Abstract
A potent, heat-labile leukotoxic material was extracted from Actinobacillus actinomycetemcomitans (strain Y4), an anaerobic gram-negative microorganism originally isolated from subgingival plaque in a patient with juvenile periodontitis. The cytopathic effects of Y4 toxin on purified monocytes were studied by the extracellular release of radioactive cytoplasmic markers and cell enzymes and by time-lapse microcinematography. Y4 toxin rapidly bound to the cells, producing dose- and time-dependent alterations culminating in cell death and release of intracellular constituents into the culture medium. The evidence to be presented suggests that the cell membrane of the monocyte may be the primary target in the development of these phenomena. Previous studies have shown that Y4 toxin also kills human polymorphonuclear leukocytes but not other cell types. It is conceivable that disruption of polymorphonuclear leukocytes and monocytes by Y4 toxin in the gingival crevice area may be relevant in the pathogenesis of juvenile periodontitis.
Full text
PDF


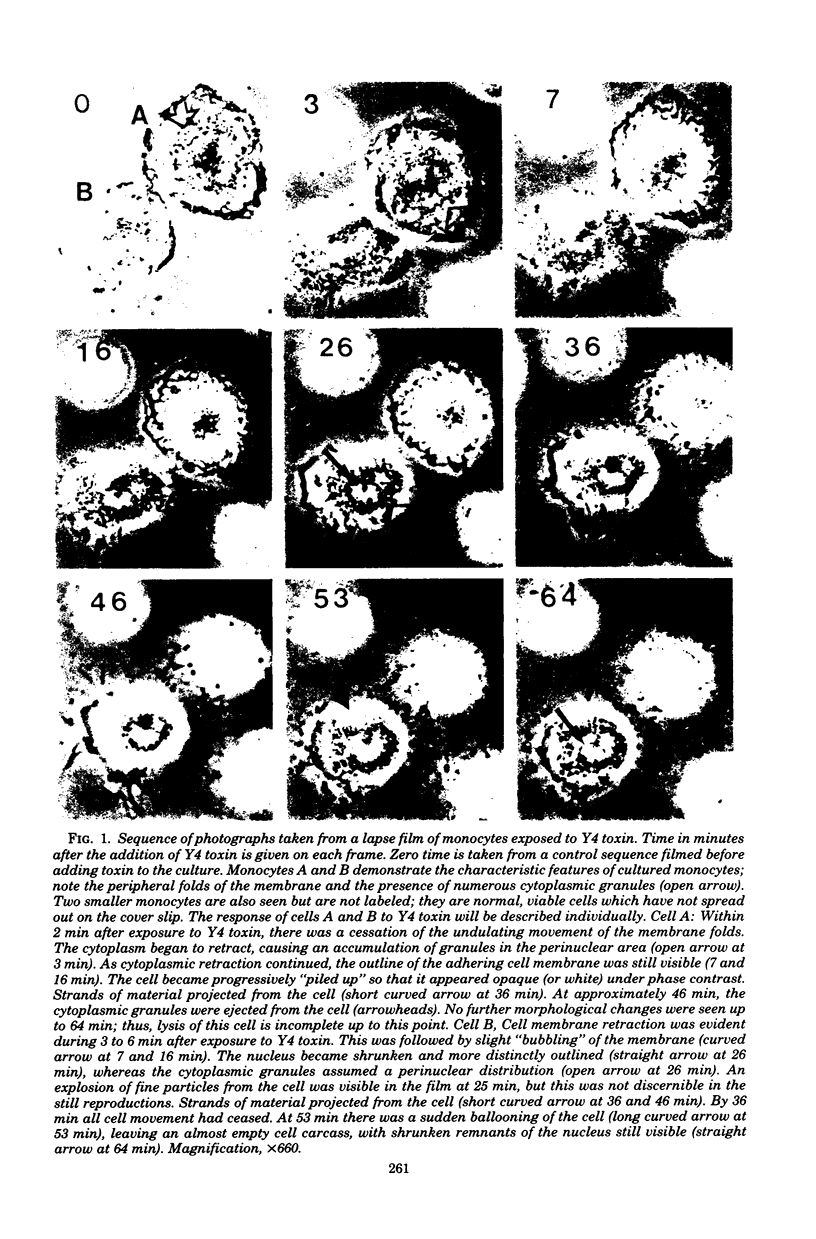
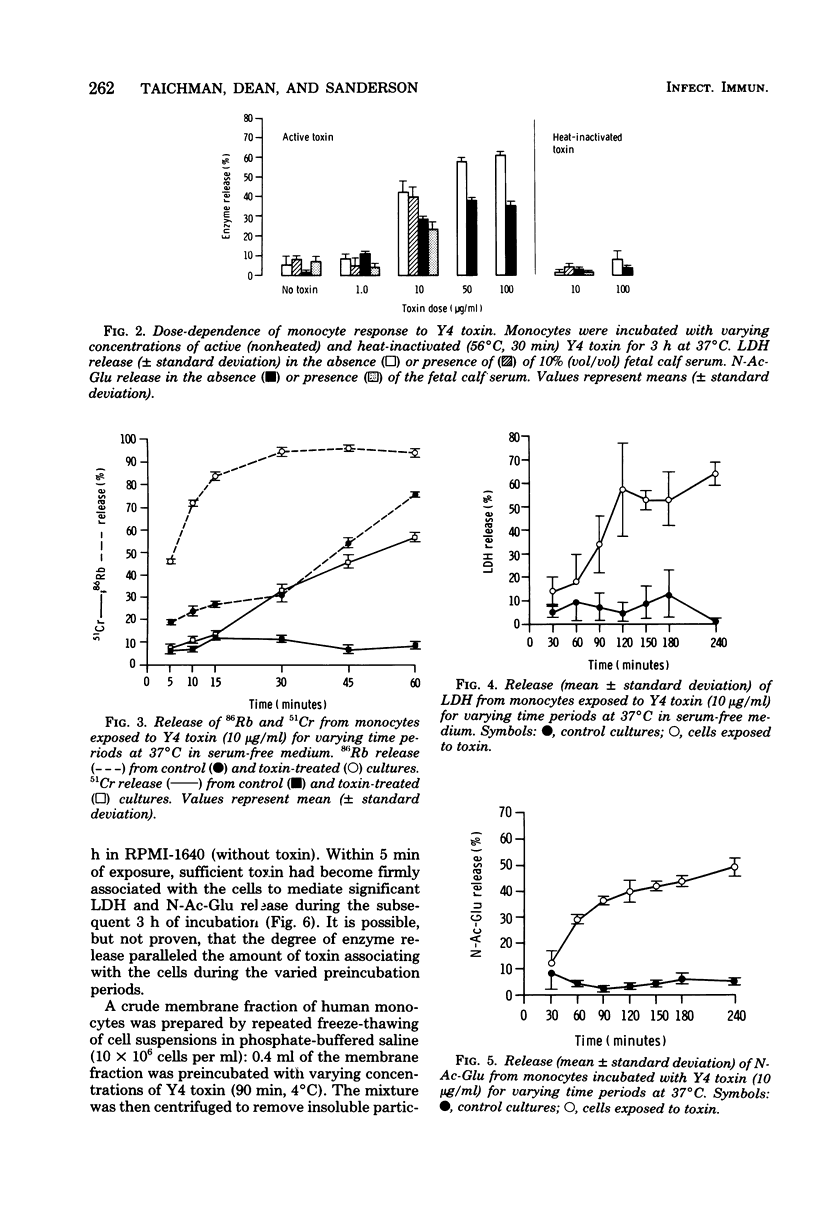
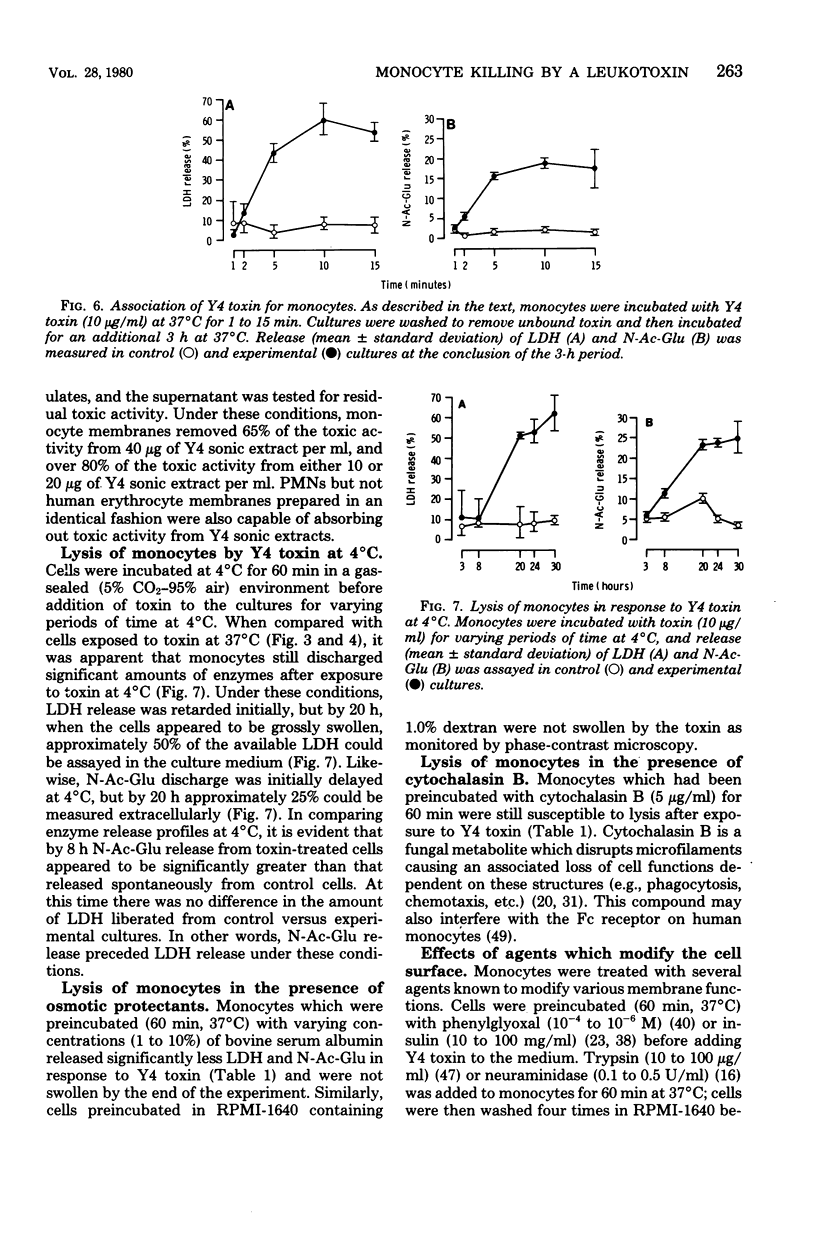
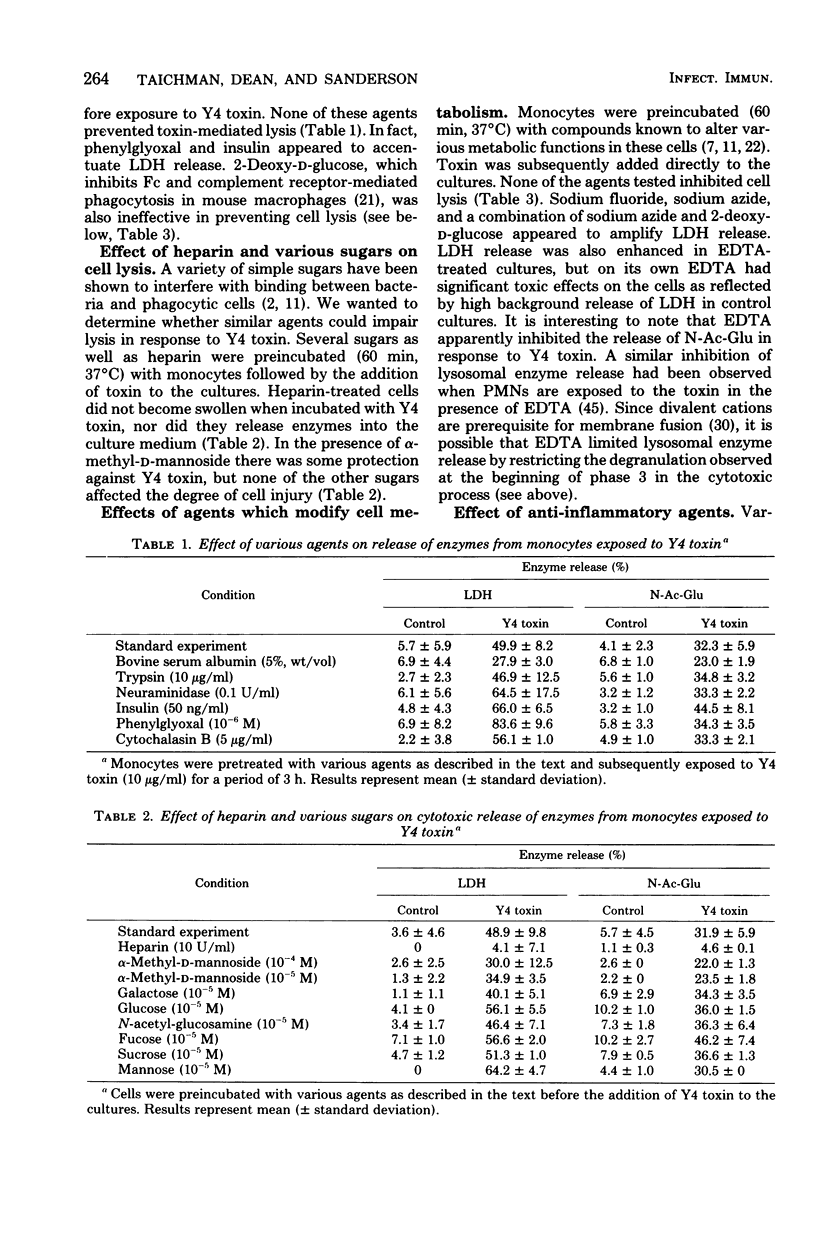
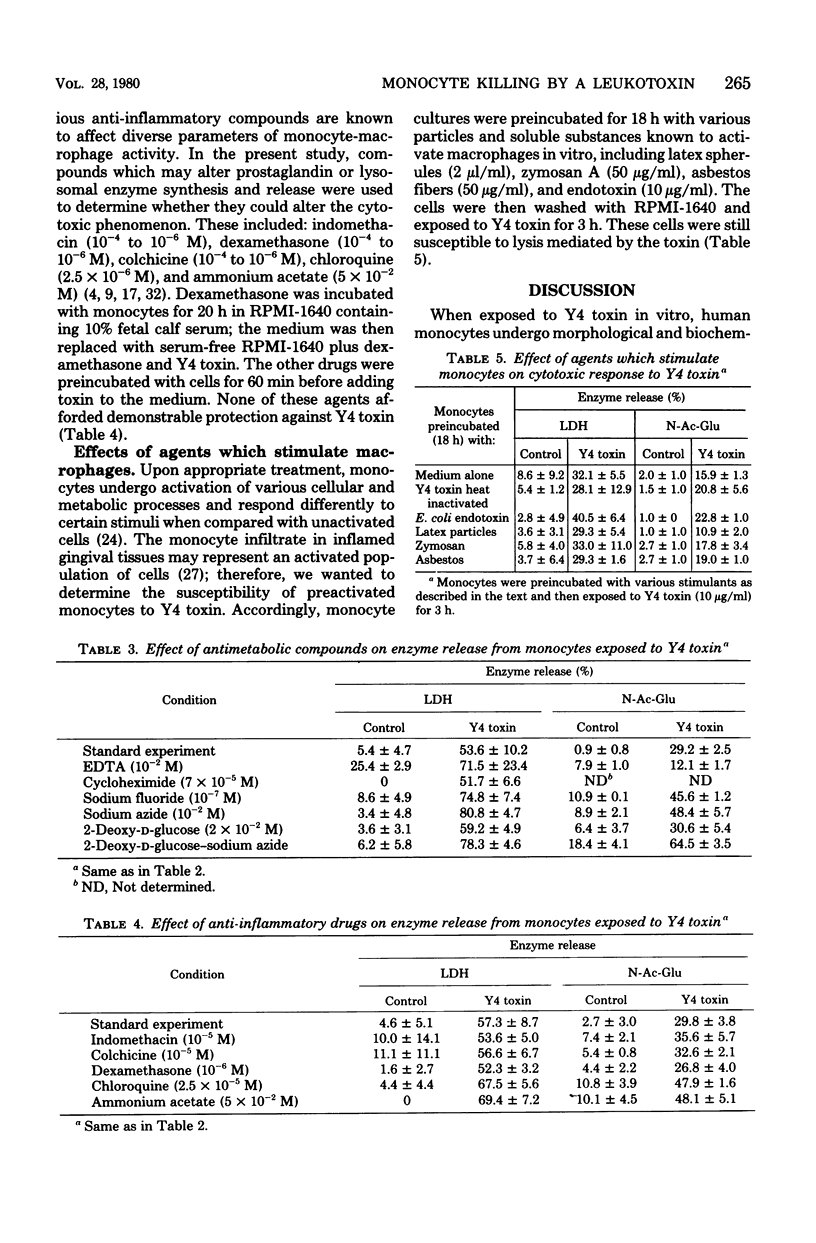



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehni P., Tsai C. C., McArthur W. P., Hammond B. F., Taichman N. S. Interaction of inflammatory cells and oral microorganisms. VIII. Detection of leukotoxic activity of a plaque-derived gram-negative microorganism. Infect Immun. 1979 Apr;24(1):233–243. doi: 10.1128/iai.24.1.233-243.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit Z., Ofek I., Goldman R., Mirelman D., Sharon N. Mannose residues on phagocytes as receptors for the attachment of Escherichia coli and Salmonella typhi. Biochem Biophys Res Commun. 1977 Sep 9;78(1):455–460. doi: 10.1016/0006-291x(77)91276-1. [DOI] [PubMed] [Google Scholar]
- Bray M. A., Gordon D. Prostaglandin production by macrophages and the effect of anti-inflammatory drugs. Br J Pharmacol. 1978 Aug;63(4):635–642. doi: 10.1111/j.1476-5381.1978.tb17276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cainciola L. J., Genco R. J., Patters M. R., McKenna J., van Oss C. J. Defective polymorphonuclear leukocyte function in a human periodontal disease. Nature. 1977 Feb 3;265(5593):445–447. doi: 10.1038/265445a0. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Page R. C., Wilde G. Defective neutrophil chemotaxis in juvenile periodontitis. Infect Immun. 1977 Dec;18(3):694–700. doi: 10.1128/iai.18.3.694-700.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R. T., Barrett A. J. Lysosomes. Essays Biochem. 1976;12:1–40. [PubMed] [Google Scholar]
- Dean R. T. Macrophage protein turnover. Evidence for lysosomal participation in basal proteolysis. Biochem J. 1979 May 15;180(2):339–345. doi: 10.1042/bj1800339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferne M., Duchan Z., Rabinowitz-Begner S., Sela M. N., Ginsburg I. The effect of leukocyte hydrolases on bacteria. XII. The release of lipopolysaccharide (LPS) from Salmonella typhi by leukocyte extracts, lysozyme, inflammatory exudates and by serum and synovial fluid and the modulation by anionic and cationic polyelectrolytes of LPS release and the sensitization of erythrocytes. Inflammation. 1978 Mar;3(1):59–80. doi: 10.1007/BF00917322. [DOI] [PubMed] [Google Scholar]
- Freimer N. B., Ogmundsdóttir H. M., Blackwell C. C., Sutherland I. W., Graham L., Weir D. M. The role of cell wall carbohydrates in binding of microorganisms to mouse peritoneal exudate macrophages. Acta Pathol Microbiol Scand B. 1978 Apr;86(2):53–57. doi: 10.1111/j.1699-0463.1978.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Hovi T., Mosher D., Vaheri A. Cultured human monocytes synthesize and secrete alpha2-macroglobulin. J Exp Med. 1977 Jun 1;145(6):1580–1589. doi: 10.1084/jem.145.6.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving J. T., Newman M. G., Socransky S. S., Heely J. D. Histological changes in experimental periodontal disease in rats mono-infected with a gram-negative organism. Arch Oral Biol. 1975 Mar;20(3):219–220. doi: 10.1016/0003-9969(75)90013-8. [DOI] [PubMed] [Google Scholar]
- Johnson W. D., Jr, Mei B., Cohn Z. A. The separation, long-term cultivation, and maturation of the human monocyte. J Exp Med. 1977 Dec 1;146(6):1613–1626. doi: 10.1084/jem.146.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop J., Ax W., Sedlacek H. H., Seiler F. R. Effect of Vibrio cholerae neuraminidase on the phagocytosis of E. coli by macrophages in vivo and in vitro. Immunology. 1978 Mar;34(3):555–563. [PMC free article] [PubMed] [Google Scholar]
- Kurland J. I., Bockman R. Prostaglandin E production by human blood monocytes and mouse peritoneal macrophages. J Exp Med. 1978 Mar 1;147(3):952–957. doi: 10.1084/jem.147.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lavine W. S., Maderazo E. G., Stolman J., Ward P. A., Cogen R. B., Greenblatt I., Robertson P. B. Impaired neutrophil chemotaxis in patients with juvenile and rapidly progressing periodontitis. J Periodontal Res. 1979 Jan;14(1):10–19. doi: 10.1111/j.1600-0765.1979.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Malawista S. E., Gee J. B., Bensch K. G. Cytochalasin B reversibly inhibits phagocytosis: functional, metabolic, and ultrastructural effects in human blood leukocytes and rabbit alveolar macrophages. Yale J Biol Med. 1971 Dec;44(3):286–300. [PMC free article] [PubMed] [Google Scholar]
- Michl J., Ohlbaum D. J., Silverstein S. C. 2-Deoxyglucose selectively inhibits Fc and complement receptor-mediated phagocytosis in mouse peritoneal macrophages. I. Description of the inhibitory effect. J Exp Med. 1976 Dec 1;144(6):1465–1483. doi: 10.1084/jem.144.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschel R. J., Rosen N., Rosen O. M., Bloom B. R. Modulation of Fc-mediated phagocytosis by cyclic AMP and insulin in a macrophage-like cell line. J Immunol. 1977 Nov;119(5):1813–1820. [PubMed] [Google Scholar]
- Mørland B., Mørland J. Selective induction of lysosomal enzyme activities in mouse peritoneal macrophages. J Reticuloendothel Soc. 1978 Jun;23(6):469–477. [PubMed] [Google Scholar]
- Newman M. G., Socransky S. S. Predominant cultivable microbiota in periodontosis. J Periodontal Res. 1977 Mar;12(2):120–128. doi: 10.1111/j.1600-0765.1977.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Newman M. G., Socransky S. S., Savitt E. D., Propas D. A., Crawford A. Studies of the microbiology of periodontosis. J Periodontol. 1976 Jul;47(7):373–379. doi: 10.1902/jop.1976.47.7.373. [DOI] [PubMed] [Google Scholar]
- Page R. C., Davies P., Allison A. C. Effects of dental plaque on the production and release of lysosomal hydrolases by macrophages in culture. Arch Oral Biol. 1973 Dec;18(12):1481–1495. doi: 10.1016/0003-9969(73)90124-6. [DOI] [PubMed] [Google Scholar]
- Page R. C., Davies P., Allison A. C. Participation of mononuclear phagocytes in chronic inflammatory diseases. J Reticuloendothel Soc. 1974 May;15(5):413–438. [PubMed] [Google Scholar]
- Page R. C., Schroeder H. E. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest. 1976 Mar;34(3):235–249. [PubMed] [Google Scholar]
- Poste G., Allison A. C. Membrane fusion. Biochim Biophys Acta. 1973 Dec 28;300(4):421–465. doi: 10.1016/0304-4157(73)90015-4. [DOI] [PubMed] [Google Scholar]
- Ringrose P. S., Parr M. A., McLaren M. Effects of anti-inflammatory and other compounds on the release of lysosomal enzymes from macrophages. Biochem Pharmacol. 1975 Mar 1;24(5):607–614. doi: 10.1016/0006-2952(75)90181-1. [DOI] [PubMed] [Google Scholar]
- Sanderson C. J. The mechanism of T cell mediated cytotoxicity. I. The release of different cell components. Proc R Soc Lond B Biol Sci. 1976 Jan 20;192(1107):221–239. doi: 10.1098/rspb.1976.0010. [DOI] [PubMed] [Google Scholar]
- Sanderson C. J. The mechanism of T cell mediated cytotoxicity. II. Morphological studies of cell death by time-lapse microcinematography. Proc R Soc Lond B Biol Sci. 1976 Jan 20;192(1107):241–255. doi: 10.1098/rspb.1976.0011. [DOI] [PubMed] [Google Scholar]
- Sanderson C. J. The uptake and retention of chromium by cells. Transplantation. 1976 Jun;21(6):526–529. [PubMed] [Google Scholar]
- Scharmann W., Jacob F., Porstendörfer J. The cytotoxic action of leucocidan from Pseudomonas aeruginosa on human polymorphonuclear leucocytes. J Gen Microbiol. 1976 Apr;93(2):303–308. doi: 10.1099/00221287-93-2-303. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H., Bianco A. R., Handwerger B. S., Kahn C. R. Demonstration that monocytes rather than lymphocytes are the insulin-binding cells in preparations of humah peripheral blood mononuclear leukocytes: implications for studies of insulin-resistant states in man. Proc Natl Acad Sci U S A. 1975 Feb;72(2):474–478. doi: 10.1073/pnas.72.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. Ultrastructure of membrane lesions in immune lysis, osmotic lysis and drug-induced lysis. Fed Proc. 1974 Oct;33(10):2116–2124. [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Slots J. The predominant cultivable organisms in juvenile periodontitis. Scand J Dent Res. 1976 Jan;84(1):1–10. doi: 10.1111/j.1600-0722.1976.tb00454.x. [DOI] [PubMed] [Google Scholar]
- Taichman N. S., Hammond B. F., Tsai C. C., Baehni P. C., McArthur W. P. Interaction of inflammatory cells and oral microorganisms. VII. In vitro polymorphonuclear responses to viable bacteria and to subcellular components of avirulent and virulent strains of Actinomyces viscosus. Infect Immun. 1978 Aug;21(2):594–604. doi: 10.1128/iai.21.2.594-604.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taichman N. S., McArthur W. P. Interaction of inflammatory cells and oral bacteria: release of lysosomal hydrolases from rabbit polymorphonuclear leukocytes exposed to gram-positive plaque bacteria. Arch Oral Biol. 1976;21(4):257–263. doi: 10.1016/0003-9969(76)90044-3. [DOI] [PubMed] [Google Scholar]
- Taichman N. S., Tsai C. C., Baehni P. C., Stoller N., McArthur W. P. Interaction of inflammatory cells and oral microorganisms. IV. In vitro release of lysosomal constituents from polymorphonuclear leukocytes exposed to supragingival and subgingival bacterial plaque. Infect Immun. 1977 Jun;16(3):1013–1023. doi: 10.1128/iai.16.3.1013-1023.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. C., Hammond B. F., Baehni P., McArthur W. P., Taichman N. S. Interaction of inflammatory cells and oral microorganisms. VI. Exocytosis of PMN lysosomes in response to gram-negative plaque bacteria. J Periodontal Res. 1978 Nov;13(6):504–512. doi: 10.1111/j.1600-0765.1978.tb00204.x. [DOI] [PubMed] [Google Scholar]
- Tsai C. C., McArthur W. P., Baehni P. C., Hammond B. F., Taichman N. S. Extraction and partial characterization of a leukotoxin from a plaque-derived Gram-negative microorganism. Infect Immun. 1979 Jul;25(1):427–439. doi: 10.1128/iai.25.1.427-439.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J. C. The presence of two Fc receptors on mouse macrophages: evidence from a variant cell line and differential trypsin sensitivity. J Exp Med. 1977 Apr 1;145(4):931–945. doi: 10.1084/jem.145.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigler Z., Schugar S. The effect of cytochalasin B and alkaloid compounds on human monocyte functions. J Reticuloendothel Soc. 1979 Mar;25(3):235–241. [PubMed] [Google Scholar]



