Abstract
Background
Despite treatment with virologically suppressive antiretroviral therapy (ART), neurocognitive impairment may persist or develop de novo in aging HIV-infected individuals. We evaluated advancing age as a predictor of neurocognitive impairment in a large cohort of previously ART-naïve individuals on long-term ART
Design
The AIDS Clinical Trials Group (ACTG) Longitudinal Linked Randomized Trials (ALLRT) was a prospective cohort study of HIV-infected individuals originally enrolled in randomized ART trials. This analysis examined neurocognitive outcomes ≥2 years after ART initiation.
Methods
All participants underwent annual neurocognitive testing consisting of Trail making A and B, the WAIS-R Digit Symbol and Hopkins Verbal Learning Tests. Uni- and multi-variable repeated measures regression models evaluated factors associated with neurocognitive performance. Predictors at parent study entry (ART naïve) included entry demographics, smoking, injection drug use, hepatitis B surface antigen, hepatitis C virus serostatus, history of stroke, ART regimen type, pre-ART nadir CD4 and plasma viral load (PVL) and as well as time-updated PVL and CD4.
Results
The cohort comprised 3,313 individuals with median pre-ART age of 38 years, 20% women; 36% Black, non-Hispanic; 22% Hispanic. Virologic suppression was maintained at 91% of follow-up visits. Neurocognitive performance improved with years of ART. After adjusting for the expected effects of age using norms from HIV–negative individuals, the odds of neurocognitive impairment at follow-up visits among the HIV-infected increased by nearly 20% for each decade of advancing age.
Conclusion
Despite continued virologic suppression and neurocognitive improvement in the cohort as a whole, older individuals were more likely to have neurocognitive impairment than younger individuals.
Keywords: HIV, cognitive impairment, dementia, aging, ART, HAND
INTRODUCTION
Effective combination antiretroviral therapy (ART) extends the life of individuals with human immunodeficiency virus (HIV), and the infected population is aging.[1] Treatment with ART can result in significant neurocognitive improvement in many individuals with HIV-associated neurocognitive disorders (HAND).[2] However, improvement varies by person and several cohort studies have demonstrated that HAND can persist despite virologic suppression and immune recovery on ART[3, 4]. A number of risk factors for HAND have been identified including older age, less education, the presence of depression, hepatitis C virus (HCV) infection, a diagnosis of AIDS (CDC 1993), and the presence of other severe medical comorbidities.[5] Recent reports indicate that more than 50% of persons living with HIV are over age 50 years of age, and 30% are over the age of 60. [1, 6] These trends raise the importance of understanding the impact of aging on the course of HAND.[7]
While several studies have evaluated the impact of aging on risk of cognitive impairment in HIV, most of these studies have been cross-sectional[8], included substantial proportions of individuals lacking viral suppression on cART[9] and used neurocognitive measures with very limited sensitivity and specificity[10]. Two longitudinal studies[11, 12] have evaluated the relationship of neurocognitive impairment to declining cognition. In the first study[11], two assessments were performed – baseline and 14-month follow-up. The number of participants was small (27 HIV+ under age 40 and 56 over age 50 years). While the rate of incident cognitive disorders was higher in HIV+ than in HIV− participants, there was no main effect of age or age by HIV interaction. The second longitudinal study[12] included 54 HIV+ and 30 HIV− individuals over one year and concluded that age and HIV infection interact to produce larger declines in verbal memory over time. Thus, important gaps in the literature include whether decline in neurocognitive function occurs in virologically suppressed participants followed for several years, and when decline occurs, at what age does it begin.
To address these gaps, we evaluated the relationship of advancing age to neurocognitive decline, after adjusting for other relevant covariates, in a cohort of previously ART-naïve individuals, a large proportion of whom were virologically suppressed for 2 or more years after initiating their first ART regimen.
METHODS
Study population
This was an analysis of prospectively collected data from The AIDS Clinical Trials Group (ACTG) Longitudinal Linked Randomized Trials (ALLRT) study. Participants were drawn from seven randomized clinical trials of antiretrovirals (Table 1), referred to here as the “parent studies” [13]. Participants agreed to long-term follow up with the purpose of evaluating clinical, virologic, immunologic and neurologic outcomes associated with treatment of HIV. Individuals were selected for this analysis if they were ART-naïve when they entered their parent studies and had at least one NPZ4 score result. Discontinuing ART was defined as stopping for at least 3 weeks prior to neurocognitive evaluation. Additionally, since normative neurocognitive data were not available for all race/ethnicity groups, we further restricted the study population to white non-Hispanic (N=1965), black non-Hispanic (N=1595) and Hispanic (N=1015). To achieve the study goal of examining individuals during long-term ART, only neurocognitive assessments done at least 2 years after ART initiation were included in this analysis.
Table 1.
Participant demographic and disease characteristics, unadjusted and adjusted odds ratios and 95 percent confidence intervals for the association between risk factors and neurocognitive impairment.
| N or MEDIAN (IQR) | UNADJUSTED | ADJUSTED† | ||||
|---|---|---|---|---|---|---|
| Variables | Odds Ratio (95% CI) |
P-value | Odds Ratio (95% CI) |
P-value | ||
| Years since ART initiation | Every 1 year increase | 3.5 (2.5, 5.4) | 0.95 (0.93,0.96) | <0.001 | 0.94 (0.92,0.96) | <0.001 |
| Sex (ref: Male) | F | 673 (20%) | 1.44 (1.25,1.68) | <0.001 | 1.35 (1.15,1.59) | <0.001 |
| Age at ART initiation | Every 10-year increase | 38* (31, 45) | 1.17 (1.10,1.25) | <0.001 | 1.18 (1.11,1.26) | <0.001 |
| Ethnicity/Race (ref: White) | White Non-Hispanic | 1,404 (42%) | n/a | n/a | n/a | n/a |
| Black Non-Hispanic | 1,172 (35%) | 0.91 (0.79,1.05) | 0.18 | 0.76 (0.65,0.89) | <0.001 | |
| Hispanic | 737 (22%) | 2.93 (2.51,3.42) | <0.001 | 2.61 (2.22,3.07) | <0.001 | |
| Years of education (ref: >12(post high school)) | ≤12 years | 1,258 (38%) | 1.71 (1.51,1.94) | <0.001 | 1.37 (1.2,1.57) | <0.001 |
| Initial ART drug class (ref: NRTI only) | NRTI+NNRTI | 536 (16%) | 0.96 (0.80,1.16) | 0.68 | n/a | n/a |
| PI+NNRTI | 1,558 (47%) | 1.08 (0.77,1.52) | 0.66 | n/a | n/a | |
| PI+NRTI | 122 (4%) | 1.04 (0.87,1.24) | 0.68 | n/a | n/a | |
| PI+NRTI+NNRTI | 974 (29%) | 0.84 (0.59,1.18) | 0.31 | n/a | n/a | |
| Smoking history on/before 1st neurocognitive evaluation (ref: No) | Yes | 1,843 (56%) | 1.08 (0.95,1.22) | 0.23 | n/a | n/a |
| Injection drug use (ref: Not reported) | reported (currently or previously) | 235 (7%) | 1.35 (1.07,1.7) | 0.01 | 1.12 (0.85,1.46) | 0.42 |
| Hepatitis B status (ref: Negative) | Positive | 83 (3%) | 1.21 (0.83,1.77) | 0.32 | n/a | n/a |
| Hepatitis C status (ref: Negative) | Positive | 258 (8%) | 1.64 (1.31,2.06) | <0.001 | 1.4 (1.08,1.82) | 0.01 |
| Nadir CD4 (ref: >350 cells/µL, n=689 (21%)) | 0–50 | 656 (20%) | 1.24 (1.02,1.50) | 0.03 | 1.09 (0.87,1.35) | 0.46 |
| 51–200 | 855 (26%) | 0.97 (0.81,1.16) | 0.75 | 0.89 (0.73,1.08) | 0.23 | |
| 201–350 | 1,113 (34%) | 1.06 (0.89,1.25) | 0.54 | 1.00 (0.84,1.20) | >0.9 | |
| Baseline HIV RNA (ref: ≤100,000 copies/ml) | >100,000 | 1,119 (34%) | 1.02 (0.90,1.16) | 0.78 | n/a | n/a |
| Parent study (ref: A5257, n=1201(36%)) | 384 | 273 (8%) | 0.65 (0.52,0.81) | <0.001 | 1.13 (0.85,1.51) | 0.41 |
| 388 | 94 (3%) | 0.77 (0.46,1.28) | 0.31 | 1.15 (0.66,2.00) | 0.62 | |
| A5014 | 18 (1%) | 0.79 (0.32,1.91) | 0.6 | 1.01 (0.43,2.37) | >0.90 | |
| A5095 | 406 (12%) | 0.59 (0.48,0.72) | <0.001 | 0.82 (0.65,1.04) | 0.1 | |
| A5142 | 323 (10%) | 0.75 (0.60,0.92) | 0.007 | 0.93 (0.73,1.18) | 0.54 | |
| A5202 | 998 (30%) | 0.80 (0.69,0.93) | 0.004 | 0.82 (0.70,0.96) | 0.01 | |
| Stroke history on/before 1st neurocognitive evaluation (ref: No) | Yes | 24 (1%) | 1.65 (0.82,3.33) | 0.16 | n/a | n/a |
| Lab toxicity with grade ≥3 before year 1 (ref: No) | Yes | 664 (20%) | 0.93 (0.79,1.09) | 0.35 | n/a | n/a |
| Adherence during year 1 (ref: 100% adherence) | <100% | 979 (30%) | 1.01 (0.89,1.15) | 0.84 | n/a | n/a |
| Time-varying CD4^ (ref: >500 cells/µL) | 0–350 | 699 (21%) | 1.31 (1.16,1.49) | <0.001 | 1.21 (1.05,1.40) | 0.008 |
| 351–500 | 816 (25%) | 1.15 (1.05,1.26) | 0.002 | 1.12 (1.01,1.24) | 0.03 | |
| Time-varying HIV RNA^ (ref: ≤200 copies/ml) | >200 | 304 (9%) | 1.09 (0.95,1.25) | 0.22 | n/a | n/a |
Abbreviations: IQR: interquartile range; CI: confidence interval; PI: protease inhibitors; NRTI: nucleoside reverse transcriptase inhibitors; NNRTI: non-nucleoside reverse transcriptase inhibitors. n/a: not applicable.
Only covariates with unadjusted p-value ≤ 0.1, are included.
For time-varying CD4 and HIV RNA, the frequencies represent these covariates at the 1st neurocognitive evaluation
12% of participants were over 50, 2% of the participants were over 60 years of age.
Neurocognitive Testing
The 4-test neurocognitive battery (NPZ4) included the Trail-making Tests A and B (TMA, TMB) [14], the WAIS-R Digit Symbol subtest (DSY) [15], and the Hopkins Verbal Learning Test - Revised (HVLT-R). [16] These tests were chosen because they are sensitive in detecting HIV-related neurocognitive changes. [17, 18] Briefly, TMA and DSY assess psychomotor speed, TMB assesses executive function, and the HVLT-R evaluates verbal learning. The HVLT-R was added to the battery to enhance its sensitivity to evolving changes in the pattern of neurocognitive impairment in the era of combination antiretroviral therapy (cART).[12] The raw score for each test was standardized using demographically-adjusted (age, sex, years of education, race/ethnicity as appropriate) normative means. A standardized score was calculated by subtracting the appropriate normative mean, then dividing by the appropriate normative standard deviation. For TMA, TMB and DSY, norms were available for White, Black and Hispanic ethnicities. [19, 20] The parent clinical trials included no HIV uninfected individuals, and therefore did not yield norms. We used the best available normative data. For the HVLT-R, existing norms were available for Whites and Blacks tested in English[19, 20], and for Hispanic participants tested in Spanish.[21] Participants completed the NPZ4 at ALLRT entry and approximately every 48 weeks thereafter (TMA, TMB and DSY were assessed in ALLRT throughout the study (since 2000); HVLT-R was added in 2006). The primary outcome was overall impairment on the NP test battery, defined as ≤ −2.0 SD on one test or ≤ −1.0 SD on two tests. We sought to determine the impact of the predictor variable and covariates on NP outcome after expected virologic and immunologic effects of cART had been achieved. Thus, the analysis included only NP scores from visits at least 2 years after cART was initiated in the parent studies.
Age and other covariates
For each participant, baseline characteristics were defined at cART initiation (at parent study entry). Ascertainment methods for clinical and laboratory variables have been previously described in detail. [13] The primary predictor was age and covariates included in the analysis were sex, race/ethnicity, IV drug use, years of education, history of smoking, history of stroke, ACTG study, initial ART regimens, CD4+ T-cell counts at baseline and post-baseline, nadir CD4 count, HIV RNA viral load at baseline and post-baseline, antiretroviral adherence (ACTG adherence questionnaire), hepatitis C status (diagnosis or antibody positive), and hepatitis B status (surface antigen positive) at first available time point.
Statistical analysis
We used unadjusted (univariate) and adjusted (multivariable) generalized estimating equations (GEE) models to identify risk factors associated with neurocognitive impairment deficits. The outcome variable was neurocognitive impairment as a binary variable based on NPZ4. In multivariable adjusted models, variables with p-value from the univariate models ≤ 0.1 were included as covariates. Repeated measures logistic regression (GEE) estimated the odds of neurocognitive impairment at an NPZ4 evaluation.
RESULTS
Baseline (pre-ART) characteristics defined at the parent study entry (ART initiation) for each participant are shown in Table 1. The median age of the 3313 HIV+ individuals was 38 years (interquartile range [22] 31, 45); 2% were over 60, 12% were over 50 years of age; 42% were white non-Hispanic; and 20% were females (N= 673). The median nadir CD4 was 221 cells/µL (IQR 80, 324). The median number of visits at which the neurocognitive tests were administered to each participant after 2 years on ART was 3 (IQR 2, 6). During follow-up, participants remained on ART at 97% of visits, 91% of HIV RNA measures were ≤200 copies/ml and 61% of CD4 counts were >500 cells/µL.
Univariable and multivariable GEE regression models on binary NPZ4 impairment are shown in Table 1. In the unadjusted model, the odds ratio (OR) of neurocognitive impairment for each decade of advancing age at parent study entry was 1.17 (95% CI 1.10–1.25, p<0.001). The OR for hepatitis C co-infection was 1.64 (1.31–2.06, p<0.001). ORs for other significant covariates included 1.44 (1.25–1.68, p<0.001) for women as compared to men; 1.71 (1.51–1.94, p<0.001) for ≤12 years of education compared to those with >12 years of education; 1.35 (1.07–1.70, p=0.01) for history of injection drug use; 1.31 (1.16–1.49, p<0.001) for those with time-varying CD4 counts less than 350 cells/µL and 1.15 (1.05–1.26, p=0.002) for time-varying CD4 counts between 351–500 cells/µL, as compared to CD4 counts >500 cells/µL. In the cohort as a whole, neurocognitive impairment rates diminished with increasing years on ART 0.95 (0.93–0.96, p<0.001).
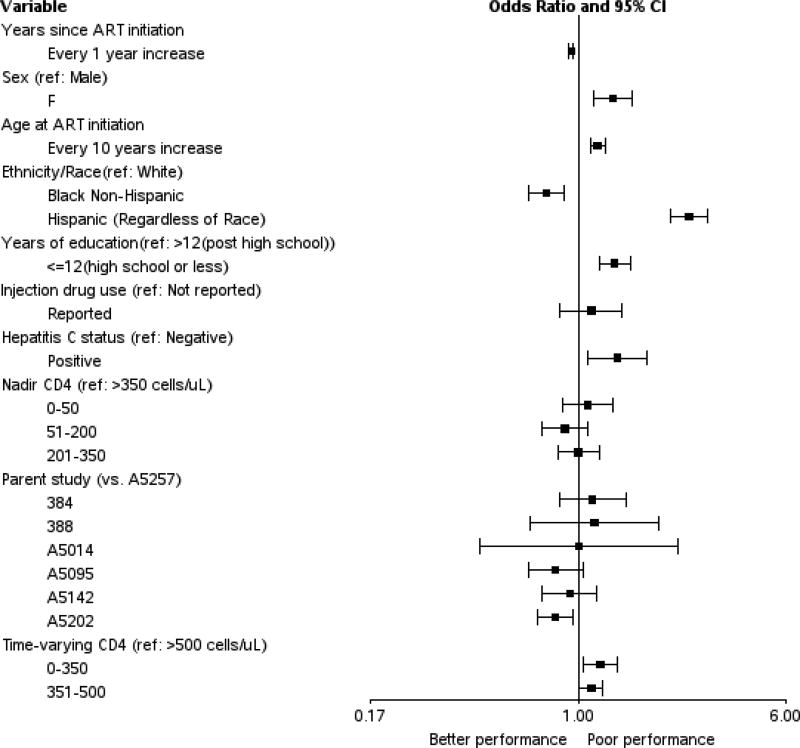
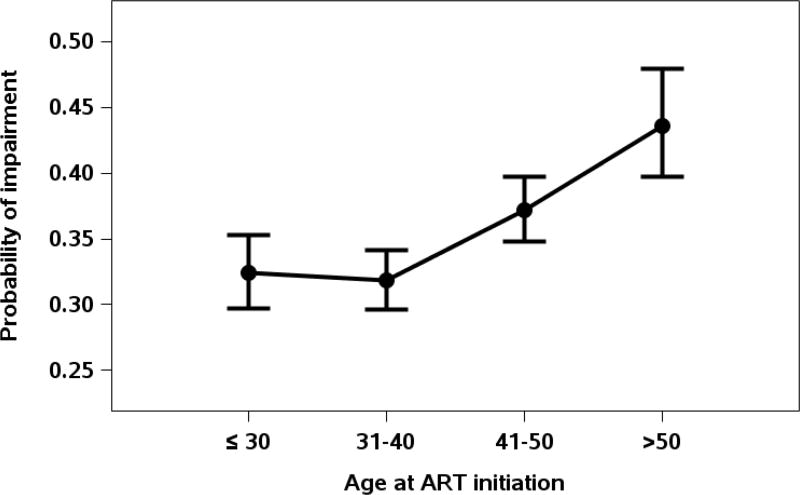
In the multivariable adjusted model (Table 1 and Figure 1), after correcting for the expected effects of age using norms from HIV–negative individuals, the odds of neurocognitive impairment at follow-up visits among our HIV-infected subjects increased by nearly 20% for each decade of advancing age at parent study entry (OR 1.18, [1.11–1.26], p<0.001). Figure 2. shows that the age-related increase in neurocognitive impairment began in the 5th decade (age 41–50). Hepatitis C infection was also associated with neurocognitive impairment (OR 1.40, [1.08–1.82], p=0.01) along with less education (OR 1.37, [1.20–1.57], p<0.001) time-varying CD4 level less than 350 cells/µL (OR 1.21, [1.05–1.40], p=0.008) and time-varying CD4 level between 351–500 cells/µL during follow up (OR 1.12, [1.01–1.24], p=0.03). Women had greater odds of impairment than men (OR 1.35, [1.15–1.59], p<0.001). Variables for which the odds of impairment were not significant in the adjusted models included injection drug use, initial ART drug class and parent study and nadir CD4 level.
Figure 1.
Forest plot showing adjusted odds ratios and 95 percent confidence intervals for the association between risk factors and neurocognitive impairment.
Figure 2.
Probability of cognitive impairment according to age decade at parent study entry.
DISCUSSION
We found that advancing age was a significant risk factor for neurocognitive impairment, even two or more years after starting initial ART treatment. This was true despite normative adjustment of neurocognitive scores for age from HIV-negative individuals, and after adjusting for a variety of covariates in multivariable models. This elevated risk of neurocognitive impairment with age was seen despite continued virologic suppression in most and despite overall neurocognitive improvement in the cohort as a whole. In contrast to a recent study that found that increased cognitive impairment began in the seventh decade [11], we observed an increase in risk beginning in the fifth decade.
Analyses showed that women were more likely than men to show neurocognitive impairment during follow-up after 2 years of cART. Some previous cross-sectional studies have reported higher frequencies of neurocognitive impairment among women as compared to men[23, 24] but others have not[25]. Another study showed no difference in longitudinal worsening between men and women[26]. There were important differences between the cohorts in these prior reports and our own, including absence of cART[24, 26] and differences in neurocognitive tests used[25]. Our cohort is unique in its size and the high proportion of individuals studied after 2 years of virally suppressive ART.
Our findings have implications for the aging HIV+ population on cART. Numerous prior studies have postulated premature or accelerated aging in HIV[27], but the exact mechanisms by which this might occur are unclear. Several mechanisms have been proposed. First, cART has been shown to magnify adverse effects on neurocognition of common comorbidities associated with aging, such as diabetes mellitus, hypertension and abdominal obesity. [28–31] Second, older individuals may require higher CNS-penetration ART regimens to benefit neurocognitively than younger individuals, as was shown in an older subgroup in a recent clinical trial.[32] Third, CNS toxicities of ART may have a greater impact in older participants than younger ones,[33].
Although a recent study [34] did not find HCV co-infection to be significantly associated with poorer neurocognitive outcomes, our study found HCV to be a significant risk factor. Of note, our study was conducted before direct-acting antiviral HCV agents were in use. Differences in findings between our study and previous studies may reflect our larger cohort size and greater power to detect an effect.
We did not find a statistically significant association between initial ART drug class and neurocognitive impairment. This contrasts with some recent studies in which efavirenz (NNRTI) based regimens were found to be associated with worse neurocognitive functioning. [35, 36] A caveat is that some of our study participants might have switched ART regimens during follow-up.
We observed a higher rate of impairment among participants of Latino/Hispanic ethnicity. Since we used normative corrections for Hispanic ethnicity, and in the case of the HVLT-R, for Spanish language, poorer performance of Hispanics is not attributable to inadequate demographic corrections. Similar findings have been reported previously, and are not completely explained by worse HIV disease characteristics or comorbidities. [37–40] These findings might be related to culturally-relevant psychosocial and biomedical factors that have not been adequately explored to date.
Strengths of this study include a large, prospective longitudinal cohort with more than 3000 participants taking randomly assigned initial antiretroviral therapy. The vast majority achieved high rates of sustained virologic suppression. Care was taken to minimize practice effects as a result of repeated testing – in the case of the HVLT-R, by using different word lists, and in the case of the other tests, by using norms corrected for practice effects. A limitation of this study, as with all observational studies, is that the observed relationships represent associations, which may not be causative. Few subjects were over age 60, limiting the generalizability of our findings to older populations. The majority of the cohort began neurocognitive testing subsequent to initiation of cART[13] thus the impact of cART initiation on neurocognition was not assessed.
Future studies should evaluate potential mediators of the adverse effects of age on neurocognitive trajectories, such as inflammation, coinfections such as syphilis and cytomegalovirus, and vascular risk factors including diabetes, hypercholesterolemia and central obesity. Novel treatments will depend in part on which factors or combinations of these factors are driving neurocognitive impairment in HIV.
Acknowledgments
The manuscript was submitted by Hamza Coban, MD. He is a fellow at Interdisciplinary Research Fellowship in NeuroAIDS (IRFN) program which is funded by NIH (MH 081482). He is also supported by funds from NIH MH 105319 (Cristian L. Achim)
The project described was supported by Award Number U01AI068636 from the National Institute of Allergy and Infectious Diseases and supported by National Institute of Mental Health (NIMH), National Institute of Dental and Craniofacial Research (NIDCR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Data in this manuscript were collected by the AIDS Clinical Trials Group (ACTG) Longitudinal Linked Randomized Trials (ALLRT) study. ALLRT was supported by the AIDS Clinical Trials Group of the National Institute for Allergy and Infectious Diseases (AI 68634, AI 38858, AI 38855, AI 069481)
We would also like to acknowledge the study coordinators at the ACTG sites and all the research participants.
Funding: This study funded by NIH grants AI 68634, AI 38858, AI 68636, AI 38855, AI069481, AI 27757
Footnotes
ClinicalTrials.gov Identifier: NCT00001137
References
- 1.UNAIDS. HIV and Aging: A special supplement to the UNIAIDS report on the global AIDS epidemic 2013. Geneva: Joint UN programme on HIV/AIDS; 2013. [Google Scholar]
- 2.Cysique LA, Vaida F, Letendre S, Gibson S, Cherner M, Woods SP, et al. Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology. 2009;73(5):342–348. doi: 10.1212/WNL.0b013e3181ab2b3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tozzi V, Balestra P, Murri R, Galgani S, Bellagamba R, Narciso P, et al. Neurocognitive impairment influences quality of life in HIV-infected patients receiving HAART. Int J STD AIDS. 2004;15(4):254–259. doi: 10.1258/095646204773557794. [DOI] [PubMed] [Google Scholar]
- 4.Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21(14):1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- 5.De Ronchi D, Faranca I, Berardi D, Scudellari P, Borderi M, Manfredi R, et al. Risk factors for cognitive impairment in HIV-1-infected persons with different risk behaviors. Arch Neurol. 2002;59(5):812–818. doi: 10.1001/archneur.59.5.812. [DOI] [PubMed] [Google Scholar]
- 6.Mahy M, Autenrieth CS, Stanecki K, Wynd S. Increasing trends in HIV prevalence among people aged 50 years and older: evidence from estimates and survey data. Aids. 2014;28(4):S453–459. doi: 10.1097/QAD.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan IL, Smith BR, Hammond E, Vornbrock-Roosa H, Creighton J, Selnes O, et al. Older individuals with HIV infection have greater memory deficits than younger individuals. Journal of neurovirology. 2013;19(6):531–536. doi: 10.1007/s13365-013-0209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valcour V, Paul R, Neuhaus J, Shikuma C. The Effects of Age and HIV on Neuropsychological Performance. Journal of the International Neuropsychological Society : JINS. 2011;17(1):190–195. doi: 10.1017/S1355617710001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cross S, Onen N, Gase A, Overton ET, Ances BM. Identifying risk factors for HIV-associated neurocognitive disorders using the international HIV dementia scale. J Neuroimmune Pharmacol. 2013;8(5):1114–1122. doi: 10.1007/s11481-013-9505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molsberry SA, Lecci F, Kingsley L, Junker B, Reynolds S, Goodkin K, et al. Mixed membership trajectory models of cognitive impairment in the multicenter AIDS cohort study. Aids. 2015;29(6):713–721. doi: 10.1097/QAD.0000000000000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seider TR, Luo X, Gongvatana A, Devlin KN, de la Monte SM, Chasman JD, et al. Verbal memory declines more rapidly with age in HIV infected versus uninfected adults. J Clin Exp Neuropsychol. 2014;36(4):356–367. doi: 10.1080/13803395.2014.892061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smurzynski M, Collier AC, Koletar SL, Bosch RJ, Wu K, Bastow B, et al. AIDS clinical trials group longitudinal linked randomized trials (ALLRT): rationale, design, and baseline characteristics. HIV Clin Trials. 2008;9(4):269–282. doi: 10.1310/hct0904-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reitan RMWD. The Halstead–Reitan neuropsychological test battery. Tucson, Arizona: Neuropsychology Press; 1985. [Google Scholar]
- 15.Wechsler D. Wechsler adult intelligence scale – revised manual. New York: The Psychological Corporation; 1981. [Google Scholar]
- 16.Belkonen S. Hopkins Verbal Learning Test. In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of Clinical Neuropsychology. New York, NY: Springer New York; 2011. pp. 1264–1265. [Google Scholar]
- 17.Tross S, Price RW, Navia B, Thaler HT, Gold J, Hirsch DA, et al. Neuropsychological characterization of the AIDS dementia complex: a preliminary report. AIDS. 1988;2(2):81–88. doi: 10.1097/00002030-198804000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Van Gorp WG, Miller EN, Satz P, Visscher B. Neuropsychological performance in HIV-1 immunocompromised patients: a preliminary report. J Clin Exp Neuropsychol. 1989;11:763–773. doi: 10.1080/01688638908400930. [DOI] [PubMed] [Google Scholar]
- 19.Heaton RK, Miller SW, Taylor M, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan battery : demographically adjusted neuropsychological norms for African American and Caucasian adults, professional manual. Lutz, Fla.: Psychological Assessment Resources; 2004. [Google Scholar]
- 20.Norman MA, Moore DJ, Taylor M, Franklin D, Jr, Cysique L, Ake C, et al. Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. J Clin Exp Neuropsychol. 2011;33(7):793–804. doi: 10.1080/13803395.2011.559157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cherner M, Suarez P, Lazzaretto D, Fortuny LA, Mindt MR, Dawes S, et al. Demographically corrected norms for the Brief Visuospatial Memory Test-revised and Hopkins Verbal Learning Test-revised in monolingual Spanish speakers from the U.S.-Mexico border region. Arch Clin Neuropsychol. 2007;22(3):343–353. doi: 10.1016/j.acn.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bunupuradah T, Chetchotisakd P, Jirajariyavej S, Valcour V, Bowonwattanuwong C, Munsakul W, et al. Neurocognitive impairment in patients randomized to second-line lopinavir/ritonavir-based antiretroviral therapy vs. lopinavir/ritonavir monotherapy. Journal of neurovirology. 2012;18(6):479–487. doi: 10.1007/s13365-012-0127-9. [DOI] [PubMed] [Google Scholar]
- 23.Hestad KA, Menon JA, Silalukey-Ngoma M, Franklin DR, Jr, Imasiku ML, Kalima K, et al. Sex differences in neuropsychological performance as an effect of human immunodeficiency virus infection: a pilot study in Zambia, Africa. J Nerv Ment Dis. 2012;200(4):336–342. doi: 10.1097/NMD.0b013e31824cc225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Royal W, 3rd, Cherner M, Burdo TH, Umlauf A, Letendre SL, Jumare J, et al. Associations between Cognition, Gender and Monocyte Activation among HIV Infected Individuals in Nigeria. PLoS One. 2016;11(2):e0147182. doi: 10.1371/journal.pone.0147182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin E, Gonzalez R, Vassileva J, Maki P. HIV+ men and women show different performance patterns on procedural learning tasks. J Clin Exp Neuropsychol. 2011;33(1):112–120. doi: 10.1080/13803395.2010.493150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson KR, Kapoor C, Robertson WT, Fiscus S, Ford S, Hall CD. No gender differences in the progression of nervous system disease in HIV infection. J Acquir Immune Defic Syndr. 2004;36(3):817–822. doi: 10.1097/00126334-200407010-00008. [DOI] [PubMed] [Google Scholar]
- 27.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCutchan JA, Marquie-Beck JA, Fitzsimons CA, Letendre SL, Ellis RJ, Heaton RK, et al. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology. 2012;78(7):485–492. doi: 10.1212/WNL.0b013e3182478d64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foley J, Ettenhofer M, Wright MJ, Siddiqi I, Choi M, Thames AD, et al. Neurocognitive functioning in HIV-1 infection: effects of cerebrovascular risk factors and age. The Clinical neuropsychologist. 2010;24(2):265–285. doi: 10.1080/13854040903482830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fabbiani M, Ciccarelli N, Tana M, Farina S, Baldonero E, Di Cristo V, et al. Cardiovascular risk factors and carotid intima-media thickness are associated with lower cognitive performance in HIV-infected patients. HIV Med. 2013;14(3):136–144. doi: 10.1111/j.1468-1293.2012.01044.x. [DOI] [PubMed] [Google Scholar]
- 31.Valcour VG, Sacktor NC, Paul RH, Watters MR, Selnes OA, Shiramizu BT, et al. Insulin resistance is associated with cognition among HIV-1-infected patients: the Hawaii Aging With HIV cohort. J Acquir Immune Defic Syndr. 2006;43(4):405–410. doi: 10.1097/01.qai.0000243119.67529.f5. [DOI] [PubMed] [Google Scholar]
- 32.Ellis RJ, Letendre S, Vaida F, Haubrich R, Heaton RK, Sacktor N, et al. Randomized trial of central nervous system-targeted antiretrovirals for HIV-associated neurocognitive disorder. Clin Infect Dis. 2014;58(7):1015–1022. doi: 10.1093/cid/cit921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Underwood J, Robertson KR, Winston A. Could antiretroviral neurotoxicity play a role in the pathogenesis of cognitive impairment in treated HIV disease? AIDS. 2015;29(3):253–261. doi: 10.1097/QAD.0000000000000538. [DOI] [PubMed] [Google Scholar]
- 34.Clifford DB, Vaida F, Kao YT, Franklin DR, Letendre SL, Collier AC, et al. Absence of neurocognitive effect of hepatitis C infection in HIV-coinfected people. Neurology. 2015;84(3):241–250. doi: 10.1212/WNL.0000000000001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciccarelli N, Fabbiani M, Di Giambenedetto S, Fanti I, Baldonero E, Bracciale L, et al. Efavirenz associated with cognitive disorders in otherwise asymptomatic HIV-infected patients. Neurology. 2011;76(16):1403–1409. doi: 10.1212/WNL.0b013e31821670fb. [DOI] [PubMed] [Google Scholar]
- 36.Ma Q, Vaida F, Wong J, Sanders CA, Kao YT, Croteau D, et al. Long-term efavirenz use is associated with worse neurocognitive functioning in HIV-infected patients. Journal of neurovirology. 2016;22(2):170–178. doi: 10.1007/s13365-015-0382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Durvasula RS, Miller EN, Myers HF, Wyatt GE. Predictors of neuropsychological performance in HIV positive women. J Clin Exp Neuropsychol. 2001;23(2):149–163. doi: 10.1076/jcen.23.2.149.1211. [DOI] [PubMed] [Google Scholar]
- 38.Rivera Mindt M, Arentoft A, Kubo Germano K, D'Aquila E, Scheiner D, Pizzirusso M, et al. Neuropsychological, cognitive, and theoretical considerations for evaluation of bilingual individuals. Neuropsychol Rev. 2008;18(3):255–268. doi: 10.1007/s11065-008-9069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heaton RK, Franklin DR, Jr, Deutsch R, Letendre S, Ellis RJ, Casaletto K, et al. Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis. 2015;60(3):473–480. doi: 10.1093/cid/ciu862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wojna V, Skolasky RL, Hechavarria R, Mayo R, Selnes O, McArthur JC, et al. Prevalence of human immunodeficiency virus-associated cognitive impairment in a group of Hispanic women at risk for neurological impairment. Journal of neurovirology. 2006;12(5):356–364. doi: 10.1080/13550280600964576. [DOI] [PubMed] [Google Scholar]