Abstract
Sizing and shaping of mesoscale architectures with nanoscale features is a key opportunity to produce the next generation of higher-performing products and at the same time unveil completely new phenomena. This review article discusses recent advances in the design of novel photonic and plasmonic structures using a biology-inspired design. The proteinaceous capsids from viruses have long been discovered as platform technologies enabling unique applications in nanotechnology, materials, bioengineering, and medicine. In the context of materials applications, the highly organized structures formed by viral capsid proteins provide a 3D scaffold for the precise placement of plasmon and gain materials. Based on their highly symmetrical structures, virus-based nanoparticles have a high propensity to self-assemble into higher-order crystalline structures, yielding hierarchical hybrid materials. Recent advances in the field have led to the development of virus-based light harvesting systems, plasmonic structures for application in high-performance metamaterials, binary nanoparticle lattices, and liquid crystalline arrays for sensing or display technologies. There is still much that could be explored in this area, and we foresee that this is only the beginning of great technological advances in virus-based materials for plasmonics and photonics applications.
Keywords: Self-assembly, Viral capsids, Plasmonics, Photonics, Nanotechnology
1 Nanomanufacturing of mesoscale devices with nanoscale features
Metamaterials have characteristics usually not found in nature (Engheta et al. 2006; Sihvola 2007). Their unique properties are defined by their organized structure, rather than by the materials properties of their individual components. Metamaterials have received focused attention in recent years, and the global market for such materials is projected to top $1.9 billion USD by 2021 (source: SPIE). There is a wide range of applications for metamaterials, extending from thermal (Maldovan 2013) to acoustic (Craster and Guenneau 2013) properties; our focus in this review lies in the electromagnetic properties of plasmonics and photonics. While photonics studies features underlying the transmission of photons, plasmonics looks at energy from the collective oscillation of free electrons (plasmons) (De Luca et al. 2011; Naik and Boltasseva 2011; Sreekanth et al. 2013, 2014, Strangi et al. 2011). Metamaterials are envisioned to find broad applicability in plasmonics and photonics, such as for super antennas, data and energy storage devices, photovoltaics and other solar technologies, light-emitting diodes (LEDs), etc. A technological hurdle to metamaterials is the availability of high-precision manufacturing technologies that facilitate mesoscale, robust assembly, while providing spatial control at the 1–100 nm level. Manufacturing and assembly technologies need to be devised that would facilitate this robust multi-scale assembly starting at the nanoscale and then smoothly proceeding to mesoscale architectures.
Top-down approaches have progressed to provide tight control of feature dimensions and impeccable reproducibility. These techniques are derived from technology implemented by the computer industry and rely on lithographic fabrication to program feature components. Photolithography (Pimpin and Srituravanich 2012), microcontact printing (or soft lithography) (Qin et al. 2010; Whitesides et al. 2001), block copolymer nanolithography (Bang et al. 2009; Lohmuller et al. 2011), nanoimprint lithography (Malloy and Litt 2011), as well as scanning-probe or dip-pen lithography (Ginger et al. 2004; Salaita et al. 2007) facilitate writing at the centimeter size scale with nanoscale features and highly controlled reproducibility. While impressive in their implementation, the technology is highly specialized and relies on complex lithographic methods. Furthermore, there are limits to the smallest dimension accessible using this technology, with examples rarely falling below 20 nm features. Thus, the barrier to entry for competing or complementary technologies is extremely high to the materials scientist and engineer.
Bottom-up approaches are in a way mimicking what is already achieved in nature: biological systems and cells are self-assembled bottom-up and, as systems, orchestrate complex functionalities. Such bottom-up approaches seek to achieve directed and controlled assembly of nanoparticle-based systems into large-scale hierarchical architectures and hybrid materials. The art and science of self-assembly has made tremendous contributions in novel materials. Technologies such as directed polymer assembly, DNA origami, and colloidal systems provide opportunities to organize composite materials in 3D space with high manufacturing precision. For example, DNA-based ‘programming’ exploits the sequence specificity of base pairing to position materials in 2D and 3D space (Doerr 2011; Endo and Sugiyama 2011; Han et al. 2011; Lin et al. 2006; Liu et al. 2011; Majumder et al. 2011; Park et al. 2006; Rajendran et al. 2012; Sacca and Niemeyer 2012; Shih and Lin 2010; Yun et al. 2012). Examples of the intricate structures that can be formed with DNA origami can be found in Fig. 1. Synthetic approaches have also been devised and include the chemical programming of hierarchical structures, such as seen in the synthesis of branched dendrimer systems (Bergamini et al. 2011; Furuta et al. 2003; Miura et al. 2011; Mynar et al. 2006; Nantalaksakul et al. 2006; Serin et al. 2002). Other developments include the use of synthetic block copolymers (Zhao et al. 2009) or application of orthogonal pairs of coiled-coil peptides (Stevens et al. 2004; Wagner et al. 2010) to induce self-assembly of nanoparticles. It is clear that self-assembly holds great potential for the manufacturing of mesoscale materials through simple solution-based bottom-up synthesis.
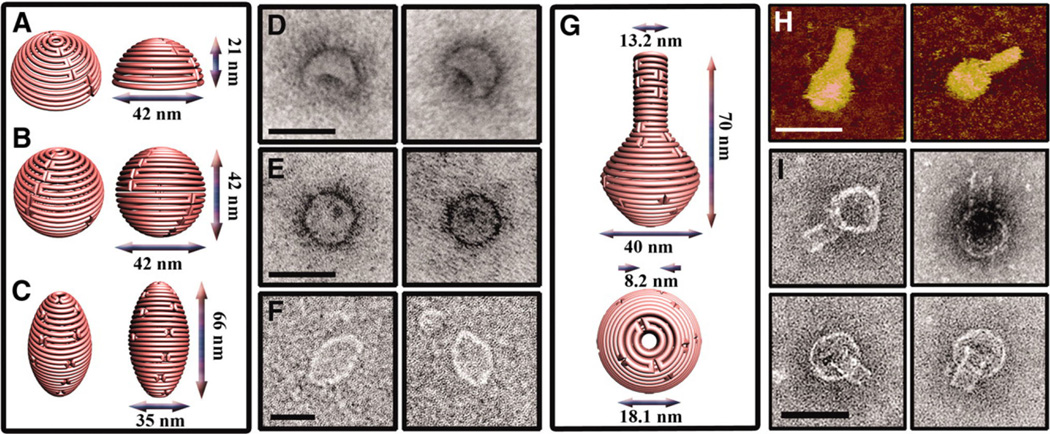
Fig. 1.
3D DNA nanostructures formed using a bottom-up approach. a–c Schematic representations of hemisphere, sphere, and ellipsoid structures, respectively. d–f TEM images of the hemisphere, sphere, and ellipsoid structures, respectively. Scale bar is 50 nm. g Schematic representation of a flask structure. h AFM images of the nanoflask. Scale bar is 75 nm. i TEM images of the nanoflask with clearly recognizable neck and rounded bottom features. Scale bar is 50 nm. Reproduced with permission from Han et al. (2011)
Virus-based nanomaterials are an example of biological building blocks that naturally master assembly across multiple length scales; coat protein units self-assemble into organized 3D nanoscale architectures. Based on the high degree of symmetry of viral capsids, such structures also show a high propensity to self-assemble into higher-order multi-particle materials, all the way to the mesoscale. This review article sets out to provide a summary of the recent advances in virus-based nanomanufacturing, while providing a perspective on future development and the potential of virus-based materials in optical devices in the fields of photonics and plasmonics.
1.1 Viruses in materials science
Since their discovery about 100 years ago, viruses have been studied by clinicians, biologists, physicists, chemists, and engineers. In the simplest form, a virus consists of a protein capsid and nucleic acid. They are ubiquitous in nature, i.e., they infect all forms of life and inhabit all environments. Virus capsids self-assemble into defined, monodisperse, and highly symmetrical 3D structures with atomic precision—a level of control not yet achieved with purely synthetic approaches. While viruses come in many shapes and sizes, including head–tail assemblies, droplet shapes, and even bottle-shaped structures, the icosahedral and helical symmetries are the most common in the lot (Wen et al. 2013).
It should be noted that viruses are highly dynamic structures that can self-assemble into varying geometries and sizes. For example, the 30 nm-sized nanoparticles formed by the plant virus brome mosaic virus (BMV) can be disassembled into coat proteins and re-assembled around artificial cargos. Using this strategy, a set of different-sized gold core capsid shell hybrid materials has been synthesized (Sun et al. 2007), where the size of the hybrid is governed not only by the size of the gold core but depends in a complex way on the electrostatic interaction between the capsid proteins and the gold cores (Šiber et al. 2010). Not only can different-sized particles be generated, but also geometries can be switched. Tubular structures, for example, can be obtained through self-assembly of cowpea chlorotic mottle virus (CCMV) coat proteins in the presence of DNA templates (Mukherjee et al. 2006), whereas in plants, in the presence of its RNA genome, CCMV forms icosahedrons. In fact a range of dominant CCMV coat protein polymorphs such as single-wall capsids, multiwall capsids, tubes, and free coat protein can be observed with their stability determined by electrostatic interactions (Vega-Acosta et al. 2014). Reconstitution experiments of tobacco mosaic virus (TMV) date back almost 60 years, yielding either disk-shaped or rod-shaped structures (Fraenkel-Conrat and Williams 1955). More recently, it was shown that TMV can also be shaped into spheres through thermal transition (Atabekov et al. 2011). It is fascinating that a single protein that in plants self-assembles into discrete, uniform, and monodisperse particles, can also assemble into a range of different structures in the test tube, a property attesting to the complicated nature of long-range interactions between the proteins (Leckband and Israelachvili 2001). These properties make virus-based materials versatile platforms for applications in nanotechnology and materials science.
Compared to their synthetic counterparts, such as liposomal, polymeric, and metallic nanoparticles, virus-based nanomaterials offer several advantages for materials design: The production of viral nanoparticles (VNPs) and their genome-free counterparts, virus-like particles (VLPs), is highly scalable through bacterial fermentation or farming in plants. Materials synthesis using VNP- or VLP-based scaffolds is typically performed under benign and ambient conditions, avoiding the use of toxic solvents or energy-consuming heating processes. Since their structures are genetically programmed, the material offers the highest possible degree of reproducibility, yielding essentially identical clones. Further, a variety of modification procedures have been established that enable the production of functionalized materials; these include the following methods: genetic engineering, bioconjugation targeting amino acids with spatial control and deterministic distribution (as opposed to synthetic materials, where labeling follows probabilistic distribution), mineralization, and encapsulation; for a review on these methodologies, we would like to refer the reader to Pokorski and Steinmetz (2011).
Chimeric virus technology is a particularly powerful strategy for structure-based engineering (and also unique to virus-based or, more generally, protein-based materials). Synthetic biology enables the addition, deletion, or exchange of amino acids to impart new functionalities. If a desired conjugation site is not available, it could be introduced with spatial precision on the inside or outside of the virus capsid through genetic insertion (Chatterji et al. 2004; Geiger et al. 2013; Wang et al. 2002). To induce self-assembling properties, affinity tags could be introduced (Bruckman et al. 2011; Chatterji et al. 2005). At the same time, electrostatics could be tuned to change self-interactions (Šiber et al. 2010), interactions with surfaces or other colloidal materials, or to enable spatially constrained materials deposition inside the viral cavity (Loo et al. 2006). Genetic engineering is so powerful indeed, that even entire proteins could be displayed on the virus capsid scaffold; for example, we demonstrated the expression of fluorescent proteins as coat protein fusions yielding optically active nanomaterials (Shukla et al. 2014).
Over the past two decades, many chemical biology approaches have been developed that allow us to tailor virus properties and enable the production of functional entities carrying cargos relevant for optoelectronics, photonics, plasmonics, light harvesting systems, etc. (discussed below). As the field of chemical virology continues to grow, efforts must be paid toward the nanomanufacturing of higher-order, hierarchical structures that would enable the fabrication of devices for application in real life. In the following sections, we will discuss crystallization and self-assembly procedures that have made headways toward these long-term goals; we will also highlight some recent work that demonstrates the opportunities of virus-based materials in photonic and plasmonic applications.
2 Hierarchical virus-based assemblies—toward metamaterials
2.1 Structure-based engineering of core–shell VLPs and their assemblies
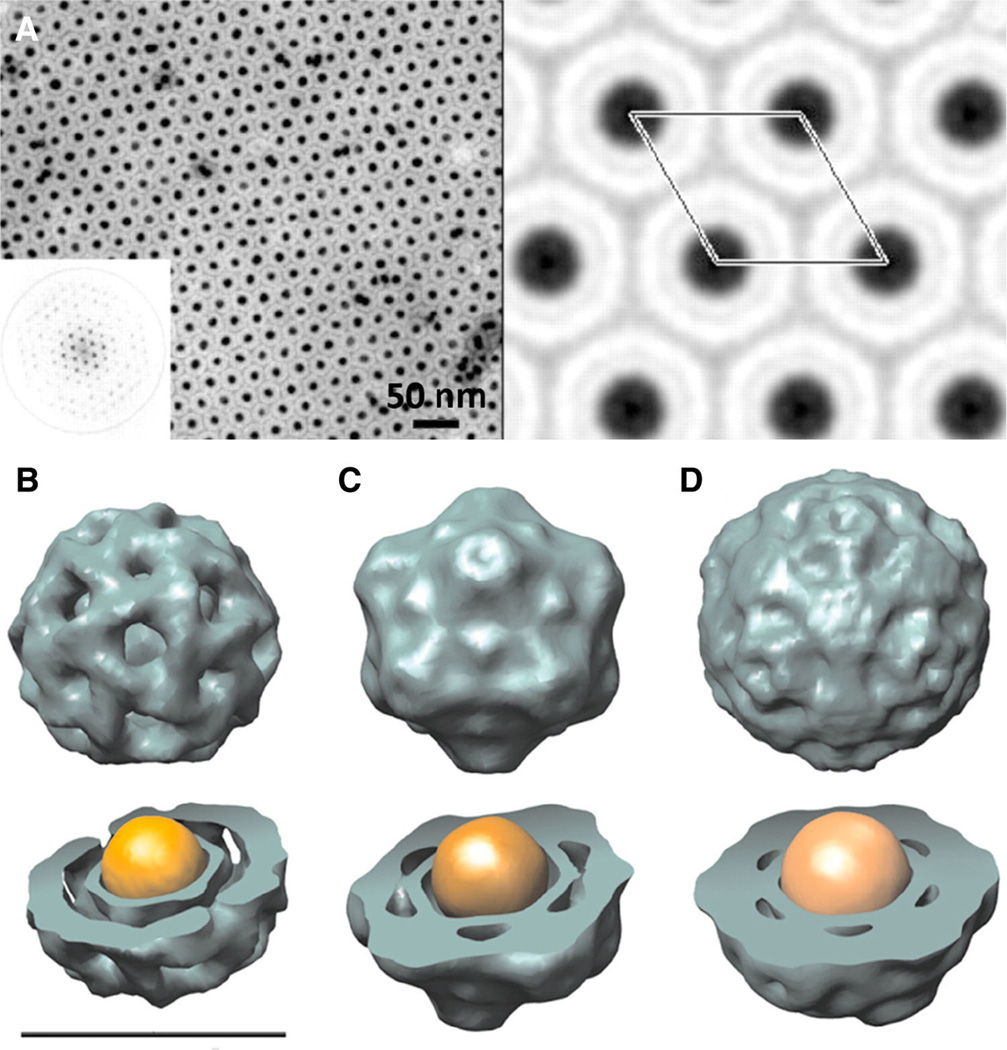
Viruses self-assemble into discrete structures, and based on their monodispersed and symmetrical structures, they also have a high propensity to self-assemble into higher-order hierarchical structures, such as crystalline 2D or 3D lattices (Natarajan and Johnson 1998). These properties also apply to cargo-loaded core–shell systems, and therefore enable the precise positioning of cargos (e.g., metals) in 2D or 3D lattices with long-range ordered precision. This concept was first demonstrated by Dragnea and colleagues, who pioneered methods enabling the synthesis of crystalline gold core–VLP shell arrays using brome mosaic virus (BMV) as a scaffold (Sun et al. 2007). Hybrid gold core-loaded VLPs were assembled resembling T = 1, pseudo T = 2, and T = 3 symmetry as a function of gold nanoparticle size (6, 9, and 12 nm, respectively) (Fig. 2). 3D crystals were grown and optical spectroscopy revealed double spectral features for the VLP-assisted gold nanoparticle array indicating the signature of multipolar coupling between adjacent gold cores leading to plasmonic band formation. This is one of few examples where plasmonic band splittings in metallodielectric materials have been observed experimentally. This pioneering work lays the foundation for further development of VLP crystals for potential application as high-performance metamaterials.
Fig. 2.
Assembly of hybrid gold core-loaded BMV. a Negatively stained TEM image of 12 nm gold-loaded BMV with Fourier transform (insert) and corresponding Fourier projection map with one unit cell highlighted. b–d 3D reconstructions of structures of BMV-gold hybrids based on TEM data as a function of gold nanoparticles size (6, 9, and 12 nm, respectively). The assemblies resemble T = 1, pseudo T = 2, and T = 3 symmetries, respectively. Scale bar is 21 nm. Reproduced with permission from Sun et al. (2007)
With regard to utilizing the interior cavity of virus-based materials for placement of cargo, several methods have been established; we differentiate two fundamental techniques. First, cargo can be encapsulated during VLP self-assembly. Here, purified coat protein monomers are mixed under self-assembly conditions (bathing condition that favor coat protein assembly) with the cargo of interest; encapsulation can be achieved through stoichiometric mixing and statistical distribution (Comellas-Aragones et al. 2007), programmed through electrostatic interactions (Sun et al. 2007), or via bio-specific interactions. For the latter, Lommel, Franzen, and colleagues conjugated artificial origin of assembly sites (OAS) to gold nanoparticles, triggering assembly of hybrid core-shell structures using coat proteins from the red clover necrotic mottle virus (RCNMV) (Loo et al. 2006, 2007). Similar strategies have also been developed by Stockley and colleagues, whose research utilizes the phage MS2; here the translational repression operator RNA stem loop sequence is fused to medically relevant cargos for encapsulation and drug delivery applications (Brown et al. 2002; Wu et al. 1995).
The second method explores the constrained synthesis of cargo inside the intact VLP. The first efforts to utilize viral cages for materials synthesis were carried out by Douglas and Young (1998). The general principle is as follows: precursor ions of the materials of interest (e.g., and ) are added to the viral capsid under condition that favor expansion and pore opening (high pH and low salt). The negatively charged precursors freely diffuse between the interior and exterior and associate with the positively charged interior capsid surfaces. Lowering of the pH leads to the formation of polyoxometalate species such as and crystallization (e.g., producing tungstate and vanadate crystals), and structural changes in the capsid structure (non-swollen, closed form) trap the inorganic material. Since viral capsids are highly monodisperse, the resulting inorganic materials have narrow size distribution. These methods have been extended and applied to a variety of virus-based scaffolds and manifold inorganic materials have been produced using icosahedrons as well as high aspect ratio filaments (such as plant virus TMV or M13 phage); it should also be noted that both the interior as well as the exterior surfaces can serve as nucleation sites; these methods are reviewed in Pokorski and Steinmetz (2011).
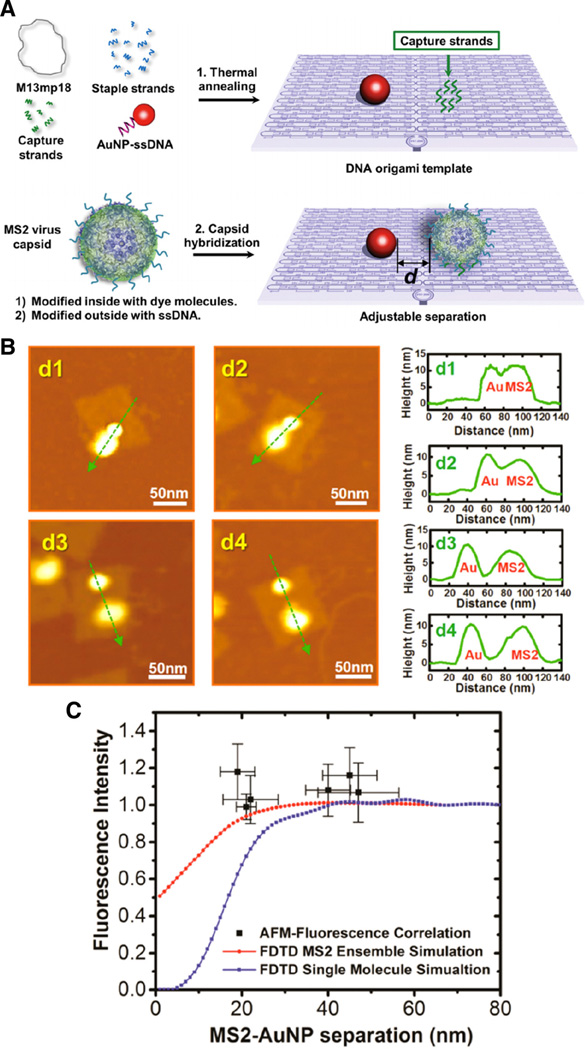
Toward applications as plasmonic devices, Francis and coworkers reported the development of MS2 phage-based core–shell hierarchical structures, in which gold nanoparticles were encapsulated inside MS2 and fluorophores were placed on the exterior surfaces. The distance of the fluorophore away from the capsid with the encapsulated gold nanoparticle was controlled through the use of oligonucleotide hairpin structures. The photonic properties were studied, and fluorescence intensity enhancement and corresponding increase in fluorescence lifetime were observed as a function of spatial placement of the plasmon and gain material (Capehart et al. 2013). Taking this approach a step further, De Yoreo, Francis, and colleagues reversed the situation and placed the fluorophores inside MS2 then positioned the fluorescent MS2 phages in proximity to gold nanoparticles antennas; this architecture was built using DNA origami. The resulting hierarchical plasmonic nanostructures enabled fluorescence enhancement through nanoparticle size and spatial placement controlling the plasmon and gain material interdistance (Fig. 3) (Wang et al. 2014). Overall, these two papers present nice examples of the protein engineering and DNA programming interface: the capsid served as the structural building block, while DNA hybridization technology was used for sequence-programmed fluorophore placement. The bioin-spired plasmonic nanostructures provide tunable design for manipulating photonic excitation and photoemission.
Fig. 3.
Assembly of hierarchical plasmonic nanostructures. a Schematic for attachment of fluorescent MS2 phages at varying distances to 10 nm gold nanoparticles using DNA origami templates. b AFM three-dimensional topography analysis and height profiles of MS2-AuNP nanostructures with varying interparticle distances. c Experimental data of fluorescence intensity from AFM-fluorescence correlation (black squares) compared to finite-difference time-domain (FDTD) numerical simulations for single-molecule and ensemble (entire collection of dye molecules within capsid) fluorescence. Results show enhanced fluorescence intensity with weak dependence on distance due to the use of the MS2 capsid as a three-dimensional molecular scaffold that maintains the fluorescent molecules outside the quenching zone. Reproduced with permission from Wang et al. (2014)
Another approach to gain control over placement of metals is to use the 3D structure of the viral capsid itself. For example, alignment of cubic gold nanoparticles along the 1 micron-long phage M13 resulted in a 1D nanoprobe for application in surface-enhanced Raman scattering (SERS). The gold-loaded M13-based SERS probes were equipped with antibodies targeting disease markers and sensing was achieved detecting the analyte at pg/mL concentrations (Lee et al. 2014). Another example demonstrating viral templating of plasmonic structures was described by Ratna and coworkers (Fontana et al. 2014): genetic engineering was used to place cysteine residues strategically into surface loops using the cowpea mosaic virus (CPMV)-based scaffold. The exposed thiol groups enabled precise placement of plasmonic gold nanoparticles with defined interparticle spacing and symmetry. The resulting idealized assembly consisted of gold nanoparticles positioned at the 125-fold axes of the pseudo T = 3 CPMV icosahedron. Simulations indicate that such idealized assemblies would give rise to a tenfold surface-averaged enhancement of the local electromagnetic field. Experimentally, assemblies with 6–12 nanoparticles per CPMV were obtained, which could be separated and purified with reasonable yields (Fontana et al. 2014). This indeed is a beautiful example of structure-based engineering of deterministic nanoparticle arrays. We envision that higher-order hierarchical assembly of such hybrid structures into devices will pave the way toward new phenomena in plasmonics.
2.2 Co-crystallization of binary virus and nanoparticle lattices—toward AB-type heterofunctional materials
Crystallization procedures have long been developed for many virus-based scaffolds and are used both in x-ray crystallography to determine their atomic structures as well as in materials design to create homogenous functional devices in 3D space. Crystallization is achieved through adjustment of the bathing conditions (salt and pH) to reduce or screen surface charges and resulting electrostatic repulsive forces and/or via the addition of precipitants such as PEG (Casselyn et al. 2001). Alternative methods include the use of charged polymers, dendrimers, or dendrons to induce co-crystallization through electrostatic coupling between the additive (polymer or dendrimer/dendron structure) and the proteinacous nanoparticles with their patchy electrostatic pattern (Kostiainen et al. 2011; Mikkilä et al. 2013).
Indeed viruses also represent an interesting case of patchy colloids (Bianchi et al. 2011) with strongly anisotropic interactions acting as multivalent junctions (Glotzer and Solomon 2007) that are purported to become the elementary bricks of self-assembled materials (Romano and Sciortino 2011). It is interesting that the quintessential patchiness of viruses, associated not only with the patchiness of the capsid protein subunits themselves but maybe even more importantly stemming from the charge distribution that conforms to the full capsid symmetry as quantified by their T number (Losdorfer Bozic and Podgornik 2013), could make them the material of choice in the rational design of novel binary materials, a venue apparently still not sufficiently explored. It is in fact the genetic programmability of the virus capsid epitope that could make them the building blocks of preference for constructing complicated self-assembled arrays with a controllable degree and type of order.
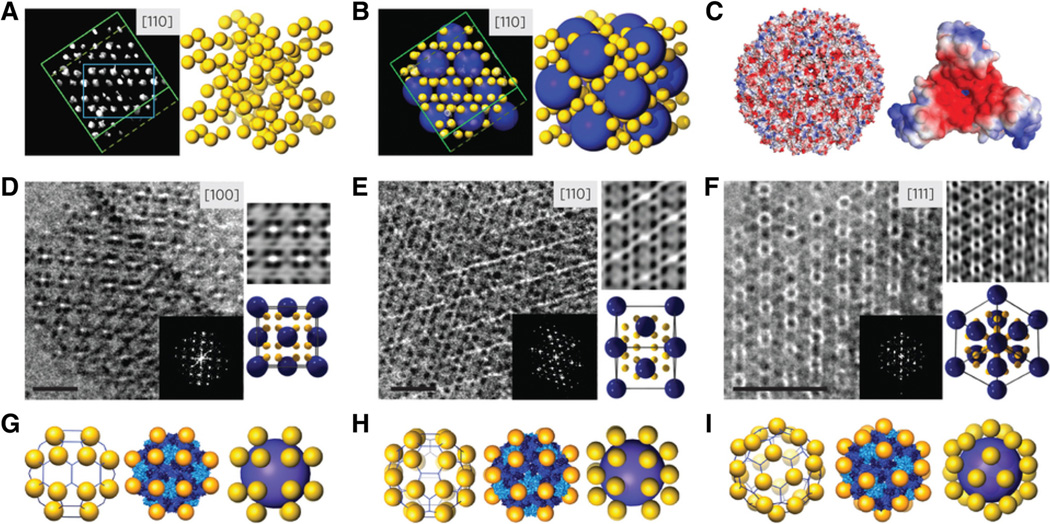
Recently, Kostiainen and colleagues took a first step into this direction and demonstrated co-crystallization experiments yielding binary nanoparticle superlattices of the AB-type (Kostiainen et al. 2013). Periodic nanostructures with lattice constants were produced using either 28 nm-sized CCMV nanoparticles or 12 nm-sized ferritin cages. CCMV and ferritin, both of which display patchy surface charges with an overall net negative charge, were mixed A and co-crystallized with positively charged gold nanopar-ticles. The patchy, but symmetrical, charge distribution led to electrostatically driven self-assembly of crystalline CCMV–gold nanoparticle binary structures yielding AB-type cubic structures, where the unit cell consists of four CCMV and 32 gold nanoparticles (Fig. 4). Further, iron oxide-loaded ferritin cages were assembled into iron oxide core ferritin shell–gold nanoparticle superlattices; freestanding structures were obtained by magnetic separation and drying and magnetic properties were demonstrated in magnetic resonance imaging applications. The development of co-crystallization yielding AB-type materials is an exciting new development and we expect it will yield many interesting structures for plasmonic and photonic opportunities in the future.
Fig. 4.
AB-type CCMV-gold nanoparticle lattice formation. a 3D cryo-electron tomography density maps of the superlattice viewed along the [110] projection axis with a unit cell outlined in cyan. Position of gold nanoparticles determined using the electron density map is shown in yellow to the right. b Schematic of electron density maps is shown in (a) with CCMV highlighted in blue and gold nanoparticles in yellow. c Illustration of the electrostatic potential map [scale goes from 0 (blue) to −9kBT/e (red)] of the CCMV capsid with negative patches found around the 60 quasi-three-fold axes of CCMV. Magnified view of one of the negative patches is shown to the right. d–f Cryo-TEM images of the superlattices viewed along the [100], [110], and [111] projection axes, respectively, with Fourier transform (inset). Scale bar is 50 nm. Inverse Fourier transform is shown in the top right and a schematic of the unit cell is shown in the bottom right, with the size of the particles reduced for clarity. g–i Position of gold around CCMV as viewed from the same direction as the corresponding TEM images above. Schematic of location of gold on CCMV frame and on the surface representation are shown to the left. Experimental particle positions determined by cryo-TEM tomography are shown to the right. Reproduced with permission from Kostiainen et al. (2013)
2.3 Self-assembly of anisotropic, high aspect ratio filamentous viruses
While the previous sections focused on the design and utilization of mostly icosahedral platforms, we now turn toward elongated high aspect ratio materials, the most prominent example being TMV. Self-assembly of the hollow nanotube-shaped TMV capsid measuring 300 × 18 nm from reconstituted coat proteins has been extensively studied in various scientific settings; relevant to this discussion is its application in light harvesting systems A (Endo et al. 2007; Ma et al. 2008; Miller et al. 2007). The 3D scaffold of the viral nanotube allows for precise spatial positioning of donor and acceptor chromophores with genetic control at the atomic level. Its engineerability in combination with its self-assembly properties makes this approach unique for facilitating efficient chromophore coupling through Försterresonance energy transfer (FRET).
Because of the interest and opportunities of fluorophoreor chromophore-modified viral capsids, our laboratory recently conducted a systematic study to evaluate the optical properties of icosahedral CPMV and rod-shaped TMV carrying fluorescent cargos; specifically, the effects of dye density, spatial placement, and conjugation chemistry on fluorescence intensity and lifetimes were studied (Wen et al. 2015). We report that for maximized fluorescence output, the fluorophore should be attached to non-aromatic groups (e.g., lysines and not tyrosines) because de-localized electrons in the ring system reduce the fluorescence intensity, on the exterior surface (as opposed to interior surface, where crowing may occur), and with sparse labeling such that the spacing between the dyes is ~10 nm (to avoid FRET). This work lays a foundation for the application of fluorescent-labeled virus-based materials in biophotonics. We envision that the design rules will enable informed design and fabrication of hybrid structures that display dyes and metals in defined arrays for applications in plasmonics.
Besides its use for placement of chromophores, gold nanoclusters can also be precisely positioned on TMV disks or intact TMV rod assemblies (Bruckman et al. 2011). Furthermore, TMV rods can be assembled from the bottom-up from colloidal gold nanoparticle surfaces (Mueller et al. 2011) or deposited standing up on gold surfaces (Royston et al. 2008). The precise placement of cargos such as metals or fluorophores has been demonstrated in a number of settings and opens the door for application of virus-based materials for photonics and plasmonics. Future studies to combine gold (plasmon material) and fluorophore (gain material) on the same viral scaffold are expected to yield materials that may provide unique emergent properties. Nevertheless, to realize incorporation into devices, higher-order structures spanning the nano- and mesoscale must be developed. We already discussed crystallization experiments focusing on icosa-hedrons (see above); in the following section we will summarize advances in viral liquid crystal research.
2.4 Virus-based liquid crystals
Three high aspect ratio materials that have been extensively studied in liquid crystal research are the plant virus TMV and the filamentous phages M13 and fd (Bruckman et al. 2011; Dogic and Fraden 2006; Endo et al. 2007; Kostiainen et al. 2013; Ma et al. 2008; Mikkilä et al. 2013; Miller et al. 2007; Mueller et al. 2011; Royston et al. 2008; Shenton et al. 1999). These materials have a propensity to self-assemble end-to-end (or head-to-tail) and side-to-side when exposed to appropriate conditions to induce long-range interactions that govern their mesoscale assembly. For instance, at acidic pH, TMV rods align tail-to-head and form long wires via the hydrophobic interactions between the dipolar ends of the virus rods (Shenton et al. 1999), while the lateral interactions are dominated by electrostatic and van der Waals forces (French et al. 2010). Through adjustment of the bathing conditions, addition of stabilizing molecules such as aniline, TMV fibers, bundles, and sheets can be obtained (Niu et al. 2007). Ordered TMV gels were actually used already in the seminal work of Bernal and Fankuchen to study the relevance of long-range electrostatic interactions for their stability (Bernal and Fankuchen 1941). In fact the canonical Poisson–Boltzmann theory of electrostatic interactions (Siber et al. 2012) was applied to the case of viruses soon after its publication (Oster 1950)and even before it was applied to any other biologically relevant materials.
In agreement with the Onsager theory (Onsager 1949, 1969) of liquid crystalline ordering, TMV forms a nematic liquid crystalline phase at a critical concentration (Dogic and Fraden 2006). The repulsive osmotic pressure in the TMV liquid crystalline arrays has been measured directly and it was shown to compare favorably with the theory of electrostatic interactions (Millman and Nickel 1980); the stability of the TMV gels was deduced to conform to the standard equilibrium between attractive and repulsive long-range interactions acting on the nanoscale (Millman et al. 1984). Further, lamellar assemblies of high aspect ratio viruses can be obtained through assembly at liquid–liquid or liquid–solid interfaces (Adams and Fraden 1998; Dogic et al. 2000). This can be induced and controlled through functionalization of the viral scaffold with solvatochromic dyes (Johnson et al. 2007) to orient the rods at liquid–liquid interfaces (solvent–water mixtures). Alignment can also be triggered through external control, such as with electric or magnetic fields (Ermolina et al. 2006; Hirai et al. 2003; Lee et al. 2002). One can thus control the nematic direction of the ordered phase and enforce the whole sample to remain in a single domain, which would not occur if allowed to order spontaneously without the symmetry-breaking external field.
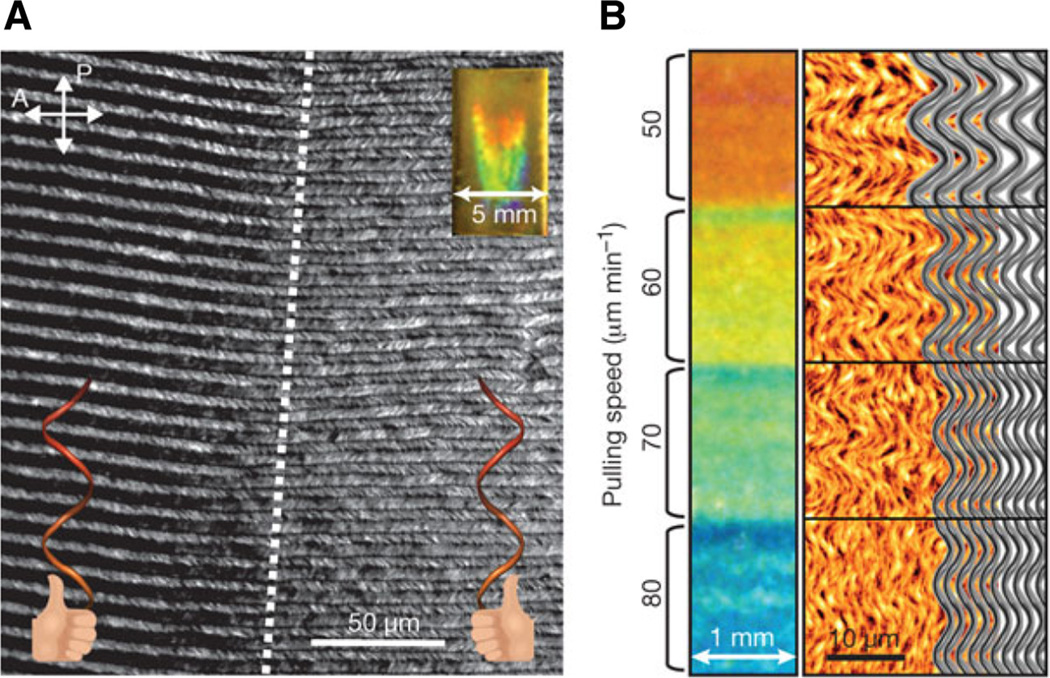
Pioneering work by Belcher and coworkers using M13 phage demonstrated that various liquid crystalline phases can be reached. For example, M13 phage-based liquid crystals present a nematic phase at low concentrations. As the concentration increases, a cholesteric liquid crystalline phase is observed. Finally, at high concentrations, the system exhibits a smectic liquid crystalline phase [reviewed in Yang et al. (2013)]. In addition to in-solution techniques, electrospinning, wetspinning, and dip coating (Brinker et al. 1991; Chung et al. 2011; Lee and Belcher 2004) have been applied to generate long-range ordered filamentous virus-based films. Recently, Lee and colleagues showed that chiral liquid crystalline M13 phage films could be obtained using dip-coating methods. By carefully adjusting phage concentration, pulling speed, bathing conditions, M13 surface chemistry, and surface chemistry of the solid support, supramolecular M13 films with nematic orthogonal twists, cholesteric helical ribbons, or smectic helicolidal nanofilaments were produced. The M13 arrays exhibited unique optical and photonic properties. When spot illuminated, the films showed iridescence (Fig. 5); these films could function as chiral reflectors, filters, and structural color matrices (Chung et al. 2011). Indeed the application of the M13 films as colorimetric sensors for sensing of the explosive trinitrotoluene (TNT) has already been demonstrated (Oh et al. 2014).
Fig. 5.
Optical properties of smectic helicolidal nanofilament structures formed by M13 phage. a Polarized optical microscopy image (cross-polar) of the grain boundary between opposite-handed ribbons (white dashed line) of the nanofilaments with iridescence on the film shown (inset). b Films formed at different pulling speeds show distinct colors due to narrower phage fibers at faster pulling speeds, as seen in the AFM images and schematic diagrams to the right. Reproduced with permission from Chung et al. (2011)
In general, filamentous viruses possess all the relevant mesophases exhibited by inorganic nematogens: isotropic, nematic, cholesteric, smectic A, smectic C, and crystalline phases (Dogic and Fraden 2006). A particularly important area of this research, relevant for self-assembly of hierarchical metamaterials, is the study of mixtures of viruses with polymers and spherical colloids (such as polystyrene spheres) that show complicated phase behavior associated with the presence of layered smectic/lamellar phases [for a detailed description see Dogic and Fraden (2006)]. In this case, the colloid component of the system can mediate depletion interactions between viruses equivalent to an additional short-range attractive component of the osmotic pressure (Asakura and Oosawa 1958; Lekkerkerker and Tuinier 2011). This might also open up new venues in the study of virus-based liquid crystals composed of a mixture of, for example, a filamentous virus and an icosahedral virus. Such heterofunctional systems exhibiting multivalent interactions between the building blocks, based on the interplay between very complicated geometry and shape-dependent long-range interactions, have yet to be investigated and might exhibit phases that would be difficult to foresee.
2.5 Perspectives and opportunities
From the review of the recent literature, it is clear that the field is gaining momentum. The establishment of chemical and synthetic virology protocols, enabling the modification of virus-based scaffolds with atomic precision and spatial control, has laid the foundation for technology development spanning applications in materials and medicine. Tunable and triggered self-organization of functional or hybrid virus assemblies into free-standing 3D crystal lattices and/or films provide the opportunity for implementation in functional devices. Viruses and their assemblies span multiple length scales: from individual amino acids, to coat proteins, to intact capsids, to hierarchical ordered assemblies.
While the technology development has grown out of its infancy, there is still “plenty of room at the bottom” to transform the next generation of photonics, plasmonics, and other devices. To date, most research has focused on the assembly of symmetrical virus-hybrid structures forming uniform 3D structures from single, identical building blocks. Recent research has made progress leading toward co-crystallized ABABAB… materials (Kostiainen et al. 2013); such periodic structure enables a higher degree of tunability and freedom to incorporate multiple functional entities into a single device. Nevertheless, in this example, the building component is the icosahedron, which due to its spheroidal shape exhibits properties of an essentially ‘scalar’ material, i.e., a material that can be described by specifying a single magnitude. We see in the richness of shapes and symmetries of viruses opportunities toward application and development of multivalent ‘vectorial’ materials and furthermore toward fabrication of ‘tensorial’ materials. The properties of vectorial material would be characterized by a magnitude and direction (such as in the case of the dipole moment), whereas tensorial materials would be characterized by multiple magnitudes and directions (such as in the case of the quadrupole moment). Viruses can by their very nature and by the inherent and highly modifiable patchiness of their epitopes serve as carriers of these “multipolar” and multivalent material properties and could present a natural generalization from two-headed Janus particles (Glotzer and Solomon 2007) to four-headed Brahma particles and then on toward the many-headed Hydra particles, if we follow the popular mythologic trend in complex colloid nomenclature.
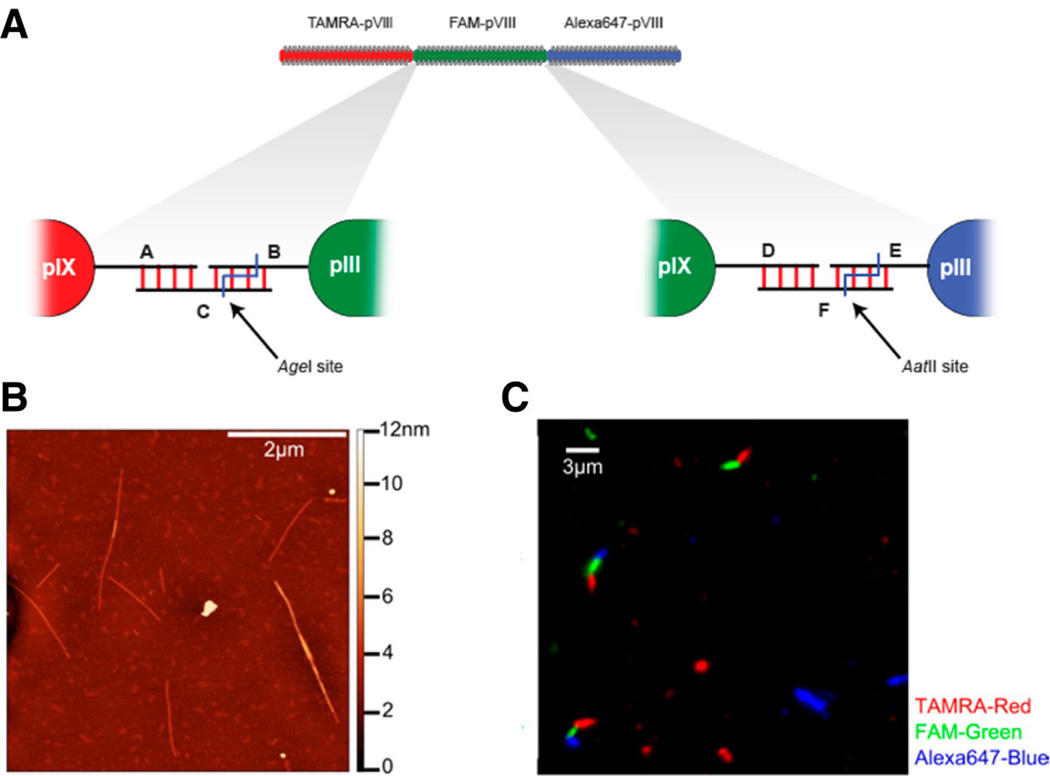
Based on their definable end structures and helical nature of the virus body, TMV and M13 are polar materials with vectorial properties, and many interesting crystalline arrays have been fabricated, most of which, however, are of the AAAAA… type. There is one notable exception, in which an ABC-type material was synthesized using M13 phage in combination with DNA hybridization technology leading to sequence controlled end-by-end assembly (Hess et al. 2013) (Fig. 6).
Fig. 6.
ABC-type organization of M13 phage. a Scheme of construction of phage trimers through DNA hybridization. b AFM image of linked phages. c Fluorescence microscopy image demonstrating ordering of the phage structures. Reproduced with permission from Hess et al. (2013)
We envision that the design and manufacturing of heterofunctional materials of the ABC-type will provide new opportunities for materials design and phenomena. ABC building blocks can be assembled with three different chemical subunits, denoted as ‘A,’ ‘B,’ and ‘C,’ attached linearly. When the ABC groupings are aligned in the same direction and repeated, i.e., “ABCABCABC….,” unlike ABABABAB… or AAAAAA… structures, it is not centrosymmetric but is consistent with vector and tensor symmetry. Such anisotropic ‘vectorial’ and ‘tensorial’ building blocks that feature properties described by magnitude(s) and direction(s) enable multivalent junctions between these building blocks, therefore opening the door for new phenomena that could not be achieved using ‘scalar’ raw materials. As a step toward this direction, we recently begun the development of Janus-type symmetry-broken nanoparticles, enabling assembly of hierarchical organizations (Wen and Steinmetz 2014). While our current study utilized symmetry-broken icosahedral building blocks formed by CPMV, the concepts could be extended to other viral platforms such as TMV. Symmetry-broken high aspect ratio nanoparticles with multipolar properties would introduce degrees of freedom that enable spatial control of connectivity and directionality as individual nanostructures are assembled into mesoscale aggregates. Like the interconnecting building blocks of a puzzle, guided assembly of symmetry-broken building blocks with different etched features will lead to ever larger structures with specified and controllable patterning.
To conclude, we envision that the development of advanced nanomanufacturing and nanopatterning techniques will enable the programming of high frequency metamaterials, capacitors, sensors, photonic materials, and batteries. It remains contagiously exciting at the virus–materials interface.
Acknowledgments
This work was supported in parts by a grant from the National Science Foundation (NSF CMMI 333651 to N. F. S) for support in the study of nanomanufacturing of virus-based materials, and a grant from the U.S. Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering under Award No. DE-SC0008176 and DE-SC0008068 (to N. F. S and R. P.) for support in the study of long-range interactions for biomolecular and inorganic nanoscale assembly. A. M. W. acknowledges support from the NIH T32 HL105338 Training Grant. This contribution is the written, peer-reviewed version of a paper presented at one of the two conferences “From Life to Life: Through New Materials and Plasmonics”—Accademia Nazionale dei Lincei in Rome on June 23, 2014, and “NanoPlasm 2014: New Frontiers in Plasmonics and NanoOptics”—Cetraro (CS) on June 16–20, 2014.
Footnotes
This contribution is the written, peer-reviewed version of a paper presented in one of the two conferences “From Life to Life: Through New Materials and Plasmonics”, Accademia Nazionale dei Lincei in Rome on June 23, 2014, and at “NanoPlasm 2014: New Frontiers in Plasmonics and NanoOptics”, Cetraro (CS) on June 16–20, 2014.
Contributor Information
Amy M. Wen, Department of Biomedical Engineering, Schools of Medicine and Engineering, Case Western Reserve University, Cleveland, OH 44106, USA
Rudolf Podgornik, Department of Physics, University of Massachusetts, Amherst, MA 01003, USA; Department of Theoretical Physics, Jožef Stefan Institute, University of Ljubljana, 1000 Ljubljana, Slovenia; Department of Physics, Faculty of Mathematics and Physics, University of Ljubljana, 1000 Ljubljana, Slovenia.
Giuseppe Strangi, Department of Physics, Case Western Reserve University, Cleveland, OH 44106, USA; Department of Physics and CNR–IPCF, University of Calabria, 87036 Rende, Italy.
Nicole F. Steinmetz, Department of Biomedical Engineering, Schools of Medicine and Engineering, Case Western Reserve University, Cleveland, OH 44106, USA Department of Radiology, School of Medicine, Case Western Reserve University, Cleveland, OH 44106, USA; Department of Materials Science and Engineering, School of Engineering, Case Western Reserve University, Cleveland, OH 44106, USA; Department of Macromolecular Science and Engineering, School of Engineering, Case Western Reserve University, Cleveland, OH 44106, USA.
References
- Adams M, Fraden S. Phase behavior of mixtures of rods (tobacco mosaic virus) and spheres (polyethylene oxide, bovine serum albumin) Biophys J. 1998;74:669–677. doi: 10.1016/S0006-3495(98)77826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura S, Oosawa F. Interaction between particles suspended in solutions of macromolecules. J Polym Sci. 1958;33:183–192. [Google Scholar]
- Atabekov J, Nikitin N, Arkhipenko M, Chirkov S, Karpova O. Thermal transition of native tobacco mosaic virus and RNA-free viral proteins into spherical nanoparticles. J Gen Virol. 2011;92:453–456. doi: 10.1099/vir.0.024356-0. [DOI] [PubMed] [Google Scholar]
- Bang J, Jeong U, du Ryu Y, Russell TP, Hawker CJ. Block copolymer nanolithography: translation of molecular level control to nanoscale patterns. Adv Mater. 2009;21:4769–4792. doi: 10.1002/adma.200803302. [DOI] [PubMed] [Google Scholar]
- Bergamini G, Ceroni P, Fabbrizzi P, Cicchi S. A multichro-mophoric dendrimer: from synthesis to energy up-conversion in a rigid matrix. Chem Commun (Camb) 2011;47:12780–12782. doi: 10.1039/c1cc16000a. [DOI] [PubMed] [Google Scholar]
- Bernal JD, Fankuchen I. X-ray and crystallographic studies of plant virus preparations : I. Introduction and preparation of specimens II. Modes of aggregation of the viruspParticles. J Gen Physiol. 1941;25:111–146. doi: 10.1085/jgp.25.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi E, Blaak R, Likos CN. Patchy colloids: state of the art and perspectives. Phys Chem Chem Phys. 2011;13:6397–6410. doi: 10.1039/c0cp02296a. [DOI] [PubMed] [Google Scholar]
- Brinker CJ, Frye GC, Hurd AJ, Ashley CS. Fundamentals of sol-gel dip coating. Thin Solid Films. 1991;201:97–108. [Google Scholar]
- Brown WL, Mastico RA, Wu M, Heal KG, Adams CJ, Murray JB, Simpson JC, Lord JM, Taylor-Robinson AW, Stockley PG. RNA bacteriophage capsid-mediated drug delivery and epitope presentation. Intervirology. 2002;45:371–380. doi: 10.1159/000067930. [DOI] [PubMed] [Google Scholar]
- Bruckman MA, Soto CM, McDowell H, Liu JL, Ratna BR, Korpany KV, Zahr OK, Blum AS. Role of hexahistidine in directed nanoassemblies of tobacco mosaic virus coat protein. ACS Nano. 2011;5:1606–1616. doi: 10.1021/nn1025719. [DOI] [PubMed] [Google Scholar]
- Capehart SL, Coyle MP, Glasgow JE, Francis MB. controlled integration of gold nanoparticles and organic fluorophores using synthetically modified MS2 viral capsids. J Am Chem Soc. 2013;135:3011–3016. doi: 10.1021/ja3078472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casselyn M, Perez J, Tardieu A, Vachette P, Witz J, Delacroix H. Spherical plant viruses: interactions in solution, phase diagrams and crystallization of brome mosaic virus. Acta Crystallogr D Biol Crystallogr. 2001;57:1799–1812. doi: 10.1107/s0907444901014949. [DOI] [PubMed] [Google Scholar]
- Chatterji A, Ochoa W, Paine M, Ratna BR, Johnson JE, Lin T. New addresses on an addressable virus nanoblock: uniquely reactive Lys residues on cowpea mosaic virus. Chem Biol. 2004;11:855–863. doi: 10.1016/j.chembiol.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Chatterji A, Ochoa WF, Ueno T, Lin T, Johnson JE. A virus-based nanoblock with tunable electrostatic properties. Nano Lett. 2005;5:597–602. doi: 10.1021/nl048007s. [DOI] [PubMed] [Google Scholar]
- Chung WJ, Oh JW, Kwak K, Lee BY, Meyer J, Wang E, Hexemer A, Lee SW. Biomimetic self-templating supramolecular structures. Nature. 2011;478:364–368. doi: 10.1038/nature10513. [DOI] [PubMed] [Google Scholar]
- Comellas-Aragones M, Engelkamp H, Claessen VI, Sommerdijk NA, Rowan AE, Christianen PC, Maan JC, Verduin BJ, Cornelissen JJ, Nolte RJ. A virus-based single-enzyme nanoreactor. Nat Nanotechnol. 2007;2:635–639. doi: 10.1038/nnano.2007.299. [DOI] [PubMed] [Google Scholar]
- Craster RV, Guenneau S, editors; Hull R, et al., editors. Acoustic metamaterials: negative refraction, imaging, lensing and cloaking. Springer series in materials science. Vol. 166. Dordrecht: Springer; 2013. [Google Scholar]
- De Luca A, Grzelczak MP, Pastoriza-Santos I, Liz-Marzan LM, La Deda M, Striccoli M, Strangi G. Dispersed and encapsulated gain medium in plasmonic nanoparticles: a multipronged approach to mitigate optical losses. ACS Nano. 2011;5:5823–5829. doi: 10.1021/nn2015562. [DOI] [PubMed] [Google Scholar]
- Doerr A. DNA origami in 3D. Nat Methods. 2011;8:453. doi: 10.1038/nmeth0611-454. [DOI] [PubMed] [Google Scholar]
- Dogic Z, Fraden S. Ordered phases of filamentous viruses. Curr Opin Colloid Interface Sci. 2006;11:47–55. [Google Scholar]
- Dogic Z, Frenkel D, Fraden S. Enhanced stability of layered phases in parallel hard spherocylinders due to addition of hard spheres. Phys Rev E. 2000;62:3925. doi: 10.1103/physreve.62.3925. [DOI] [PubMed] [Google Scholar]
- Douglas T, Young M. Host-guest encapsulation of materials by assembled virus protein cages. Nature. 1998;393:152–155. [Google Scholar]
- Endo M, Sugiyama H. Recent progress in DNA origami technology. Curr Protoc Nucleic Acid Chem. 2011:18. doi: 10.1002/0471142700.nc1208s45. Chapter 12(Unit12) [DOI] [PubMed] [Google Scholar]
- Endo M, Fujitsuka M, Majima T. Porphyrin light-harvesting arrays constructed in the recombinant tobacco mosaic virus scaffold. Chemistry. 2007;13:8660–8666. doi: 10.1002/chem.200700895. [DOI] [PubMed] [Google Scholar]
- Engheta N, Ziolkowski RW. Metamaterials: Physics and Engineering Explorations. New York: Wiley; 2006. [Google Scholar]
- Ermolina I, Milner J, Morgan H. Dielectrophoretic investigation of plant virus particles: cow pea mosaic virus and tobacco mosaic virus. Electrophoresis. 2006;27:3939–3948. doi: 10.1002/elps.200500928. [DOI] [PubMed] [Google Scholar]
- Fontana J, Dressick WJ, Phelps J, Johnson JE, Rendell RW, Sampson T, Ratna BR, Soto CM. Virus-templated plasmonic nanoclusters with icosahedral symmetry via directed self-assembly. Small. 2014;10:3058–3063. doi: 10.1002/smll.201400470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel-Conrat H, Williams RC. Reconstitution of active tobacco mosaic virus from its inactive protein and nucleic acid components. Proc Natl Acad Sci USA. 1955;41:690–698. doi: 10.1073/pnas.41.10.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French RH, Parsegian VA, Podgornik R, Rajter RF, Jagota A, Luo J, Asthagiri D, Chaudhury MK, Chiang Y-m, Granick S, Kalinin S, Kardar M, Kjellander R, Langreth DC, Lewis J, Lustig S, Wesolowski D, Wettlaufer JS, Ching W-Y, Finnis M, Houlihan F, von Lilienfeld OA, van Oss CJ, Zemb T. Long range interactions in nanoscale science. Rev Mod Phys. 2010;82:1887–1944. [Google Scholar]
- Furuta P, Brooks J, Thompson ME, Frechet JM. Simultaneous light emission from a mixture of dendrimer encapsulated chromophores: a model for single-layer multichromophoric organic light-emitting diodes. J Am Chem Soc. 2003;125:13165–13172. doi: 10.1021/ja0371348. [DOI] [PubMed] [Google Scholar]
- Geiger FC, Eber FJ, Eiben S, Mueller A, Jeske H, Spatz JP, Wege C. TMV nanorods with programmed longitudinal domains of differently addressable coat proteins. Nanoscale. 2013;5:3808–3816. doi: 10.1039/c3nr33724c. [DOI] [PubMed] [Google Scholar]
- Ginger DS, Zhang H, Mirkin CA. The evolution of dip-pen nanolithography. Angew Chem Int Ed Engl. 2004;43:30–45. doi: 10.1002/anie.200300608. [DOI] [PubMed] [Google Scholar]
- Glotzer SC, Solomon MJ. Anisotropy of building blocks and their assembly into complex structures. Nat Mater. 2007;6:557–562. doi: 10.1038/nmat1949. [DOI] [PubMed] [Google Scholar]
- Han D, Pal S, Nangreave J, Deng Z, Liu Y, Yan H. DNA origami with complex curvatures in three-dimensional space. Science. 2011;332:342–346. doi: 10.1126/science.1202998. [DOI] [PubMed] [Google Scholar]
- Hess GT, Guimaraes CP, Spooner E, Ploegh HL, Belcher AM. Orthogonal labeling of M13 minor capsid proteins with DNA to self-assemble end-to-end multiphage structures. ACS Synth Biol. 2013;2:490–496. doi: 10.1021/sb400019s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai M, Koizumi M, Han R, Hayakawa T, Sano Y. Right-/left-circular orientation of biological macromolecules under magnetic field gradient. J Appl Crystallogr. 2003;36:520–524. [Google Scholar]
- Johnson HR, Hooker JM, Francis MB, Clark DS. Solubilization and stabilization of bacteriophage MS2 in organic solvents. Biotechnol Bioeng. 2007;97:224–234. doi: 10.1002/bit.21245. [DOI] [PubMed] [Google Scholar]
- Kostiainen MA, Hiekkataipale P, de la Torre JA, Nolte RJM, Cornelissen JJLM. Electrostatic self-assembly of virus-polymer complexes. J Mater Chem. 2011;21:2112–2117. [Google Scholar]
- Kostiainen MA, Hiekkataipale P, Laiho A, Lemieux V, Seitsonen J, Ruokolainen J, Ceci P. Electrostatic assembly of binary nanoparticle superlattices using protein cages. Nat Nanotechnol. 2013;8:52–56. doi: 10.1038/nnano.2012.220. [DOI] [PubMed] [Google Scholar]
- Leckband D, Israelachvili J. Intermolecular forces in biology. Q Rev Biophys. 2001;34:105–267. doi: 10.1017/s0033583501003687. [DOI] [PubMed] [Google Scholar]
- Lee S-W, Belcher AM. Virus-based fabrication of micro- and nanofibers using electrospinning. Nano Lett. 2004;4:387–390. [Google Scholar]
- Lee SW, Mao C, Flynn CE, Belcher AM. Ordering of quantum dots using genetically engineered viruses. Science. 2002;296:892–895. doi: 10.1126/science.1068054. [DOI] [PubMed] [Google Scholar]
- Lee HE, Lee HK, Chang H, Ahn HY, Erdene N, Lee HY, Lee YS, Jeong DH, Chung J, Nam KT. Virus templated gold nanocube chain for SERS nanoprobe. Small. 2014;10:3007–3011. doi: 10.1002/smll.201400527. [DOI] [PubMed] [Google Scholar]
- Lekkerkerker HNW, Tuinier R. Colloids and the depletion interaction. Lecture Notes in Physics. 2011:833. [Google Scholar]
- Lin C, Liu Y, Rinker S, Yan H. DNA tile based self-assembly: building complex nanoarchitectures. Chem Phys Chem. 2006;7:1641–1647. doi: 10.1002/cphc.200600260. [DOI] [PubMed] [Google Scholar]
- Liu W, Zhong H, Wang R, Seeman NC. Crystalline two-dimensional DNA-origami arrays. Angew Chem Int Ed Engl. 2011;50:264–267. doi: 10.1002/anie.201005911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmuller T, Aydin D, Schwieder M, Morhard C, Louban I, Pacholski C, Spatz JP. Nanopatterning by block copoly-mer micelle nanolithography and bioinspired applications. Biointerphases. 2011;6:MR1–MR12. doi: 10.1116/1.3536839. [DOI] [PubMed] [Google Scholar]
- Loo L, Guenther RH, Basnayake VR, Lommel SA, Franzen S. Controlled encapsidation of gold nanoparticles by a viral protein shell. J Am Chem Soc. 2006;128:4502–4503. doi: 10.1021/ja057332u. [DOI] [PubMed] [Google Scholar]
- Loo L, Guenther RH, Lommel SA, Franzen S. Encapsidation of nanoparticles by red clover necrotic mosaic virus. J Am Chem Soc. 2007;129:11111–11117. doi: 10.1021/ja071896b. [DOI] [PubMed] [Google Scholar]
- Losdorfer Bozic A, Podgornik R. Symmetry effects in electrostatic interactions between two arbitrarily charged spherical shells in the Debye-Huckel approximation. J Chem Phys. 2013;138:074902. doi: 10.1063/1.4790576. [DOI] [PubMed] [Google Scholar]
- Ma YZ, Miller RA, Fleming GR, Francis MB. Energy transfer dynamics in light-harvesting assemblies templated by the tobacco mosaic virus coat protein. J Phys Chem B. 2008;112:6887–6892. doi: 10.1021/jp8006393. [DOI] [PubMed] [Google Scholar]
- Majumder U, Rangnekar A, Gothelf KV, Reif JH, LaBean TH. Design and construction of double-decker tile as a route to three-dimensional periodic assembly of DNA. J Am Chem Soc. 2011;133:3843–3845. doi: 10.1021/ja1108886. [DOI] [PubMed] [Google Scholar]
- Maldovan M. Sound and heat revolutions in phononics. Nature. 2013;503:209–217. doi: 10.1038/nature12608. [DOI] [PubMed] [Google Scholar]
- Malloy M, Litt LC. Technology review and assessment of nanoimprint lithography for semiconductor and patterned media manufacturing. J Micro Nanolithogr MEMS MOEMS. 2011;10:032001–032013. [Google Scholar]
- Mikkilä J, Rosilo H, Nummelin S, Seitsonen J, Ruokolainen J, Kostiainen MA. Janus-dendrimer-mediated formation of crystalline virus assemblies. ACS Macro Lett. 2013;2:720–724. doi: 10.1021/mz400307h. [DOI] [PubMed] [Google Scholar]
- Miller RA, Presley AD, Francis MB. Self-assembling light-harvesting systems from synthetically modified tobacco mosaic virus coat proteins. J Am Chem Soc. 2007;129:3104–3109. doi: 10.1021/ja063887t. [DOI] [PubMed] [Google Scholar]
- Millman BM, Nickel BG. Electrostatic forces in muscle and cylindrical gel systems. Biophys J. 1980;32:49–63. doi: 10.1016/S0006-3495(80)84915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman BM, Irving TC, Nickel BG, Loosley-Millman ME. Interrod forces in aqueous gels of tobacco mosaic virus. Biophys J. 1984;45:551–556. doi: 10.1016/S0006-3495(84)84192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y, Momotake A, Takeuchi K, Arai T. The use of dendrimers as high-performance shells for round-trip energy transfer: efficient trans-cis photoisomerization from an excited triplet state produced within a dendrimer shell. Photochem Photobiol Sci. 2011;10:116–122. doi: 10.1039/c0pp00272k. [DOI] [PubMed] [Google Scholar]
- Mueller A, Eber FJ, Azucena C, Petershans A, Bittner AM, Gliemann H, Jeske H, Wege C. Inducible site-selective bottom-up assembly of virus-derived nanotube arrays on RNA-equipped wafers. ACS Nano. 2011;5:4512–4520. doi: 10.1021/nn103557s. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Pfeifer CM, Johnson JM, Liu J, Zlotnick A. Redirecting the coat protein of a spherical virus to assemble into tubular nanostructures. J Am Chem Soc. 2006;128:2538–2539. doi: 10.1021/ja056656f. [DOI] [PubMed] [Google Scholar]
- Mynar JL, Lowery TJ, Wemmer DE, Pines A, Frechet JM. Xenon biosensor amplification via dendrimer-cage supramole-cular constructs. J Am Chem Soc. 2006;128:6334–6335. doi: 10.1021/ja061735s. [DOI] [PubMed] [Google Scholar]
- Naik GV, Boltasseva A. A comparative study of semiconductor-based plasmonic metamaterials. Metamaterials. 2011;5:1–7. [Google Scholar]
- Nantalaksakul A, Dasari RR, Ahn TS, Al-Kaysi R, Bardeen CJ, Thayumanavan S. Dendrimer analogues of linear molecules to evaluate energy and charge-transfer properties. Org Lett. 2006;8:2981–2984. doi: 10.1021/ol0608956. [DOI] [PubMed] [Google Scholar]
- Natarajan P, Johnson JE. Molecular packing in virus crystals: geometry, chemistry, and biology. J Struct Biol. 1998;121:295–305. doi: 10.1006/jsbi.1998.3982. [DOI] [PubMed] [Google Scholar]
- Niu Z, Bruckman MA, Li S, Lee LA, Lee B, Pingali SV, Thiyagarajan P, Wang Q. Assembly of tobacco mosaic virus into fibrous and macroscopic bundled arrays mediated by surface aniline polymerization. Langmuir. 2007;23:6719–6724. doi: 10.1021/la070096b. [DOI] [PubMed] [Google Scholar]
- Oh JW, Chung WJ, Heo K, Jin HE, Lee BY, Wang E, Zueger C, Wong W, Meyer J, Kim C, Lee SY, Kim WG, Zemla M, Auer M, Hexemer A, Lee SW. Biomimetic virus-based colourimetric sensors. Nat Commun. 2014;5:3043. doi: 10.1038/ncomms4043. [DOI] [PubMed] [Google Scholar]
- Onsager L. The effects of shape on the interaction of colloidal particles. Ann N Y Acad Sci. 1949;51:627–659. [Google Scholar]
- Onsager L. The motion of ions: principles and concepts. Science. 1969;166:1359–1364. doi: 10.1126/science.166.3911.1359. [DOI] [PubMed] [Google Scholar]
- Oster G. Two-phase formation in solutions of tobacco mosaic virus and the problem of long-range forces. J Gen Physiol. 1950;33:445–473. doi: 10.1085/jgp.33.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Pistol C, Ahn SJ, Reif JH, Lebeck AR, Dwyer C, LaBean TH. Finite-size, fully addressable DNA tile lattices formed by hierarchical assembly procedures. Angew Chem Int Ed Engl. 2006;45:735–739. doi: 10.1002/anie.200503797. [DOI] [PubMed] [Google Scholar]
- Pimpin A, Srituravanich W. Review on micro- and nano-lithography techniques and their applications. Eng J. 2012:16. [Google Scholar]
- Pokorski JK, Steinmetz NF. The art of engineering viral nanoparticles. Mol Pharm. 2011;8:29–43. doi: 10.1021/mp100225y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin D, Xia Y, Whitesides GM. Soft lithography for micro- and nanoscale patterning. Nat Protoc. 2010;5:491–502. doi: 10.1038/nprot.2009.234. [DOI] [PubMed] [Google Scholar]
- Rajendran A, Endo M, Sugiyama H. DNA origami: synthesis and self-assembly. Curr Protoc Nucleic Acid Chem Chapter. 2012;12 doi: 10.1002/0471142700.nc1209s48. Unit 12 19 11-18. [DOI] [PubMed] [Google Scholar]
- Romano F, Sciortino F. Colloidal self-assembly: patchy from the bottom up. Nat Mater. 2011;10:171–173. doi: 10.1038/nmat2975. [DOI] [PubMed] [Google Scholar]
- Royston E, Ghosh A, Kofinas P, Harris MT, Culver JN. Self-assembly of virus-structured high surface area nanomaterials and their application as battery electrodes. Langmuir. 2008;24:906–912. doi: 10.1021/la7016424. [DOI] [PubMed] [Google Scholar]
- Sacca B, Niemeyer CM. DNA origami: the art of folding DNA. Angew Chem Int Ed Engl. 2012;51:58–66. doi: 10.1002/anie.201105846. [DOI] [PubMed] [Google Scholar]
- Salaita K, Wang Y, Mirkin CA. Applications of dip-pen nanolithography. Nat Nanotechnol. 2007;2:145–155. doi: 10.1038/nnano.2007.39. [DOI] [PubMed] [Google Scholar]
- Serin JM, Brousmiche DW, Frechet JM. Cascade energy transfer in a conformationally mobile multichromophoric den-drimer. Chem Commun (Camb) 2002:2605–2607. doi: 10.1039/b207905d. [DOI] [PubMed] [Google Scholar]
- Shenton W, Douglas T, Young M, Stubbs G, Mann S. Inorganic-organic nanotube composites from template mineralization of tobacco mosaic virus. Adv Mater. 1999;11:253–256. [Google Scholar]
- Shih WM, Lin C. Knitting complex weaves with DNA origami. Curr Opin Struct Biol. 2010;20:276–282. doi: 10.1016/j.sbi.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S, Dickmeis C, Nagarajan AS, Fischer R, Commandeur U, Steinmetz NF. Molecular farming of fluorescent virus-based nanoparticles for optical imaging in plants, human cells and mouse models. Biomater Sci. 2014;2:784–797. doi: 10.1039/c3bm60277j. [DOI] [PubMed] [Google Scholar]
- Siber A, Bozic AL, Podgornik R. Energies and pressures in viruses: contribution of nonspecific electrostatic interactions. Phys Chem Chem Phys. 2012;14:3746–3765. doi: 10.1039/c1cp22756d. [DOI] [PubMed] [Google Scholar]
- Šiber A, Zandi R, Podgornik R. Thermodynamics of nanospheres encapsulated in virus capsids. Phys Rev E. 2010;81:051919. doi: 10.1103/PhysRevE.81.051919. [DOI] [PubMed] [Google Scholar]
- Sihvola A. Metamaterials in electromagnetics. Metamaterials. 2007;1:2–11. [Google Scholar]
- Sreekanth KV, De Luca A, Strangi G. Experimental demonstration of surface and bulk plasmon polaritons in hypergratings. Sci Rep. 2013;3:3291. doi: 10.1038/srep03291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekanth KV, Krishna KH, De Luca A, Strangi G. Large spontaneous emission rate enhancement in grating coupled hyperbolic metamaterials. Sci Rep. 2014;4:6340. doi: 10.1038/srep06340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MM, Flynn NT, Wang C, Tirrell DA, Langer R. Coiled-coil peptide-based assembly of gold nanoparticles. Adv Mater. 2004;16:915–918. [Google Scholar]
- Strangi G, De Luca A, Ravaine S, Ferrie M, Bartolino R. Gain induced optical transparency in metamaterials. Appl Phys Lett. 2011;98:251912. [Google Scholar]
- Sun J, DuFort C, Daniel MC, Murali A, Chen C, Gopinath K, Stein B, De M, Rotello VM, Holzenburg A, Kao CC, Dragnea B. Core-controlled polymorphism in virus-like particles. Proc Natl Acad Sci USA. 2007;104:1354–1359. doi: 10.1073/pnas.0610542104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Acosta JR, Cadena-Nava RD, Gelbart WM, Knobler CM, Ruiz-Garcia J. Electrophoretic mobilities of a viral capsid, its capsid protein, and their relation to viral assembly. J Phys Chem B. 2014;118:1984–1989. doi: 10.1021/jp407379t. [DOI] [PubMed] [Google Scholar]
- Wagner SC, Roskamp M, Pallerla M, Araghi RR, Schlecht S, Koksch B. Nanoparticle-induced folding and fibril formation of coiled-coil-based model peptides. Small. 2010;6:1321–1328. doi: 10.1002/smll.200902067. [DOI] [PubMed] [Google Scholar]
- Wang Q, Lin T, Johnson JE, Finn MG. Natural supramolecular building blocks: cysteine-added mutants of cowpea mosaic virus. Chem Biol. 2002;9:813–819. doi: 10.1016/s1074-5521(02)00166-7. [DOI] [PubMed] [Google Scholar]
- Wang D, Capehart SL, Pal S, Liu M, Zhang L, Schuck PJ, Liu Y, Yan H, Francis MB, De Yoreo JJ. Hierarchical assembly of plasmonic nanostructures using virus capsid scaffolds on DNA origami templates. ACS Nano. 2014;8:7896–7904. doi: 10.1021/nn5015819. [DOI] [PubMed] [Google Scholar]
- Wen AM, Steinmetz NF. The aspect ratio of nanoparticle assemblies and the spatial arrangement of ligands can be optimized to enhance the targeting of cancer cells. Adv Healthc Mater. 2014;3:1739–1744. doi: 10.1002/adhm.201400141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen AM, Rambhia PH, French RH, Steinmetz NF. Design rules for nanomedical engineering: from physical virology to the applications of virus-based materials in medicine. J Biol Phys. 2013;39:301–325. doi: 10.1007/s10867-013-9314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen AM, Infusino M, De Luca A, Kernan DL, Czapar AE, Strangi G, Steinmetz NF. Interface of Physics and Biology: engineering Virus-Based Nanoparticles for Biophotonics. Bioconjug Chem. 2015 doi: 10.1021/bc500524f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Soft lithography in biology and biochemistry. Annu Rev Biomed Eng. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- Wu M, Brown WL, Stockley PG. Cell-specific delivery of bacteriophage-encapsidated ricin A chain. Bioconjug Chem. 1995;6:587–595. doi: 10.1021/bc00035a013. [DOI] [PubMed] [Google Scholar]
- Yang SH, Chung WJ, McFarland S, Lee SW. Assembly of bacteriophage into functional materials. Chem Rec. 2013;13:43–59. doi: 10.1002/tcr.201200012. [DOI] [PubMed] [Google Scholar]
- Yun JM, Kim KN, Kim JY, Shin DO, Lee WJ, Lee SH, Lieberman M, Kim SO. DNA origami nanopatterning on chemically modified graphene. Angew Chem Int Ed Engl. 2012;51:912–915. doi: 10.1002/anie.201106198. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Thorkelsson K, Mastroianni AJ, Schilling T, Luther JM, Rancatore BJ, Matsunaga K, Jinnai H, Wu Y, Poulsen D, Frechet JM, Alivisatos AP, Xu T. Small-molecule-directed nanoparticle assembly towards stimuli-responsive nanocomposites. Nat Mater. 2009;8:979–985. doi: 10.1038/nmat2565. [DOI] [PubMed] [Google Scholar]