Abstract
The Tamaulipan rock rattlesnake (Crotalus lepidus morulus) is a montane snake that occurs in the humid pine-oak forest and the upper cloud forest of the Sierra Madre Oriental in southwestern Tamaulipas, central Nuevo Leon, and southeastern Coahuila in Mexico. Venom from this rattlesnake was fractionated by High-Performance Liquid Chromatography for the purpose of discovering disintegrin molecules. Disintegrins are non-enzymatic, small molecular weight peptides that interfere with cell-cell and cell-matrix interactions by binding to various cell receptors. Eleven fractions were collected by anion exchange chromatography and pooled into six groups (I, II, III, IV, V, and VI). Proteins of the six groups were analyzed by SDS-PAGE and western blot using antibodies raised against a disintegrin.
The antibodies recognized different protein bands in five (II, III, IV, V, and VI) of six groups in a molecular mass range of 7 to 105 kDa. Western blot analysis revealed fewer protein bands in the higher molecular mass range and two bands in the disintegrin weight range in group II compared with the other four groups. Proteins in group II were further separated into nine fractions using reverse phase C18 chromatography. Fraction 4 inhibited platelet aggregation and was named morulustatin, which exhibited a single band with a molecular mass of approximately 7 kDa. Mass spectrometry analysis of fraction 4 revealed the identification of disintegrin peptides LRPGAQCADGLCCDQCR (MH+ 2035.84) and AGEECDCGSPANCCDAATCK (MH+ 2328.82). Morulustatin inhibited ADP-induced platelet aggregation in human whole blood and was concentration-dependent with an IC50 of 89.5 nM ± 12.
Keywords: Crotalus lepidus morulus, disintegrin, hemostasis, morulustatin, platelets, venom
Introduction
Snake venoms are complex mixtures of proteins and peptides possessing a variety of biological activities [5]. Viperid (family Viperidae) venom proteins belong to only a few major protein families, including enzymes and proteins without enzymatic activity [4]. In particular, common rattlesnake (Crotalus and Sistrurus genera) venom components include metalloproteinases, serine proteases, phospholipases A2 (PLA2s), L-amino acid oxidases (LAAOs), Cysteine-Rich Secretory Proteins (CRISPs), C-type lectin-like, disintegrins and vasoactive peptides [5,6,10].
Viperid venoms may contain well over 100 protein components [25]. These venom proteins may exhibit important pharmacological activities that humans could exploit [13], i. e. some metalloproteinases, serine proteases and disintegrins isolated from snake venoms have served as a basis for the manufacture of drugs designed for the diagnosis and treatment of cardiovascular and cerebrovascular disorders. Also, some venom toxins, in particular, disintegrins, have also been described as having antimetastasis activities, which is the leading causes of death in patients with cancer [26]. Metastasis is determined by several stages that involve adhesion, migration, invasion of lymph nodes and vessels, causing the tumor cells to leave the vasculature with the help of metalloproteinases, and arriving at the tissue target [4, 10, 19, 20, 25, 28]. Many studies have shown disintegrins to inhibit cancer cell migration in vitro, and inhibit lung tumor colonization by cancer cells in vivo [1, 8, 10, 13, 15].
Disintegrins are peptides rich in cysteines that contain in their structure a sequence of amino acids located at the tip of a flexible hairpin loop of type RGD, KGD, MLD, KTS, ECD, VGD, MGD and WGD, which recognize integrin receptors [15]. Integrins are heterodimeric (αβ) cell receptors containing an extracellular and a transmembrane domain, as well as a short cytoplasmic tail in each subunit. These receptors are present in platelet membranes (i.e. αIIbβ3, αvβ3 and α2β1), where they allow interactions between extracellular adhesion molecules and the intracellular actin cytoskeleton [15]. Integrins are significant classes of cell surface receptors implicated in cell-cell and cell-matrix interactions. The subfamilies of integrins β1 and β3 play a crucial role in tumor invasion and dissemination. Contortrostatin, a disintegrin from Agkistrodon contortrix contortrix venom, is an example of an effective inhibitor of β1-integrin-mediated adhesion in metastatic melanoma cells [30].
Disintegrins are divided into five different subgroups according to their polypeptide length and number of disulfide bonds. The subgroups include small (41-52 amino acids, 4 disulfide linkages), medium (∼70 amino acids, 6 disulfide linkages), large (∼84 amino acids, 7 disulfide linkages), homodimeric and heterodimeric disintegrins [16]. Dimeric disintegrins contain subunits of about 67 residues each with ten cysteines involved in the formation of four intra-chain disulfide bonds and two interchain cysteine linkages [3]. Many studies have shown that disintegrins are capable of inhibiting platelet aggregation induced by collagen, adenosine diphosphate (ADP), arachidonic acid, ristocetin, and thrombin [16, 21]
Disintegrins are described in the venoms of many Crotalids [24]. The genus Crotalus is a group of rattlesnakes highly representative in Mexico. It is composed of around 31 species, 16 of them being endemic [2]. Some of these Mexican species are relatively small montane rattlesnakes inhabiting the pine–oak forests of all of the major mountainous regions of Mexico. Although the venom composition of some species of rattlesnake is very well known [5,12], there is little or no information for many species of montane rattlesnakes, particularly for the many species occupying the Mexican highlands [9]. The Tamaulipas rock rattlesnake (Crotalus lepidus morulus) is a relatively small, montane rattlesnake that occurs in the humid pine-oak forest and the upper cloud forest of the Sierra Madre Oriental in southwestern Tamaulipas, central Nuevo Leon, and southeastern Coahuila in Mexico [7]. Crotalus lepidus morulus venom can produce hemorrhage and edema in mice [3,9]. Furthermore, it shows myotoxic, proteolytic (over azocasein casein and gelatin) and fibrinogenolytic activities [3, 16]. In addition, individual Tamaulipan rock rattlesnakes collected in Northeast of Mexico have been reported to have venom with both intra and inter-specific variation in regards to protein content and biological activities [9].
Considering that disintegrins can prevent platelet aggregation via inhibition of a surface glycoprotein receptor (αIIbβ3) activity, they offer a unique opportunity for studying platelet-platelet and platelet-endothelium interactions [18]. The goal of this study was to identify a disintegrin inhibiting ADP-induced platelet aggregation present in the venom of the Tamaulipan rock rattlesnake.
Materials and Methods
Snakes
Crotalus lepidus morulus snake were obtained from Galeana, Nuevo Leon, México (24° 50′ 0″ N / 100° 4′ 0″ W) (FIG.1). They are currently housed at the herpetarium of the Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León, México.
Figure 1. Location of Capture Site of the Tamaulipan Rock Rattlesnake (Crotalus lepidus morulus) Galeana, State of Nuevo Leon Location in Mexico.
Venom
Venom from three C. l. morulus was extracted by allowing the snakes to bite into a parafilm stretched over a disposable plastic cup. The venom samples were pooled, centrifuged (Eppendorf Model 5417R, USA) (500 gfor 10 min), and filtered through a 0.45 μm filter. The venom was lyophilized (Labconco Freezone 6, USA) and stored at 4°C (Revco, Thermo Electron Corporation, USA). Additionally, pooled venom from Crotalus lepidus klauberi (supplied by the National Natural Toxins Research Center) was used as control.
Protein concentration determination
Protein concentrations were determined by standard methods [14] at 280 nm using an extinction coefficient of 1 mg/mL. A Beckman's DU7400 spectrophotometer (USA) was used to measure absorbance.
Protein purification
Anion exchange chromatography
Two hundred microliters of venom sample (40 mg/mL) were fractionated by anion exchange chromatography. Venom was separated using a Waters® DEAE 5PW column (7.5 × 75 mm) column on a Waters High Performance Liquid Chromatography (HPLC) system. The column was equilibrated with 0.02M Tris-HCl buffer, pH 8.0. Fractions were eluted using 0.02M Tris–HCl, buffer, pH 8.0 containing 0.5M NaCl, at a flow rate of 1 mL/min. A Waters 2487 tunable detector (USA) was used to monitor absorbance at 280 nm. Fractions were analyzed by western blot and probed with antibodies raised against recombinant disintegrin mojastin. Fractions were pooled into six groups (I, II, II, IV, V, and VI) according to their elution times. Proteins in group II were further fractionated by reverse phase C18 chromatography.
Reverse phase C18 chromatography
Proteins of the anion exchange group II were further fractionated by reverse phase C18 chromatography. Two hundred microliters of venom sample (2 mg/mL) were separated using a Grace Vydac Reverse Phase C18 (250 × 4.6 mm) column. The column was equilibrated using 0.1% trifluoroacetic acid (TFA), and the elution of fractions was accomplished using an 80% acetonitrile in 0.1% TFA gradient over 60 min, with a flow rate of 1 mL/min. A Waters 2487 tunable detector was used to monitor absorbance at 280 nm.
SDS-PAGE
A mixture containing 10 μL of venom sample (2 mg/ml of crude venom or 1 mg/ml of HPLC fractions), 5 μl of 2× sample buffer (Novex, Invitrogen) and 1μl of 10× sample reducing agent NUPAGE (Invitrogen™, USA) was heated to 100 °C for 5 min and loaded on to a10-20% Tricine acrylamide gels (Invitrogen™, USA). Samples were separated for 90 min at 125 V. Gels were stained with Simply Blue Safe Stain (Invitrogen) and distained overnight in water. Molecular Weight Standards (See Blue Plus2 Prestained, Invitrogen, USA) were used to estimate molecular masses.
Identification of disintegrins by western blot
Crude venoms and venom C18 fractions were separated by SDS-PAGE under the conditions described above. After electrophoresis, proteins were transferred to a 0.2 μm Immobilon PVDF membrane (previously washed with 100% methanol) using a Trans-blot SD semi-dry transfer cell (BIO-RAD). After transference at 25 V: 100 mA for 1 h, the membrane was blocked with 5% non-fat dry milk diluted in 1× Tris (hydroxymethyl) aminomethane buffer (TBST), pH 8.4, for 2 h. The membrane was then rinsed three times with 1× TBST, pH 8.4, and incubated for 12 h at room temperature (with gentle shaking) with rabbit (Oryctolagus cuniculus) antibodies against recombinant-mojastin diluted 1:1000 in 1× TBST, pH 8.4. After three washes with 1× TBST, pH 8.4, the membrane was incubated at room temperature for 1 h with biotinyalted anti-rabbit IgG (generated in goat), diluted 1:20,000 in 1× TBST. The membrane was again rinsed three times with 1× TBST, pH 8.4, and incubated for 1 h at room temperature with ExtrAvidin-Peroxidase (Sigma), diluted 1:2000. Finally, the membrane was washed with 1× TBST, pH 8.4, (three times) and developed by adding 3,3-diaminobenzidine (DAB+UREA H2O2) (Sigma Fast, USA) for 1 h.
Sample preparation, LC-MS/MS and data analysis
Disintegrin samples were reduced in 1% sodium deoxycholate in 50 mM ammonium bicarbonate (pH 7.5) and heated to 99°C for 5 min. The mixture was then allowed to cool down to 25°C and 5 mM DTT was added and incubated for 30 min at 56°C, followed by alkylation with 15 mMiodoacetamide for 1 hr at 25°C in the dark. Excess iodoacetamide was neutralized using 5 mM DTT incubated for 15 min at 25°C. Proteins were digested overnight with sequencing grade modified trypsin (enzyme:protein ratio of 1:50) at 37 °C. The digested mixture was acidified with 1% Formic Acid (FA) final concentration. The samples were desalted using C18 (Waters) cartridge, and then evaporated to dryness in a SpeedVac (ThermoFisher Scientific, USA).
Peptides were analyzed by online reverse phase chromatography coupled with an electrospray ionization interface to acquire MS (measuring intensity and m/z ratio for peptides) and MS/MS (fragmentation spectra of peptides) scans. A nanoflow HPLC system (Eksigent, Thermo Fisher Scientific, USA) was used for online reversed-phase chromatographic separation; peptides were loaded on 5 mm long trap column (inner diameter 300 μm) in buffer A (0.2% FA) and separated on 18 cm long fused silica capillary analytical column (inner diameter 150 μm), both packed with 3um 200A Magic AQ C18 reverse-phase material (MichromBioResources, Inc, CA, USA). Peptides were eluted by an increasing concentration of buffer B (0.2% FA in Acetonitrile (ACN)) – from 5 to 40% in 100 min. Following the gradient elution, the column was washed with 80% buffer B and re-equilibrated with 5% buffer B. Peptides were eluted into the mass spectrometer at a flow rate of 600 nl/min. The total run time was approximately 125 min, including sample loading and column conditioning. Peptides were analyzed using an automated data-dependent acquisition on a LTQ-Orbitrap Elite mass Spectrometer (USA). Each mass Spectrometry (MS) scan was acquired at a resolution of 240,000 fwhm (at 400 m/z) for mass range 300-2,000 Th with the lock mass option enabled (m/z: 445.120025) and was followed by up to 12 MS/MS data dependent scans on the most intense ions using collision induced activation (CID). AGC (Automatic Gain Control) target values for MS and MS/MS scans were set to 1e6 (max fill time 500 ms) and 1e5 (max fill time 50 ms) respectively. The precursor isolation window was set to 2 Th with CID normalized collision energy of 35; the dynamic exclusion window was set to 60 seconds.
MS data were analyzed using MASCOT software version and searched against the Uniprot/SwissProt database (http://www.uniprot.org/). Search criteria included a static modification of cysteine residues of +57.0214 Da; a variable modification of +15.9949 Da to include potential oxidation of methionines. Searches were performed with semi-tryptic digestion, allowing a maximum of two missed cleavages on the peptides and analyzed from the sequence database. The false discovery rate (FDR) for peptide, protein, and site identification was set to 1%.
Platelet aggregation assay
A Chrono-Log Whole-Blood Aggregometer™ was used to monitor platelet aggregation, by impedance, of human whole blood incubated with the reverse phase fractions (FIG. 6) [8, 23]. Four hundred and fifty microliters of 10% citrated human blood was incubated, at 3 7°C, with equal amounts of 0.15 M saline solution, at least 5 min prior to testing. Ten microliters of venom fractions (1 mg/mL) were incubated with the blood sample for 2 min. An electrode was inserted in the blood sample, and 90 s later, 10 μL of a 1 mM ADP solution were added to the blood sample to promote platelet aggregation. As a control, platelet aggregation was initiated by 10 μL of ADP (10 μM final concentration), and the percentage of impedance reflecting percentage of aggregation was measured. The percent inhibition of platelet aggregation was calculated by the following equation: [(C-E/C] ×100, where C is the units of platelet aggregation (ohms) for the control, and E is the unit of platelet aggregation (ohms) for the experimental fraction. The negative control consisted of whole blood incubated with 10 μL of 0.15 M saline solution. The extent of the inhibition of platelet aggregation was assessed by comparing the maximal aggregation induced by the control dose of agonist (10 μM ADP) and expressed as 50% inhibition concentration (IC50) as determined from the equation of the line generated by the dose-response curve produced for the various disintegrin concentrations.
Figure 6. Reverse Phase C18 Chromatography of Anion Exchange Group II Proteins and SDS-PAGE and Western Blot Analysis of Reverse Phase, Disintegrin-Active, Fraction 4 (Morulustatin).
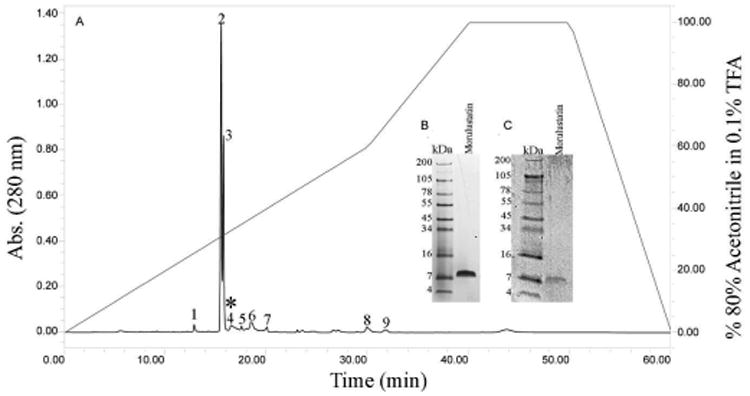
A) Two hundred microliters of venom sample (2 mg/mL) were separated using a Grace Vydac Reverse Phase C18 (250 × 4.6 mm) column. The column was equilibrated using 0.1% trifluoroacetic acid (TFA), and the elution of fractions were accomplished using 80% acetonitrile in 0.1% TFA gradient over 60 min, with a flow rate of 1 mL/min. A Waters 2487 tunable detector was used to monitor absorbance at 280 nm. The fractions were tested for inhibition of platelet aggregation. Disintegrin activity was detected in fraction 4*. B) Morulustatin ran under reducing-conditions in a 10-20% Tricine acrylamide gel. Protein was stained with Simply Blue Safe Stain (Invitrogen). C) Western blot of morulustatin with anti-r-mojasitin disintegrin polyclonal antibodies.
Results and Discussion
In the current work, the focus was to identify a disintegrin that inhibits ADP-induced platelet aggregation present in the venom of a poorly studied rattlesnake, C. l. morulus. Due to the limited amount of venom sample, only the inhibition of platelet aggregation was assayed with the isolated disintegrin, morulustatin.
Crude venom was initially fractionated by anion exchange chromatography (FIG. 2), and antibodies against a disintegrin from the venom of the Mohave rattlesnake were used in a western blot to identify the fractions that contained disintegrins (FIG. 5; unpublished data). Eleven fractions were collected by anion exchange chromatography from C. l. morulus venom (FIG. 2). For comparison purposes, venom of C. l. klauberi was fractioned by anion exchange as well. The venom of C. l. klauberi displayed more fractions (14 fractions) than C. l. morulus venom (11 fractions) (FIGS. 2 and 3). Differences within these two crude venoms can also be observed by SDS-PAGE with C. l. morulus having more peptides at the low molecular weight range (FIG. 4). Crotalus lepidus morulus showed a complex electrophoretic pattern with bands ranging from ∼7 to 100 kDa under reducing conditions (FIG. 4). The two more intense bands were seen in molecular weights of ∼48 kDa and ∼11 kDa. These two bands could correspond with a metalloproteinase (∼54 kDa) and a galactose-specific lectin (∼14 kDa) and/or a phospholipase A2 D49a (∼14 kDa) already reported by Martinez-Romero et al. [17] in a pool of C. l. morulus venom from four snakes collected in Tejocote, Santiago, Nuevo Leon.
Figure 2. Anion Exchange Chromatography of C. L. Morulus Venom from Galeana, Nuevo Leon, Mexico.
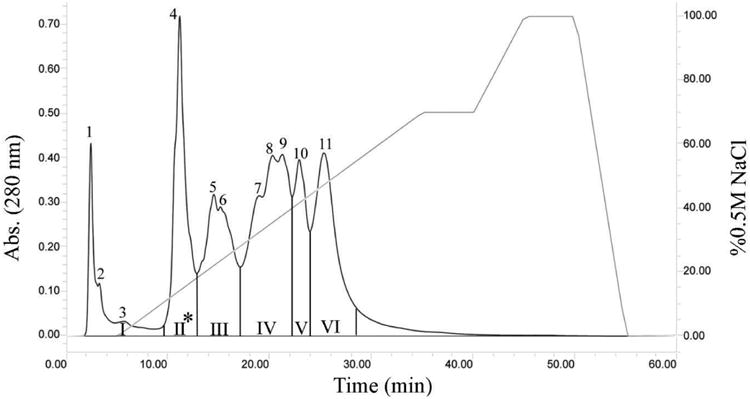
Two hundred microliters of venom sample (40 mg/mL) were fractionated using a Waters DEAE 5PW column (7.5 × 75 mm). The column was equilibrated with 0.02M Tris-HCl buffer, pH 8.0, and the venom proteins were eluted using 0.02 M Tris–HCl buffer, pH 8.0 gradient over 60 min, with a flow rate of 1 mL/min on a Waters high-performance liquid chromatography system. A Waters 2487 tunable detector was used to monitor the absorbance at 280 nm. Fractions were pooled into six groups (I, II, II, IV, V, and VI) according to their retention times. Proteins in group II were further fractionated by reverse phase C18 chromatography.
Figure 5. Western Blot Analysis of Crude C. l. klauber (C.L.K.) and C. l. Morulus (C.L.M) Venoms and Anion Exchange Protein Groups (i, ii, iii, iv, v, vi) of C. l. Morulus Venom.
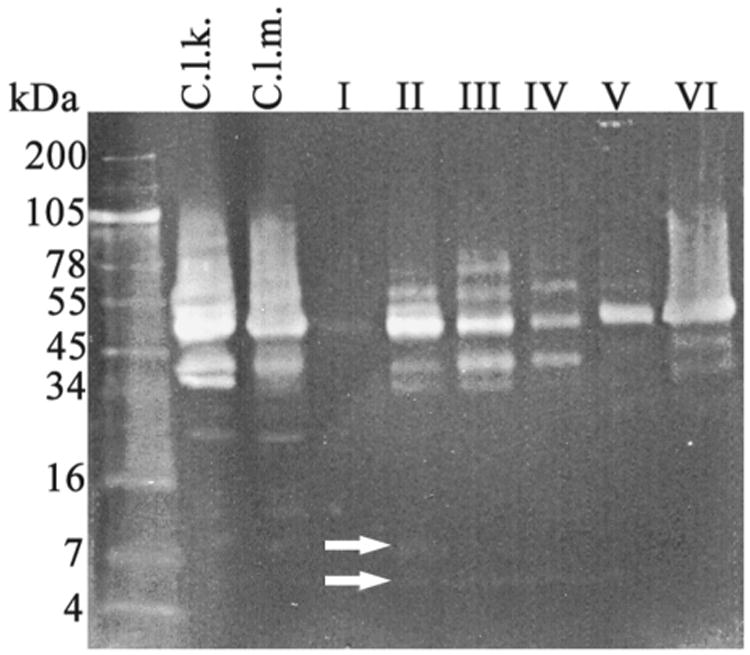
Samples were separated under reducing-conditions in a 10-20% Tricine acrylamide gel at 120V for 90 min and electrotransfered to a nitrocellulose membrane. Proteins were incubated in tandem starting with rabbit antibodies against recombinant-Mojastin (1:1000) followed by anti-rabbit IgG Biotin (generated in goat), diluted 1:20,000, and then with ExtrAvidin-Peroxidase (1:2000). Recognized bands were visualized with DAB+UREA H2O2. White arrows point to disintegrins found in fractions II-IV. Only fraction II contain the disintegrin, morulustatin, indicated by the top arrow.
Figure 3. Anion Exchange Chromatography of C. l. klauberi Venom.
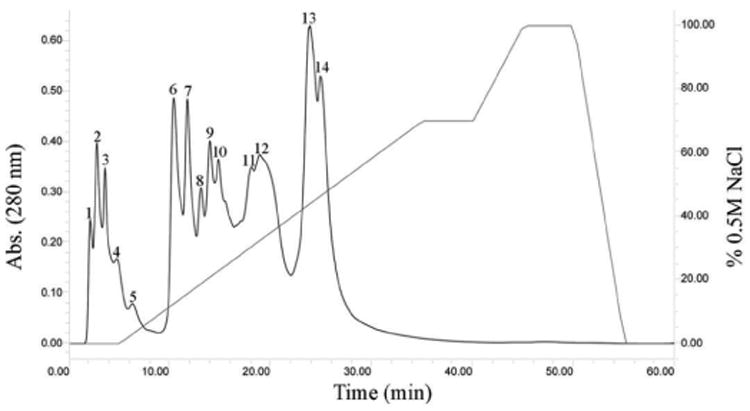
Two hundred microliters of venom sample (40 mg/mL) were fractionated using a Waters DEAE 5PW column (7.5 × 75 mm). The column was equilibrated with 0.02M Tris-HCl buffer, pH 8.0, and the venom proteins were eluted using 0.02 M Tris–HCl buffer, pH 8.0 gradient over 60 min, with a flow rate of 1 mL/min on a Waters high-performance liquid chromatography system. A Waters 2487 tunable detector was used to monitor the absorbance at 280 nm.
Figure 4. SDS-PAGE Analysis of Crude C. l. klauberi (C.L.K) and C. l. morulus (C.L.M) Venoms and Anion Exchange Protein Groups (I, II, III, IV, V, VI) of C. l. morulus Venom.
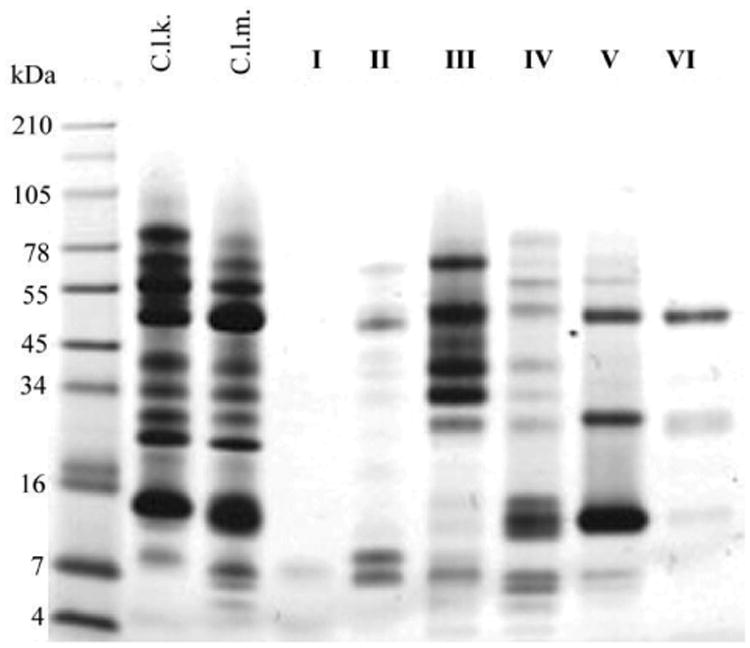
A total of 20 μg of crude venom and 10 μg of venom fractions were separated under reducing-conditions in a 10-20% Tricine acrylamide gel. The gel was run at 120V for 90 min using a BioRad PowerPak system. Proteins were stained with Simply Blue Safe Stain (Invitrogen). Numbers on the left and right of the gel indicated migration of the molecular mass markers.
The eleven fractions, collected from the C. l. morulus venom, were pooled into six groups (I, II, II, IV, V, and VI) according to their similarities in the elution profile (FIG. 2). Proteins of the six groups were analyzed by SDS-PAGE (FIG. 4) and western blot using antibodies against recombinant-mojastin, a recombinant disintegrin (FIG. 5). Mojastin is a monomeric medium-sized disintegrin (molecular mass of 7. 44 kDa) isolated from C. s. scutulatus venom, capable of inhibiting the three processes involved in platelet function (adhesion, activation, and aggregation) [22,23]. Antibodies against recombinant-mojastin (unpublished data) recognized proteins at molecular masses of ∼7, 20, and 34-100 kDa, in both whole C. l. morulus venom and most of its anion exchange fractions (FIG. 5). The ranges of proteins recognized by antibodies most likely represent disintegrin or disintegrin-like domains linked to P-II and P-III SVMPs, respectively, and medium-sized monomeric disintegrins. This cross-reactivity suggests an important sequence similarity between mojastin and disintegrins from the venom of C. l. morulus. In addition, venom of C. l. klauberi appears to react with the antibodies with a stronger intensity than C. l. morulus, indicating a higher quantity of P-II and P-III SVMPs in the venom of C. l. klauberi (FIG. 5). In the fractions of C. l. morulus, antibodies recognized different bands in five (II, II, IV, V, and VI) of the six groups in a range of molecular masses of ∼7 to 100 kDa (FIG. 5). Group II showed fewer protein bands by SDS-PAGE compared to the other four western blot positive groups, and only this group exhibited two bands (arrows) with a molecular weight expected for medium-sized disintegrin (∼7 kDa) (FIG. 5). The reason for using disintegrin antibodies in this study was to identify the venom fractions containing disintegrins and to avoid assaying a sample that was not pure and in very limited supply. Once group II (FIG. 2) was identified as having the most positive bands for possible disintegrins by western blot, it was fractionated by reverse phase (FIG. 6). All fractions were tested for platelet activity, and fraction 4 was the only one inhibiting platelet aggregation (FIG. 8A and B).
Figure 8. The Effect of Morulustatin on ADP-Induced Platelet Aggregation.
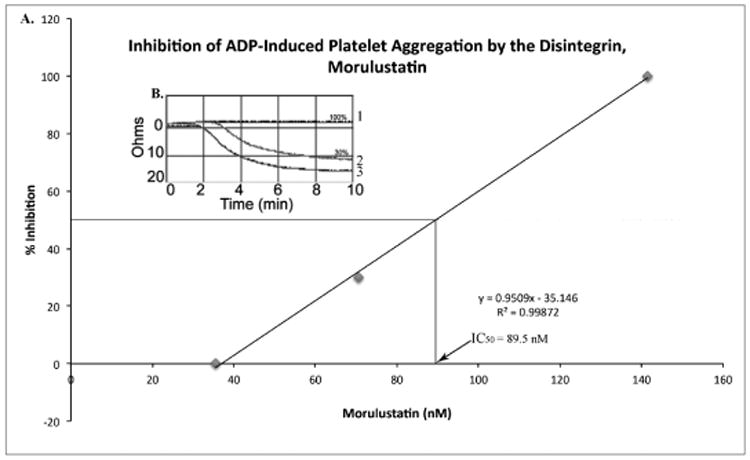
A) The dose dependent curve for the inhibition of platelet aggregation. The equation of the line was used to calculate the IC50 value of morulustatin. B) Platelet aggregation profile. Morulustatin was able to inhibit ADP-induced platelet aggregation in human whole blood by 100% in a concentration range of 565.8 to 141.4 nM (1), and 30% at a concentration of 70.7 nM (2), and 0% at 35.35 nM (3). Human whole blood without morulustatin was used as control (3).
By SDS-PAGE, fraction 4 exhibited a unique band with an apparent molecular mass of approximately 7 kDa under reducing conditions and was recognized by the disintegrin antibody in a western blot (FIG. 6B). The LC-MS/MS mass spectrometry analysis of fraction 4 (designated morulustatin) revealed the identification of peptides LRPGAQCADGLCCDQCR (MH+ 2035.84) and AGEECDCGSPANCCDAATCK (MH+ 2328.82) having similar peptide sequences to disintegrin molecules horridistatin and viridin, respectively (FIG. 7).
Figure 7. LC-MS/MS of Morulustatin: Peptide LRPGAQ-CADGLCCDQCR, MH+ 2035.84 and Peptide AGEECDCGSPANC-CDAATCK, MH+ 2328.82.
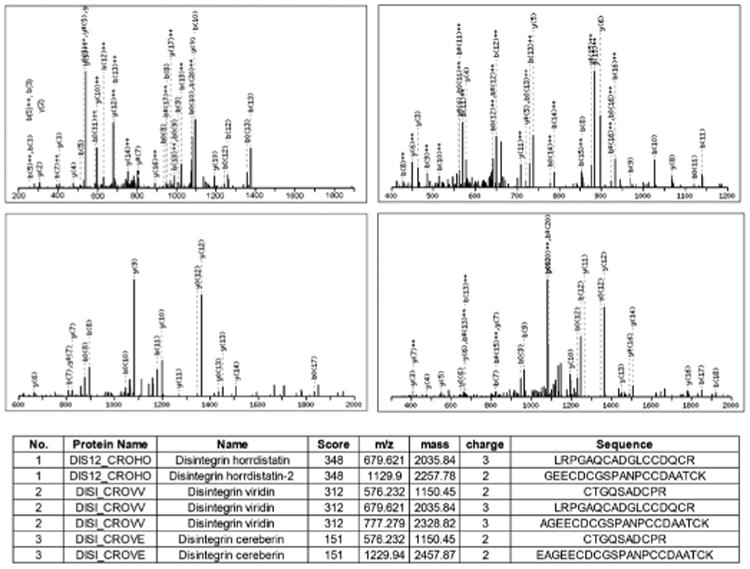
The characteristic peptide bond fragment ions, type b and y ions are labeled. Eight microliters of sample were injected in an Eksigent HPLC system using a reverse phase C18 liquid chromatography column packed with 3 μm C18 Magic beads (Michrom; 150 μmi.d. and 18 cm of bed length) coupled online with a Velos Orbitrap hybrid mass spectrometer. The mass spectrometer was operated in the data-dependent mode, in which a full scan MS was followed by MS/MS scans of the 6 most abundant ions with +2 to +3 charge states.
Morulustatin inhibited ADP-induced platelet aggregation in human whole blood, and this effect was concentration-dependent with an IC50 value of 89.5 nM ± 12 (FIGS. 8A and B). Monomeric and homodimeric disintegrins have significant inhibitory activities on APD-induced platelet aggregation (less than 400 nM) while most heterodimeric disintegrins show greater IC50 values [19,]. Scarborough et al. [24] isolated and characterized eleven disintegrins from North American Pit Viper venoms; these peptides revealed to be potent inhibitors of ADP-induced platelet aggregation with IC50 values ranging from 93 ± 14 nM (Cerastin) to 289 ± 35 nM (Cereberin). However, recent studies demonstrate that Crotalus spp disintegrins inhibit ADP-induced platelet aggregation with IC50 values as low as 13.8 nM, 16.2 nM, 17.5 nM and 19.3 nM for mojastin-2, horridistatin, mojastin-1 and crotatroxin, respectively [11, 21, 22]. The IC50 value displayed by morulustatin (89.5 nM) is less in comparison with other Crotalus spp. disintegrins, suggesting that morulustatin has a lower specificity for the human platelet integrin, αIIbβ3.This is not surprising since it is known that gene duplication and subsequent evolution are considered to play a role in the diversification of venom toxins giving rise to isoforms displaying differences in affinity to specific substrates [29].
Considering that a single snake venom may contain more than one disintegrin [15, 21, 22, 27] and that C. l. morulus venom may vary intra-specifically in composition [3], it is very possible the existence of other disintegrins, besides morulustatin, in the venom of the Tamaulipan rock rattlesnake.
Conclusions and Recommendations
This study is the first report of the isolation, molecular mass estimation, and the partial amino acid sequence of a medium size disintegrin, morulustatin, found in the venom of the Tamaulipan rock rattlesnake. Morulustatin was able to inhibit ADP-induced platelet aggregation with an IC50 value of 89.5 nM ± 12. Disintegrins have attracted many researchers for their ability to inhibit platelet aggregation and cell-matrix interaction rendering them useful not only as antithrombotic drugs but also as anticancer drugs.
Acknowledgments
Funding for the project was provided by NIH-ORIP/BMRG, Viper Resource Grant #s 8P40OD01960-10 and 3P40OD01096-10S1 (NNTRC, Texas A&M University-Kingsville); the Department of Chemistry at Texas A&M University-Kingsville, Kingsville, Texas, USA and CDCH/Universidad Central de Venezuela PG: 09-8760-2013/1. We want to thank the NNTRC personnel for their assistance. We would also like to thank TAMUK campus housing for providing accommodations for visiting scientists.
Bibliographic References
- 1.Arruda-Macedo JK, Fox JW, DE Souza-Castro M. Disintegrins from Snake Venoms and their Applications in Cancer Research and Therapy. Curr Protein Pept Sci. 2015;16:532–548. doi: 10.2174/1389203716666150515125002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaman KR, Hayes W. Rattlesnakes: Research trends and annotated checklist. In: Hayes WK, Beaman KR, Cardwell MD, Bush SP, editors. The Biology of Rattlesnakes. Loma Linda University Press; Loma Linda, California: 2008. pp. 537–550. [Google Scholar]
- 3.Borja M, Lazcano D, Martínez-Romero G, Morlett J, Sánchez E, Cepeda-Nieto AC, Garza-Garcia Y, Zugasti-Cruz A. Intra-specific Variation in the Protein Composition and Proteolytic Activity of Venom of Crotalus lepidus morulus from the Northeast of Mexico. Copeia. 2013;4:707–716. [Google Scholar]
- 4.Braud S, Bon C, Wisner A, Calvete JJ, Marcinkiewicz C, Monleon D, Esteve V, Celda B, Juárez P, Sanz L. Snake venom proteins acting on hemostasis. Biochimie. 2000;82:851–859. doi: 10.1016/s0300-9084(00)01178-0. [DOI] [PubMed] [Google Scholar]
- 5.Calvete JJ, Marcinkiewicz C, Monleon D, Esteve V, Celda B, Juárez P, Sanz L. Snake venom disintegrins: Evolution of structure and function. Toxicon. 2005;45:1063–1074. doi: 10.1016/j.toxicon.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 6.Calvete JJ, Juárez P, Sanz L. Snake venomics. Strategy and applications. J Mass Spectrom. 2007;42:1405–1414. doi: 10.1002/jms.1242. [DOI] [PubMed] [Google Scholar]
- 7.Campbell JA, Lamar WW. The Venomous Reptiles of Latin America. Cornell University Press; Ithaca: 1989. Rattlesnakes: Genus Crotalus Linnaeus 1758; pp. 350–351. [Google Scholar]
- 8.DA Silva M, Lucena M, Aguilar I, Rodríguez-Acosta A, Salazar AM, Sánchez EE, Girón ME, Carvajal Z, Arocha-Piñango CL, Guerrero B. Anti-platelet effect of cumanastatin-1, a disintegrin isolated from venom of South American Crotalus rattlesnake. Thromb Res. 2009;123:731–739. doi: 10.1016/j.thromres.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Forstner MRJ, Hilsenbeck RA, Scuddaty JF. Geographic variation in whole venom profiles from the Mottled Rock Ratllesnake (Crotalus lepidus lepidus) in Texas. J Herpetol. 1997;31:277–287. [Google Scholar]
- 10.Fox JW, Serrano SM. Approaching the golden age of natural product pharmaceuticals from venom libraries: an overview of toxins and toxin-derivatives currently involved in therapeutic or diagnostic applications. Curr Pharm Des. 2007;3:2927–2934. doi: 10.2174/138161207782023739. [DOI] [PubMed] [Google Scholar]
- 11.Galán JA, Sánchez EE, Rodríguez-Acosta A, Soto JG, Bashir S, Mclane MA, Paquette-Straub C, Pérez JC. Inhibition of lung tumor colonization and cell migration with the disintegrincrotatroxin 2 isolated from the venom of Crotalus atrox. Toxicon. 2008;51:1186–1196. doi: 10.1016/j.toxicon.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glenn JL, Straight RC. Venom properties of the rattlesnakes (Crotalus) inhabiting the Baja California region of Mexico. Toxicon. 1985;23:769–775. doi: 10.1016/0041-0101(85)90007-8. [DOI] [PubMed] [Google Scholar]
- 13.Kohn LK, Pavam CH, Veronese D, Coelho F, DE Carvalho JE, Almeida WP. Antiproliferative effect of Baylis-Hillman adducts and a new phthalide derivative on human tumor cell lines. Eur J Med Chem. 2006;41:738–744. doi: 10.1016/j.ejmech.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 15.Lucena SE, Romo K, Suntravat M, Sánchez EE. Anti-angiogenic activities of two recombinant disintegrins derived from the Mohave and Prairie rattlesnakes. Toxicon. 2014;78:10–17. doi: 10.1016/j.toxicon.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mclane MA, Sanchez EE, Wong A, Paquette-Straub C, Perez JC. Disintegrins. Curr. Drug. Targets. Cardiovasc Haematol Disord. 2004;4:327–355. doi: 10.2174/1568006043335880. [DOI] [PubMed] [Google Scholar]
- 17.Martínez-Romero G, Rucavado A, Lazcano D, Gutiérrez JM, Borja M, Lomonte B, Garza-García Y, Zugasti-Cruz A. Comparison of venom composition and biological activities of the subspecies Crotalus lepidus lepidus, Crotalus lepidus klauberiand Crotalus lepidus morulus from Mexico. Toxicon. 2013;71:84–95. doi: 10.1016/j.toxicon.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Marsh N, Williams V. Practical applications of snake venom toxins in haemostasis. Toxicon. 2005;45:1171–1181. doi: 10.1016/j.toxicon.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 19.Nanjaraj Urs AN, Yariswamy M, Ramakrishnan C, Jos V, Suvilesh KN, Savitha MN, Velmurugan D, Vishwanath BS. Inhibitory potential of three zinc chelating agents against the proteolytic, hemorrhagic, and myotoxic activities of Echiscarinatus venom. Toxicon. 2015;93:68–78. doi: 10.1016/j.toxicon.2014.11.224. [DOI] [PubMed] [Google Scholar]
- 20.Price JA., 3rd Microplate fluorescence protease assays test the inhibition of select North American snake venoms' activities with an anti-proteinase library. Toxicon. 2015;103:145–154. doi: 10.1016/j.toxicon.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Sánchez EE, Galán JA, Powell RL, Reyes SR, Soto JG, Russell WK, Russell DH, Pérez JC. Disintegrin, hemorrhagic, and proteolytic activities of Mohave rattlesnake, Crotalus scutulatus scutulatus venoms lacking Mojave toxin. Comp Biochem Physiol C Toxicol Pharmacol. 2005;141:124–132. doi: 10.1016/j.cca.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Sánchez EE, Galán JA, Russell WK, Soto JG, Russell DH, Pérez JC. Isolation and characterization of two disintegrins inhibiting ADP-induced human platelet aggregation from the venom of Crotalus scutulatus scutulatus (Mohave Rattlesnake) Toxicol Appl Pharmacol. 2006;212:59–68. doi: 10.1016/j.taap.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Sánchez EE, Lucena SE, Reyes S, Soto JG, Cantu E, Lopez-Johnston JC, Guerrero B, Salazar AM, Rodríguez-Acosta A, Galán JA, Tao WA, Pérez JC. Cloning, expression, and hemostatic activities of a disintegrin, r-mojastin 1, from the mohave rattlesnake (Crotalus scutulatus scutulatus) Thromb Res. 2010;126:211–219. doi: 10.1016/j.thromres.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scarborough RM, Rose JW, Naughton MA, Phillips DR, Nannizzi L, Arfsten A, Campbell AM, Charo IF. Characterization of the integrin specificities of disintegrins isolated from American pit viper venoms. J Biol Chem. 1993;268:1058–1065. [PubMed] [Google Scholar]
- 25.Serrano SM, Shannon JD, Wang D, Camargo AC, Fox JW. A multifaceted analysis of viperid snake venoms by two-dimensional gel electrophoresis: an approach to understanding venom proteomics. Proteomics. 2005;5:501–510. doi: 10.1002/pmic.200400931. [DOI] [PubMed] [Google Scholar]
- 26.Vyas VK, Brahmbhatt K, Bhatt H, Parmar U. Therapeutic potential of snake venom in cancer therapy: current perspectives. Asian Pacific J Trop Biomed. 2013;3:156–162. doi: 10.1016/S2221-1691(13)60042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wermelinger LS, Geraldo RB, Frattani FS, Rodrigues CR, Juliano MA, Castro HC, Zingali RB. Integrin inhibitors from snake venom: Exploring the relationship between the structure and activity of RGD-peptides. Arch Biochem Biophys. 2009;482:25–32. doi: 10.1016/j.abb.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 28.Yang RS, Tang CH, Chuang WJ, Huang TH, Peng HC, Huang TF, Fu WM. Inhibition of tumor formation by snake venom disintegrin. Toxicon. 2005;45:661–669. doi: 10.1016/j.toxicon.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Reza MA, Minh Le TN, Swarup S, Manjunatha K. Molecular evolution caught in action: gene duplication and evolution of molecular isoforms of prothrombin activators in Pseduonajat extilis (brown snake) J Thromb Haemost. 2006;4:1346–1353. doi: 10.1111/j.1538-7836.2006.01969.x. [DOI] [PubMed] [Google Scholar]
- 30.Trikha M, De Clerck YA, Markland FS. Contortrostatin, a snake venom disintegrin, inhibits beta 1 integrin-mediated human metastatic melanoma cell adhesion and blocks experimental metastasis. Cancer Res. 1994:15–4993. [PubMed] [Google Scholar]


