Abstract
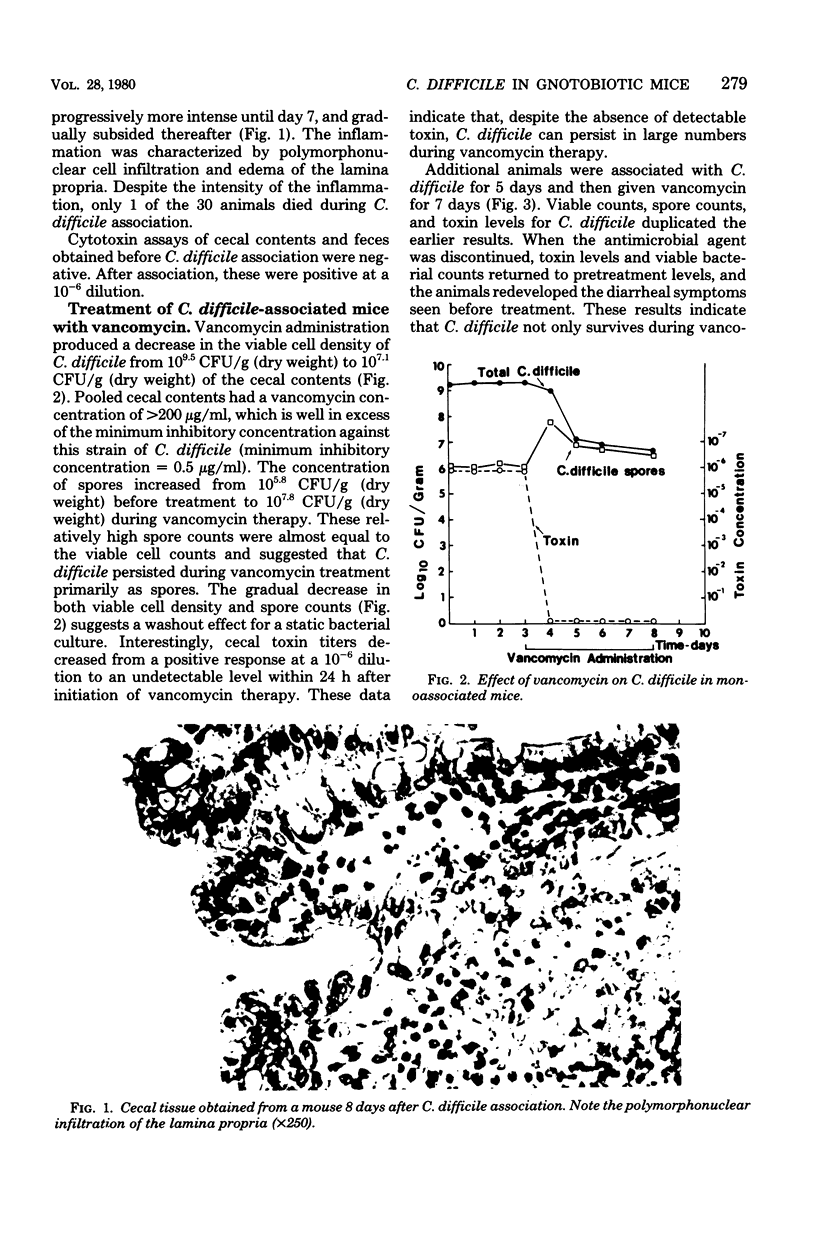
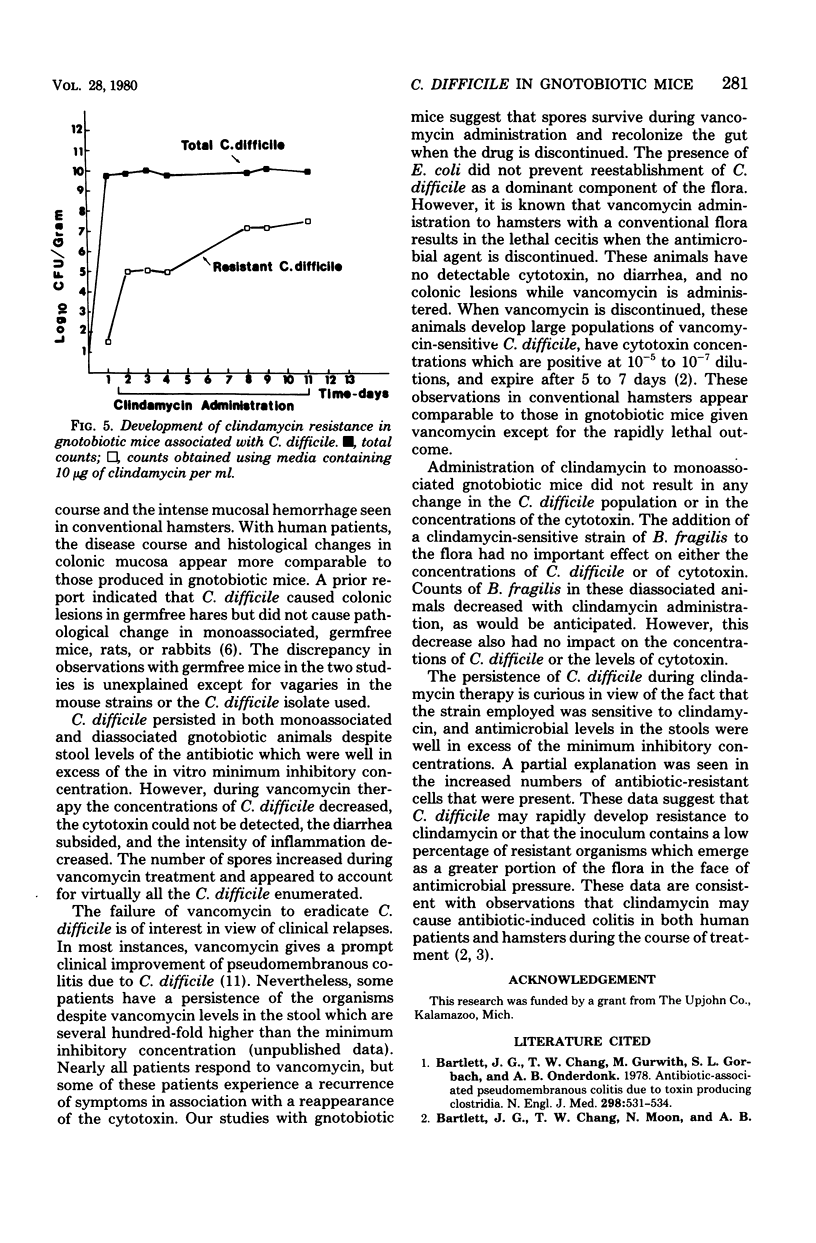
Germfree mice associated with Clostridium difficile developed intestinal disease characterized by polymorphonuclear cell infiltration of the lamina propria, diarrhea, and cecal cytotoxin concentrations positive at a 10(-6) dilution. The numbers of viable bacteria never exceeded 10(10) colony-forming units per g (dry weight). Despite the high toxin levels and chronic inflammation over a 30-day period, the mortality rate was low (less than 2%). Daily treatment of these animals with two oral doses of 2 mg of vancomycin resulted in stool levels of greater than 200 micrograms/ml, well in excess of the minimum inhibitory concentration for C. difficile. This therapy decreased viable cell density by 2 to 3 logs and increased the spore counts from 10(5.8) to 10(7.8) colony-forming units per g (dry weight) by day 7, and animals were free of detectable toxin. However, once therapy was stopped, viable bacteria and spore counts and cytotoxin concentrations returned to previous levels. Treatment of mice with concentrations of clindamycin shown to be inhibitory in vitro had no effect on C. difficile toxin titers or bacterial counts, although the appearance of a clindamycin-resistant population was noted. These data indicate that vancomycin, given orally, decreases the concentration of toxin, but C. difficile survive as spores. By contrast, large populations of vegetative cells and high cytotoxin levels persist when clindamycin is used, even at an inhibitory concentration.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett J. G., Chang T. W., Gurwith M., Gorbach S. L., Onderdonk A. B. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978 Mar 9;298(10):531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- Bartlett J. G., Chang T. W., Moon N., Onderdonk A. B. Antibiotic-induced lethal enterocolitis in hamsters: studies with eleven agents and evidence to support the pathogenic role of toxin-producing Clostridia. Am J Vet Res. 1978 Sep;39(9):1525–1530. [PubMed] [Google Scholar]
- Bartlett J. G., Onderdonk A. B., Cisneros R. L., Kasper D. L. Clindamycin-associated colitis due to a toxin-producing species of Clostridium in hamsters. J Infect Dis. 1977 Nov;136(5):701–705. doi: 10.1093/infdis/136.5.701. [DOI] [PubMed] [Google Scholar]
- Chang T. W., Bartlett J. G., Gorbach S. L., Onderdonk A. B. Clindamycin-induced enterocolitis in hamsters as a model of pseudomembranous colitis in patients. Infect Immun. 1978 May;20(2):526–529. doi: 10.1128/iai.20.2.526-529.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabard J., Dubos F., Martinet L., Ducluzeau R. Experimental reproduction of neonatal diarrhea in young gnotobiotic hares simultaneously associated with Clostridium difficile and other Clostridium strains. Infect Immun. 1979 Apr;24(1):7–11. doi: 10.1128/iai.24.1.7-11.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie T. J., Tally F. P., Bartlett J. G., Gorbach S. L. Rapid microbiological assay for chloramphenicol and tetracyclines. Antimicrob Agents Chemother. 1976 Jun;9(6):874–878. doi: 10.1128/aac.9.6.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusk R. H., Fekety R., Silva J., Browne R. A., Ringler D. H., Abrams G. D. Clindamycin-induced enterocolitis in hamsters. J Infect Dis. 1978 Apr;137(4):464–475. doi: 10.1093/infdis/137.4.464. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Hermos J. A., Dzink J. L., Bartlett J. G. Protective effect of metronidazole in experimental ulcerative colitis. Gastroenterology. 1978 Mar;74(3):521–526. [PubMed] [Google Scholar]
- Taylor N. S., Bartlett J. G. Partial purification and characterization of a cytotoxin from Clostridium difficile. Rev Infect Dis. 1979 Mar-Apr;1(2):379–385. doi: 10.1093/clinids/1.2.379. [DOI] [PubMed] [Google Scholar]
- Tedesco F., Markham R., Gurwith M., Christie D., Bartlett J. G. Oral vancomycin for antibiotic-associated pseudomembranous colitis. Lancet. 1978 Jul 29;2(8083):226–228. doi: 10.1016/s0140-6736(78)91741-5. [DOI] [PubMed] [Google Scholar]



