 52.4 ± 1.9 | 64.2 ± 3.0 |
| Right primary somatosensory cortex | 38 | −22 | 46 | 3.57 | 50 | 55.1 ± 2.0 | 67.1 ± 2.6 |
Locations are in Montreal Neurological Institute (MNI) space.
Within the right primary somatosensory cortex, rCBF was positively correlated with migraine attack frequency (r = 0.40, P = 0.033) and cutaneous allodynia (CA) symptom profile (r = 0.46, P = 0.031) (Fig. 3). In contrast, no significant correlations were observed in rCBF with the duration of migraine (r = 0.36, 0.10) or the patients' age (r = 0.10, P = 0.66). Within the left primary somatosensory cortex, no significant correlations were observed between rCBF and the patients' headache characteristics (migraine frequency: r = 0.23, P = 0.15; CA symptom: r = 0.10, P = 0.66; duration: r = 0.08, P = 0.72; age: r = −0.14, P = 0.53) (Fig. 3).
Figure 3.
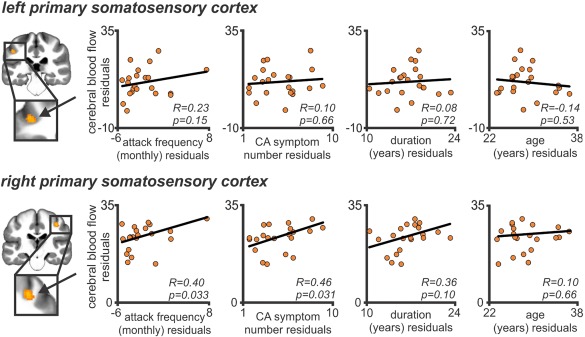
Partial correlation between cerebral blood flow (CBF) values within the primary somatosensory cortex and clinically reported headache characteristics (controlling for global CBF). Clinical variables include (from left to right) migraine frequency (per month), number of cutaneous allodynia (CA) symptoms, duration of migraine (years), and age of the patients (years). Note that the right primary somatosensory cortex was positively correlated with migraine attack frequency. [Color figure can be viewed at http://wileyonlinelibrary.com]
Finally, we are confident that movement did not have significantly impact our results as there were no significant movement‐related differences between healthy controls and migraineurs in translation (mean [±STD] mm; x, controls: 0.12 ± 0.11, migraineurs: 0.11 ± 0.19, P = 0.95; y, controls: 0.10 ± 0.09, migraineurs: 0.11 ± 0.12, P = 0.75; z, controls: 0.25 ± 0.37, migraineurs: 0.31 ± 0.42, P = 0.61) or rotation (mean [±STD] degrees; roll, controls: 0.005 ± 0.008, migraineurs: 0.005 ± 0.005, P = 0.86; pitch, controls: 0.002 ± 0.010, migraineurs: 0.002 ± 0.001, P = 0.89; yaw, controls: 0.003 ± 0.005, migraineurs: 0.002 ± 0.003, P = 0.37).
DISCUSSION
Consistent with our hypothesis, these data reveal that pediatric and young adult migraine patients have greater rCBF within the primary somatosensory cortex (S1) as compared to healthy controls. Furthermore, analogous with our previous adult investigation [Hodkinson et al., 2015], we found that resting rCBF within the right S1 was positively correlated with migraine frequency. In addition, somatosensory blood flow was positively correlated with cutaneous allodynia symptom profile. Analogous findings were also reported with other headache characteristics, specifically, no association between resting rCBF and duration or age were observed. However, in contrast to our initial hypothesis, we found no relationship between resting rCBF within the left S1 and migraine frequency. These results support the idea that pediatric and young adult patients exhibit similar patterns of altered rCBF within the somatosensory cortices, and that these changes are related to the degree of migraine frequency.
S1 and Migraine
Over recent years, brain imaging investigations have highlighted the role of S1 in pain processing; stimulus localization and intensity [Timmermann et al., 2001; Torquati et al., 2002], discrimination [Bornhövd et al., 2002; Moulton et al., 2005; Torquati et al., 2002], and perceived spread [Macefield et al., 2007]. However, we, and others have shown alterations in S1 in the interictal period (i.e., the absence of migraine‐related head pain). Instead, these resting changes may be the consequence of repetitive headache pain that, over time, induce morphometric and functional changes in S1. Indeed, a number of primary afferent nociceptive drivers may contribute to these changes including: (1) sensitization within the trigeminal primary afferent [Strassman et al., 1996], that may perpetuate via the trigeminothalamic tract to contribute to the somatosensory hyperexcitability [Lang et al., 2004; Schwedt et al., 2014] and loss of habituation [Stankewitz et al., 2013] and (2) cephalic cutaneous allodynia observed in most migraine patients [Bigal et al., 2008; Burstein et al., 2000; Lipton et al., 2008]. Indeed, we observed a significant relationship between blood flow in S1 and the number of cutaneous allodynia symptoms. Although we did not report a significant relationship in our previous adult investigation, it is pertinent to note that we observed a positive linear trend [Hodkinson et al., 2015]. These peripheral nociceptive drivers seem to produce changes in S1 in the homunculus where the head, and more particularly the forehead is represented in healthy subjects [DaSilva et al., 2002] and in migraine patients [Maleki et al., 2012].
Although a number of authors have reported blood flow increases in S1 during the interictal phase of migraine [Hodkinson et al., 2015; Kassab et al., 2009], there are conflicting findings across different investigations. In addition to other brain sites, positron emission tomography (PET) investigations have reported both blood flow increases [Kassab et al., 2009] and decreases [Kim et al., 2010] within S1 in migraine patients as compared with healthy controls. In striking contrast, the application of pCASL has yielded consistent findings with our previous study [Hodkinson et al., 2015], further supporting the role of S1 in migraine and the use of pCASL as an objective and reliable method to assess slowly evolving changes in cerebral blood flow [Hodkinson et al., 2013]. Within the right S1, we also found a consistent relationship between resting blood flow and migraine attack frequency; that is, the more frequent the attack, the greater the blood flow [Hodkinson et al., 2015]. This result may simply reflect laterality, a question that has not been closely evaluated in the migraine literature. However, is it worth noting that our migraine cohort exhibited predominate left‐sided migraine attacks, and therefore, it is not surprising that we found a greater blood flow difference and attack frequency association within the contralateral (right) somatosensory cortex.
In conjunction with altered baseline rCBF, numerous brain imaging investigations have reported increased S1 responsiveness in the interictal phase of migraine patients. For example, in response to noxious heat stimulation, high‐frequency migraine patients were associated with significantly greater activation within S1 as compared with the low‐frequency group [Maleki et al., 2012]. Similarly, Schwedt et al. [2014] employed blood oxygen level dependent (BOLD) contrast and reported significant increases in S1 activation in migraine patients' as compared with healthy controls; however, activation strength was not correlated with migraine frequency. In contrast, Lang et al. [2004] employed a magnetoencephalogram (MEG) in migraine patients and reported, in response to electrical stimulation, increased S1 excitability (N20m population) as compared to healthy controls that was positively associated with migraine frequency but not disease duration or age‐related affect. Although these findings are highly supportive of migraine attack‐induced changes, it is pertinent to note that equivalent dipole moment strength of N20m has been shown to be significantly associated with age in adult populations [Huttunen et al., 1999]. This is an important consideration given our pediatric and young adult population, and therefore whether these findings are directly applicable remain relatively unclear. Taken together, these resting blood flow changes and altered responsivity within S1 suggest that they are more likely to be the consequence of repetitive migraine attacks, rather than a component of the disease etiology.
Other Changes in S1 in Migraineurs
Given these functional alternations, it is conceivable that altered resting regional metabolism may be accompanied by volumetric and/or structural changes, or vice versa. However, it is pertinent to note that the association between changes in perfusion and tissue volume has yielded mixed results. Some investigations report a causal relationship between cerebral blood flow and grey matter volume [Fierstra et al., 2010; Várkuti et al., 2011], whereas others do not [Chen et al., 2011]. The reasons may vary from patient subtype and medications to magnetic sequences. However, in our group of adults and children using multiple approaches and the same magnets, we observe similar results. Thus, while it does remain in dispute, this section highlights the morphometric changes observed within the primary somatosensory cortex in migraine patients. Our focus on somatosensory volumetric and structural changes provides the reader with an overview of the potential contributions of associated morphometric changes. Indeed, we have included evidence for S1 functionally related structural changes in high‐ and low‐frequency migraine patients [Maleki et al., 2012].
Although numerous investigations have employed voxel‐based morphometry (VBM) to examine grey matter structure within migraine patients, to the best of our knowledge, no study to date has shown alterations within S1 cortex as compared with healthy controls [Jin et al., 2013; Rocca et al., 2006; Schmidt‐Wilcke et al., 2008; Valfre et al., 2008]. Instead, consistent reductions in grey matter volume have been observed in higher brain sites, including the dorsolateral prefrontal cortex [Rocca et al., 2006; Schmidt‐Wilcke et al., 2008], and it may be these reductions in grey matter volume that drive the functional alterations observed in migraine. For example, migraine patients' exhibit altered pain modulation [Sandrini et al., 2006] and sensory processing, both of which can be modulated by the dorsolateral prefrontal cortex [Lorenz et al., 2003; O'Reilly, 2010; Sandrini et al., 2006; Youssef et al., 2016]. Alternatively, it may reflect technical constraints. Indeed, the majority of investigations employing a recently developed morphometric analysis, surface‐based morphometry (SBM), have reported alterations in S1 cortical thickness in migraine patients. Measures of SBM have been associated with high consistency throughout aging [Fjell et al., 2009], and increased age‐related sensitivity as compared with traditional VBM methods [Hutton et al., 2009]. Nonetheless, recent SBM investigations have reported inconsistent findings in migraine patients as compared with healthy controls, reporting increased [DaSilva et al., 2007a; Kim et al., 2014], reduced [Hougaard et al., 2016], or no change [Datta et al., 2011] in S1 cortical thickness. In contrast, Maleki et al. [2012] compared high‐ with low‐frequency migraine patients. High‐frequency migraine patients were associated with increased S1 cortical thickness in conjunction with increased noxious‐induced activation; this is a critical distinction and highlights a potential adaptive response of S1 to repeated migraine attacks [Maleki et al., 2012]. Although, these functionally related structural changes may reflect the observed changes in resting metabolic state in migraine, direct evidence for neural plasticity, and synaptic remodeling is required.
S1 Across Age in Migraineurs
Notwithstanding the contribution of adult investigations, little is known about the developing brain in migraine patients. This is surprising given that pediatric cohorts offer the unique opportunity to investigate migraine at the earliest stage of the disease, evaluating whether these altered resting changes develop with disease or whether it represents a relatively stable biomarker that prompts an individual to frequent migraine attacks. Instead, this may reflect technical, biological, and recruitment constraints in pediatric investigations as compared with imaging adults [Thukral, 2015]. To the best of our knowledge, this study is the first to quantitatively compare resting rCBF in pediatric and young adult migraine patients as compared with healthy controls. Here, we report similar findings with our previous adult investigation [Hodkinson et al., 2015] and speculate that these altered resting rCBF changes are the consequence of repeated attacks, although we cannot rule out the possibility that altered resting metabolic demand may be further modified by migraine disease, or both. Interestingly, morphometric changes in a pediatric cohort also report similar findings with adult populations. Specifically, Rocca et al. [2014] reported no S1 grey matter changes as compared with healthy controls, where reductions were observed in other brain sites including the dorsolateral prefrontal cortex.
Collectively, the few pediatric migraine investigations that have been undertaken reveal similar resting functional and structural alterations as those reported in adult populations. Of course, whether these resting brain alterations precede the migraine disease, or whether it is a consequence of repeated migraine attacks remains unclear. However, the finding that similar changes are observed in adults as in children raises the question as to whether the changes are a consequence of repeated migraine attacks versus a predisposing condition of the disease state. With respect to the former, (1) the region involved includes the somatosensory cortex involving the head suggesting a correlation of nociceptive drive enhancing dendritic complexity, cortical thickening, and a substrate that may have associated increased blood flow; (2) we observe changes across age; and (3) adult patients with increased frequency show increased changes in S1 cortex [Maleki et al., 2012]. It should be noted that repeated motor stimuli increase cortical structure and morphometry in the motor regions with training [Hänggi et al., 2015], a process that may parallel the repeated sensory nociceptive attacks. However, the dynamic range of cortical changes in S1 is noted in another study of disease reversal [Hubbard et al., 2016] from chronic to episodic migraine. There is no way of defining the predisposing versus specific nociceptive drive. Despite these, investigating migraine at the earliest stage (i.e., pediatric populations) will provide greater insight into answering these questions, and whether these resting alterations are reversible following recovery.
LIMITATIONS
There are a number of limitations of this investigation worth noting. First, an important biological consideration is the differential trajectories of cerebral blood flow throughout development in age and gender when comparing to adult investigations [Satterthwaite et al., 2014]. In this investigation, we accounted for the differential T1 relaxation time of blood within age and gender; however, a potential limitation is that we assume the model derived by Wu et al. [2010]. Second, pubertal status can influence the evolution of cerebral blood flow [Satterthwaite et al., 2014]. Here, we did not account for the circulatory hormonal levels for these subjects, and therefore, potential influence of neuroendocrine factors driving these observed results remains unclear. Finally, given the intrinsic resolution of ASL, the effects of partial volume due to heterogeneous raw voxels are problematic and hence can influence the blood flow values reported in this investigation [Asllani et al., 2008].
CONCLUSIONS
These data reveal that pediatric and young adult migraine patient populations are associated with increased resting blood flow within the primary somatosensory cortex. Furthermore, consistent with adult populations, the degree of blood flow was positively related to migraine frequency, suggesting that repeated migraine attacks over time result in abnormal resting metabolic demand, likely reflecting neuronal and/or glial adaptive or maladaptive alterations.
ACKNOWLEDGMENTS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would also like to thank Adriana Johnson for her assistance with recruitment and data collection.
REFERENCES
- Ashkenazi A, Silberstein S, Jakubowski M, Burstein R (2007): Improved identification of allodynic migraine patients using a questionnaire. Cephalalgia 27:325–329. 2004. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 24 Suppl 1:9–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asllani I, Borogovac A, Brown TR (2008): Regression algorithm correcting for partial volume effects in arterial spin labeling MRI. Magn Reson Med 60:1362–1371. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA (2010): Glial and neuronal control of brain blood flow. Nature 468:232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barmettler G, Brawn J, Maleki N, Scrivani S, Burstein R, Becerra L, Borsook D (2015): A new electronic diary tool for mapping and tracking spatial and temporal head pain patterns in migraine. Cephalalgia 35:417–425. [DOI] [PubMed] [Google Scholar]
- Bartolini M, Baruffaldi R, Paolino I, Silvestrini M (2005): Cerebral blood flow changes in the different phases of migraine. Funct Neurol 20:209–211. [PubMed] [Google Scholar]
- Bigal ME, Ashina S, Burstein R, Reed ML, Buse D, Serrano D, Lipton RB (2008): Prevalence and characteristics of allodynia in headache sufferers: A population study. Neurology 70:1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornhövd K, Quante M, Glauche V, Bromm B, Weiller C, Büchel C (2002): Painful stimuli evoke different stimulus–response functions in the amygdala, prefrontal, insula and somatosensory cortex: A single‐trial fMRI study. Brain 125:1326–1336. [DOI] [PubMed] [Google Scholar]
- Burstein R, Yarnitsky D, Goor‐Aryeh I, Ransil BJ, Bajwa ZH (2000): An association between migraine and cutaneous allodynia. Ann Neurol 47:614–624. [PubMed] [Google Scholar]
- Chen JJ, Rosas HD, Salat DH (2011): Age‐associated reductions in cerebral blood flow are independent from regional atrophy. NeuroImage 55:468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MH, Wen SL, Zhou HJ, Lian‐Fang B, Li JF, Xie LJ (2013): Evaluation of headache and regional cerebral flood flow in patients with migraine. Clin Nucl Med 38:874–877. [DOI] [PubMed] [Google Scholar]
- Dai W, Garcia D, de Bazelaire C, Alsop DC (2008): Continuous flow‐driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med 60:1488–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaSilva AF, Granziera C, Snyder J, Hadjikhani N (2007a): Thickening in the somatosensory cortex of patients with migraine. Neurology 69:1990–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaSilva AF, Granziera C, Tuch DS, Snyder J, Vincent M, Hadjikhani N (2007b): Interictal alterations of the trigeminal somatosensory pathway and periaqueductal gray matter in migraine. Neuroreport 18:301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaSilva AFM, Becerra L, Makris N, Strassman AM, Gonzalez RG, Geatrakis N, Borsook D (2002): Somatotopic activation in the human trigeminal pain pathway. J Neurosci 22:8183–8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta R, Detre JA, Aguirre GK, Cucchiara B (2011): Absence of changes in cortical thickness in patients with migraine. Cephalalgia 31:1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedittis G, Da Passano CF, Granata G, Lorenzetti A, Giuffre R (1999): CBF changes during headache‐free periods and spontaneous/induced attacks in migraine with and without aura: A TCD and SPECT comparison study. J Neurosurg Sci 43:141. [PubMed] [Google Scholar]
- Fierstra J, Poublanc J, Han JS, Silver F, Tymianski M, Crawley AP, Fisher JA, Mikulis DJ (2010): Steal physiology is spatially associated with cortical thinning. J Neurol Neurosurg Psychiatry 81:290. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Walhovd KB (2009): High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex 19:2001–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ (1994): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2:189–210. [Google Scholar]
- Hänggi J, Langer N, Lutz K, Birrer K, Mérillat S, Jäncke L (2015): Structural brain correlates associated with professional handball playing. PLoS One 10:e0124222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herscovitch P, Raichle ME (1985): What is the correct value for the brain–blood partition coefficient for water? J Cereb Blood Flow Metab 5:65–69. [DOI] [PubMed] [Google Scholar]
- Hodkinson DJ, Krause K, Khawaja N, Renton TF, Huggins JP, Vennart W, Thacker MA, Mehta MA, Zelaya FO, Williams SC, Howard MA (2013): Quantifying the test‐retest reliability of cerebral blood flow measurements in a clinical model of on‐going post‐surgical pain: A study using pseudo‐continuous arterial spin labelling. NeuroImage Clin 3:301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodkinson DJ, Veggeberg R, Wilcox SL, Scrivani S, Burstein R, Becerra L, Borsook D (2015): Primary somatosensory cortices contain altered patterns of regional cerebral blood flow in the interictal phase of migraine. PLoS One 10:e0137971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodkinson DJ, Wilcox SL, Veggeberg R, Noseda R, Burstein R, Borsook D, Becerra L (2016): Increased amplitude of thalamocortical low‐frequency oscillations in patients with migraine. J Neurosci 36:8026–8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougaard A, Amin FM, Arngrim N, Vlachou M, Larsen VA, Larsson HB, Ashina M (2016): Sensory migraine aura is not associated with structural grey matter abnormalities. NeuroImage Clin 11:322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard CS, Becerra L, Smith JH, DeLange JM, Smith RM, Black DF, Welker KM, Burstein R, Cutrer FM, Borsook D (2016): Brain changes in responders vs. non‐responders in chronic migraine: Markers of disease reversal. Front Hum Neurosci 10:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton C, Draganski B, Ashburner J, Weiskopf N (2009): A comparison between voxel‐based cortical thickness and voxel‐based morphometry in normal aging. NeuroImage 48:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen J, Wikstrom H, Salonen O, Ilmoniemi RJ (1999): Human somatosensory cortical activation strengths: Comparison between males and females and age‐related changes. Brain Res 818:196–203. [DOI] [PubMed] [Google Scholar]
- Jakubowski M, Silberstein S, Ashkenazi A, Burstein R (2005): Can allodynic migraine patients be identified interictally using a questionnaire? Neurology 65:1419–1422. [DOI] [PubMed] [Google Scholar]
- Jin C, Yuan K, Zhao L, Zhao L, Yu D, von Deneen KM, Zhang M, Qin W, Sun W, Tian J (2013): Structural and functional abnormalities in migraine patients without aura. NMR Biomed 26:58–64. [DOI] [PubMed] [Google Scholar]
- Kassab M, Bakhtar O, Wack D, Bednarczyk E (2009): Resting brain glucose uptake in headache‐free migraineurs. Headache 49:90–97. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim JB, Suh SI, Seo WK, Oh K, Koh SB (2014): Thickening of the somatosensory cortex in migraine without aura. Cephalalgia 34:1125–1133. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim S, Suh SI, Koh SB, Park KW, Oh K (2010): Interictal metabolic changes in episodic migraine: A voxel‐based FDG‐PET study. Cephalalgia 30:53–61. [DOI] [PubMed] [Google Scholar]
- Lang E, Kaltenhauser M, Neundorfer B, Seidler S (2004): Hyperexcitability of the primary somatosensory cortex in migraine–a magnetoencephalographic study. Brain 127:2459–2469. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Bigal ME, Ashina S, Burstein R, Silberstein S, Reed ML, Serrano D, Stewart WF (2008): Cutaneous allodynia in the migraine population. Ann Neurol 63:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz J, Minoshima S, Casey KL (2003): Keeping pain out of mind: The role of the dorsolateral prefrontal cortex in pain modulation. Brain 126:1079–1091. [DOI] [PubMed] [Google Scholar]
- Lu H, Donahue MJ, van Zijl PC (2006): Detrimental effects of BOLD signal in arterial spin labeling fMRI at high field strength. Magn Reson Med 56:546–552. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Gandevia SC, Henderson LA (2007): Discrete changes in cortical activation during experimentally induced referred muscle pain: A single‐trial fMRI study. Cereb Cortex 17:2050–2059. [DOI] [PubMed] [Google Scholar]
- Maleki N, Becerra L, Brawn J, Bigal M, Burstein R, Borsook D (2012): Concurrent functional and structural cortical alterations in migraine. Cephalalgia 32:607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton EA, Keaser ML, Gullapalli RP, Greenspan JD (2005): Regional intensive and temporal patterns of functional MRI activation distinguishing noxious and innocuous contact heat. J Neurophysiol 93:2183–2193. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC (2010): The What and How of prefrontal cortical organization. Trends Neurosci 33:355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescot A, Becerra L, Pendse G, Tully S, Jensen E, Hargreaves R, Renshaw P, Burstein R, Borsook D (2009): Excitatory neurotransmitters in brain regions in interictal migraine patients. Mol Pain 5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca MA, Ceccarelli A, Falini A, Colombo B, Tortorella P, Bernasconi L, Comi G, Scotti G, Filippi M (2006): Brain gray matter changes in migraine patients with T2‐visible lesions. A 3‐T MRI study. Stroke 37:1765–1770. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Messina R, Colombo B, Falini A, Comi G, Filippi M (2014): Structural brain MRI abnormalities in pediatric patients with migraine. J Neurol 261:350–357. [DOI] [PubMed] [Google Scholar]
- Sandrini G, Rossi P, Milanov I, Serrao M, Cecchini A, Nappi G (2006): Abnormal modulatory influence of diffuse noxious inhibitory controls in migraine and chronic tension‐type headache patients. Cephalalgia 26:782–789. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Shinohara RT, Wolf DH, Hopson RD, Elliott MA, Vandekar SN, Ruparel K, Calkins ME, Roalf DR, Gennatas ED, Jackson C, Erus G, Prabhakaran K, Davatzikos C, Detre JA, Hakonarson H, Gur RC, Gur RE (2014): Impact of puberty on the evolution of cerebral perfusion during adolescence. Proc Natl Acad Sci USA 111:8643–8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt‐Wilcke T, Ganssbauer S, Neuner T, Bogdahn U, May A (2008): Subtle grey matter changes between migraine patients and healthy controls. Cephalalgia 28:1–4. [DOI] [PubMed] [Google Scholar]
- Schwedt TJ, Chong CD, Chiang CC, Baxter L, Schlaggar BL, Dodick DW (2014): Enhanced pain‐induced activity of pain‐processing regions in a case‐control study of episodic migraine. Cephalalgia 34:947–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004): Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23(Suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- Sonal Sekhar M, Sasidharan S, Joseph S, Kumar A (2012): Migraine management: How do the adult and paediatric migraines differ? Saudi Pharma J 20:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriani S, Feggi L, Battistella PA, Arnaldi C, De Carlo L, Stipa S (1997): Interictal and ictal phase study with Tc 99m HMPAO brain SPECT in juvenile migraine with aura. Headache 37:31–36. [DOI] [PubMed] [Google Scholar]
- Stankewitz A, Schulz E, May A (2013): Neuronal correlates of impaired habituation in response to repeated trigemino‐nociceptive but not to olfactory input in migraineurs: An fMRI study. Cephalalgia 33:256–265. [DOI] [PubMed] [Google Scholar]
- Strassman AM, Raymond SA, Burstein R (1996): Sensitization of meningeal sensory neurons and the origin of headaches. Nature 384:560–564. [DOI] [PubMed] [Google Scholar]
- Thukral BB (2015): Problems and preferences in pediatric imaging. Indian J Radiol Imag 25:359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermann L, Ploner M, Haucke K, Schmitz F, Baltissen R, Schnitzler A (2001): Differential coding of pain intensity in the human primary and secondary somatosensory cortex. J Neurophysiol 86:1499–1503. [DOI] [PubMed] [Google Scholar]
- Torquati K, Pizzella V, Della Penna S, Franciotti R, Babiloni C, Rossini PM, Romani GL (2002): Comparison between SI and SII responses as a function of stimulus intensity. Neuroreport 13:813–819. [DOI] [PubMed] [Google Scholar]
- Valfre W, Rainero I, Bergui M, Pinessi L (2008): Voxel‐based morphometry reveals gray matter abnormalities in migraine. Headache 48:109–117. [DOI] [PubMed] [Google Scholar]
- Várkuti B, Cavusoglu M, Kullik A, Schiffler B, Veit R, Yilmaz Ö, Rosenstiel W, Braun C, Uludag K, Birbaumer N, Sitaram R (2011): Quantifying the link between anatomical connectivity, gray matter volume and regional cerebral blood flow: An integrative MRI study. PLoS One 6:e14801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z (2012): Improving cerebral blood flow quantification for arterial spin labeled perfusion MRI by removing residual motion artifacts and global signal fluctuations. Magn Reson Imag 30:1409–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Aguirre GK, Rao H, Wang J, Fernandez‐Seara MA, Childress AR, Detre JA (2008): Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imag 26:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WC, Fernandez‐Seara M, Detre JA, Wehrli FW, Wang J (2007): A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn Reson Med 58:1020–1027. [DOI] [PubMed] [Google Scholar]
- Wu WC, Jain V, Li C, Giannetta M, Hurt H, Wehrli FW, Wang DJ (2010): In vivo venous blood T1 measurement using inversion recovery true‐FISP in children and adults. Magn Reson Med 64:1140–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef AM, Macefield VG, Henderson LA (2016): Cortical influences on brainstem circuitry responsible for conditioned pain modulation in humans. Hum Brain Mapp 37:2630–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]


