Abstract
Older adults with heart failure have multiple chronic conditions and a large number and range of symptoms. A fundamental component of heart failure self-care management is regular symptom monitoring. Symptom monitoring can be facilitated by cost-effective, easily accessible technologies that are integrated into patients’ lives. Technologies that are tailored to older adults by incorporating gerontological design principles are called gerontechnologies. Gerontechnology is an interdisciplinary academic and professional field that combines gerontology and technology with the goals of improving prevention, care, and enhancing the quality of life for older adults. The purpose of this article is to discuss the role of gerontechnologies, specifically the use of mobile applications available on smartphones and tablets as well as remote monitoring systems, for outpatient disease management among older adults with heart failure. While largely unproven, these rapidly developing technologies have great potential to improve outcomes among older persons.
Keywords: Aging, Cardiology, Gerontology, Tablets, Gerontechnology, Older adults, Mobile phone, Mobile applications
Introduction
Heart failure currently affects 5.7 million Americans [1] and is a leading cause of hospital admissions and 30-day readmissions [2–6]. Heart failure is also the fastest growing cardiovascular condition in the USA [7], largely due to an aging population and improved survival after myocardial infarction [8]. As such, heart failure is a large public health concern.
The management of heart failure is often complicated by the presence of multiple chronic conditions that share common symptoms, including edema, fatigue, exercise intolerance, and shortness of breath. These symptoms are regularly present, even after a hospitalization, when more than 40 % of patients report no improvement in dyspnea, anxiety, fatigue, and pain [9]. Major clinical guidelines recommend the inclusion of symptom monitoring as part of routine self-care management of patients with heart failure [10]. This can be challenging because symptom changes can be so insidious that patients have difficulty recognizing and responding to them, as well as differentiating them from symptoms of other chronic conditions including, depression [11], atrial fibrillation, diabetes, and chronic kidney disease [12]. Despite these difficulties, active participation in self-care management is crucial in heart failure because patients who are involved in their own care are more likely to have improved survival, decreased readmission rates, and better quality of life [13–15].
Concurrent with an increase in the prevalence of heart failure and the challenges of self-care management is the need for novel, easy-to-use “real-world” mechanisms for outpatient self-care management support. The use of remote monitoring with smartphone and tablet technology is particularly relevant for patients with heart failure because signs and symptoms can be quickly assessed remotely and deterioration can be quickly addressed. Smartphone and tablet mHealth apps are well positioned for monitoring and managing health conditions because for many patients they are already integrated into patients’ lives. In addition to being portable and convenient and facilitating care at the point-of-need [16], smartphones also have the advantage of being able to extract data from multiple devices, direct user input, transmit data to a server, and facilitate a two-way communication between patients and providers [17]. Using mHealth applications for disease management is also not new and has been used for patients with diabetes [18–20], hypertension [21, 22], chronic obstructive pulmonary disease [23], and cardiac arrhythmias [24–26].
Many of the previous studies of telemonitoring and remote devices in HF have been negative. One of the driving reasons may be that the technologies being tested were not well integrated into regularly used devices, such as smartphones or tablets. As such, the technologies may have caused increased treatment burden, especially in the post-hospital period [27], and relatively low adherence rates as was found in the largest clinical trials in this field [28••, 29••]. For example, in the Telemonitoring to Improve Heart Failure Outcomes (Tele-HF), study participants had to make daily calls on a landline phone and use a touch-tone telephone keypad to respond to questions on general health and heart failure symptoms [28••]. While the Better Effectiveness After Transition—Heart Failure (BEAT-HF) [29••] study used more cutting-edge technology to measure blood pressure, weight, and heart rate, it still did not test technologies that are integrated into patient’s everyday lives. What we can learn from these two trials is that the type, nature, and interoperability of the technology is important in terms of patient and provider usability and that novel disease management strategies need to be thoroughly evaluated prior to adoption [28••, 29••]. In terms of evaluating studies, the types of technology that are being tested inform the interpretation of the study findings (Table 1) [28••, 29••, 31–34].
Table 1.
Study characteristics of select telehealth and gerontechnology interventions
| Article | Study design and duration | Sample size | Mean age (years) | Population | Monitored parameters | Primary and secondary endpoints | Results |
|---|---|---|---|---|---|---|---|
| Chaudhry [30] Chaudhry [28••] Tele-HF USA |
RCT, multicenter (2006–2009) 24 mon, 6 month follow-up |
N = 1653 (IG: 826, CG: 827) | IG: 61 CG: 61 |
Patients with an HF hospitalization with previous month | IG: telephone-based interactive voice response that collected daily information about symptoms and weight CG: usual care |
Primary: death/readmission within 180 days, Secondary: hospitalization or death, LOS, number of hospitalizations |
No differences in primary or secondary endpoints |
| Koehler [31] Germany |
RCT, multicenter, 24-month follow-up |
N = 710 (IG: 354, CG: 356) | IG: 67 CG: 67 |
Stable chronic HF patients | IG: remote monitoring of ECG, blood pressure, body weight CG: usual care |
Primary: CV mortality Secondary: composite (CV mortality/HF admission), HF-admissions, LOS, NYHA class, QOL and depression |
Improved physical function in IG, no difference between groups in all-cause mortality, CV mortality, HF-admissions, LOS, NYHA class or composite (CV mortality/HF admission) |
| Ong [29••] BEAT-HF USA |
RCT (2011–2013) 6-month follow-up |
N = 1437 IG: 715 CG: 722 |
IG: 73 CG: 74 |
Hospitalized patients with HF | IG: 1) predischarge HF education, 2) telephone coaching, and 3) home telemonitoring of weight, blood pressure, heart rate and symptoms CG: usual care |
Primary: All-cause readmission within 180 days Secondary: All-cause readmission within 30 days, All-cause mortality, QOL |
No differences in All-cause 180- or 30-day readmission, 180-day mortality, Improved QOL at 180 days in intervention group |
| Piotrowicz [32], Poland |
RCT, single center 8 weeks follow-up |
N = 152 (IG: 77, CG: 75) | IG: 56, CG: 61 | Hospitalized patients with HF with NYHA class II or III | IG: home-based remote monitored cardiac rehab; patient edu, psych support CG: standard cardiac rehab, patient edu, psych support |
Primary: NYHA class, QOL, peak VO2, 6 MWT | Greater improvement in NYHA class in IG, Greater improvement in 6 MWT in CG, No difference between groups in exercise duration, peak VO2 and QOL |
| Scherr et al. [33] Austria |
RCT, 6 mon follow-up |
N = 120 (IG: 66, CG: 54) | IG: 66, CG: 67 | Heart failure patient with acute worsening and hospital admission lasting > 24 h | IG: Remote automated monitoring of BP, body weight, pharmacological treatment CG: pharmacological treatment |
Primary: hospitalization for worsening HF or death from cardiovascular cause Secondary: LOS, NYHA class, LVEF, Composite (CV mortality/HF admission) |
Shorter LOS in IG, median improved in NYHA from III to II in IG group only, no difference between groups in other outcomes |
| Seto et al. [34] Canada |
RCT, single center, 6 mon follow-up |
N = 100 (IG: 50, CG: 50) | IG: 55, CG: 52 | Ambulatory patients with heart failure | IG: remote monitoring of ECG, blood pressure, body weight; standard care CG: standard care |
Primary: BNP, self-care, QOL Secondary: number of ER visits, LVEF, NYHA class, medication prescriptions, blood test results |
No difference between groups except for overall QOL |
Abbreviations: BNP brain natriuretic peptide, CG control group, CV cardiovascular, ECG electrocardiogram, ER emergency room, HF heart failure, IG intervention group, LOS length of stay, LVEF left ventricular ejection fraction, NYHA New York Heart Association functional class, peak VO2 peak oxygen consumption, QOL quality of life, RCT randomized controlled trial, 6 MWT 6-min walk test
The purpose of this article is to discuss how specific gerontechnologies can benefit older adults with heart failure with symptom monitoring and self-care management. For discussion in this article, we use the term gerontechnologies, rather than telemonitoring, to make the distinction between current remote monitoring devices and mHealth applications that are available on smartphones and tablets.
Definition of Gerontechnology
Gerontechnology is an interdisciplinary field that combines gerontology and technology. In this field, gerontological design principles are integrated with research on biological, psychological, social, and medical aspects of aging towards the goal of improving the quality of life of older adults [35, 36]. Gerontechnologies have the potential to support independent living and social participation by improving health and well-being [37]. Traditionally, gerontechnology has focused on the application of (1) advanced technologies to address motor and cognitive disability, (2) wearable systems to recognize problems related to reduced functional capacity, and (3) technological aids to compensate for deficits and increase the level of autonomy at home [35]. More recently, gerontechnologies have expanded in scope beyond biotechnology devices and now include modalities such as tablets and smartphones [38].
Technology Use Among Older Adults
Across the United States, the use of mobile devices is experiencing significant year-to-year growth [39•]. In 2013, almost 60 % of American adults reported owning a smartphone [39•], though older adults overall—defined as those 65 and older—have lower smartphone adoption levels (18 %). While a quarter of adults ages 65–74 years own an smartphone, this proportion declines rapidly over age 75 [40•]. Despite lower overall smartphone adoption, however, the proportion of older adults owning a smartphone increased 5 % between 2012 and 2014 [40•]. In addition, 27 % of older adults own a tablet, e-book reader, or both [40•].
These results demonstrate that older adults (65 and older) are not a homogeneous group. Ownership of tablet and smartphone technologies generally falls along the lines of income and education. More highly educated and more affluent older adults with annual household incomes of $75,000 or more tend to have more technology assets and a more positive view towards online platforms [40•]. In contrast, less affluent older adults with incomes less than $30,000 annually and more disabilities are often disconnected from the digital world of technologies [40•]. Seniors with a college degree (compared to those who have not attended college) are three times as likely to own both an e-book reader and a tablet, and those with annual incomes of $75,000 or more are around four times as likely to own each device compared to those with household incomes of less than $30,000 per year [40•].
Many of the same benefits that smartphones and tablets can have on symptom monitoring and self-care management can also help address other relevant concerns in older age by supporting social connectedness and subsequently decreasing social isolation [38]. A recent study confirmed that the use of tablet technologies, such as iPads, has the potential to reduce social isolation, renew prior relationships, and enhance familial communication [38]. Regular use of an iPad was also associated with an increase in iPad competency and technological ability [38].
Potential Applications of Gerontechnologies for Older Adults With Heart Failure
Remote Monitoring for Arrhythmias
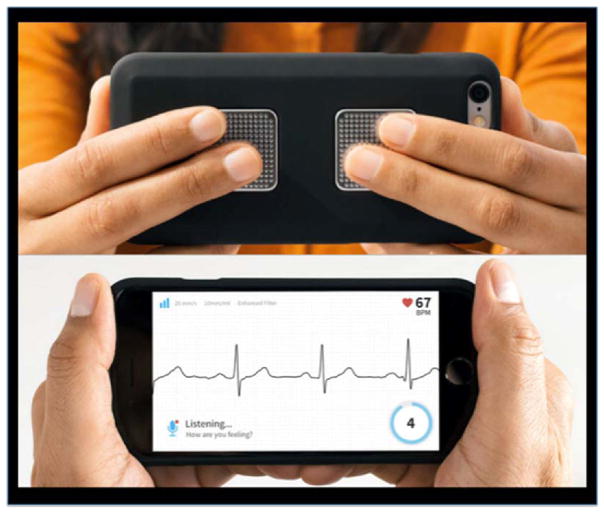
Atrial fibrillation is highly prevalent among older adults with heart failure and other cardiovascular conditions [41]. Symptoms of atrial fibrillation, including fatigue, palpitations, dyspnea, dizziness, and exercise intolerance are also commonly associated with heart failure [42]. Gerontechologies such as Kardia™ Mobile, an FDA-approved smartphone technology (Fig. 1), are designed to help patients distinguish when symptoms may more relate to an arrhythmia rather than heart failure [42] by capturing a single-lead electrocardiogram recording through two electrodes on the back of an iPhone [43]. When both electrodes make contact with the skin, a 30-s single channel electrocardiogram automatically records, the ECG data is sent over a WiFi or cellular network, and transmissions are automatically uploaded to the Kardia™ “cloud.” This technology eliminates the need for adhesive ECG electrodes, skin patches placed on the chest, and complicated lead changes by the patient. Rather, rapid, real time capture of an ECG can occur immediately at the onset of symptoms, which can provide a significant improvement for symptom evaluation. The efficacy of Kardia™ Mobile on patient reported and clinical outcomes is currently being tested in the iHEART randomized controlled trial (Clinicaltrials.gov ID: NCT02731326) [44].
Fig. 1.
Kardia™ Mobile (image used with permission from AliveCor)
Remote Monitoring of Physical Activity
Given the importance of physical activity to the maintenance of function, smartphones and commercially available wearable activity trackers are increasingly being used to measure and promote movement [45, 46]. A recent mixed-methods evaluation found that commercially available wearable activity trackers (Fitbit Zip, Misfit Shine, Jawbone Up 24, and Withings Pulse) (Fig. 2) that sync with smartphones were perceived as useful and acceptable in a small sample of 32 adults over age 50 [47]. Another study by McMahon and colleagues evaluated the short-and long-term experiences of adults >70 years old with the Fitbit One activity tracker and found that it was easy to use, useful, and acceptable at 10 weeks and 8 months [48], though there were lower survey ratings by participants >80 years old.
Fig. 2.
Examples of commercially wearable activity trackers from Fitbit, Misfit, Jawbone, and Withings
Another example is the self-administered 6 MWT mobile application (SA-6MWTapp) for patients with heart failure that permits administration of a 6-min-walk test (6 MWT) anywhere and transmits the results wirelessly to a cloud server [49]. The 6 MWT is an important measure of functional capacity that highly correlates with the results of cardiopulmonary exercise testing [50]. The SA-6MWTapp leverages smartphone technology with integrated accelerometers and GPS tracking to monitor physical activity and longitudinally collect data on patients’ 6 MWT. Currently, the SA-6MWTapp is not yet available commercially.
Comprehensive Remote Monitoring Systems Using Mobile Phones and Tablets
One of the earlier studies to test a mobile phone-based telemonitoring system was conducted by Seto and colleagues [34]. This study included taking daily weight and blood pressure readings, weekly single-lead ECGs, and daily symptom questions on a mobile phone over 6 months with feedback both to the patient and the cardiology provider (Table 1). A more recent example is the remote monitoring system called iGetBetter [15] (Partners, Boston), which enables patients to self-monitor by being able to take their weight using a Bluetooth scale, blood pressure, and heart rate measurements. Patients can view their results on an iPad mini tablet computer (Apple Inc, Cupertino, CA, USA) equipped with cellular internet connectivity [15].
Challenges for Implementation With Older Adults
Given an aging population with a high prevalence of disease, there has been tremendous growth in the number of gerontechnologies targeted towards older adults with heart failure. Though many gerontechnologies hold promise for supporting outpatient disease management, there are also a number of specific barriers for implementation in this patient population, including physical limitations from existing health conditions such as sensory impairments, cognitive changes, arthritis, and vision impairments. These age- and disease-related physical limitations inhibit the ability of older adults to utilize the functions of the technology and receive maximum benefit.
Other commonly perceived challenges of getting older adults to use gerontechnologies include user acceptance [40•], cost [51], technology self-efficacy [52] (perceived belief with regard to coping and possibility for success in action [53]), technology experience [52], burdens/workload associated with device use [27], and education/training on the device itself [40•]. For smartphones specifically, having to do manual data entry into a mHealth app is a barrier [33]. Many of these applications are also inaccessible to patients with low health and technology literacy [54]. Potentially the biggest challenge is supporting patients over 75 years of age who have had less access to smartphone, tablet, and remote monitoring technologies throughout their lives. Because of this limitation, many studies that have evaluated these technologies have not included older adults. One way to address many of these barriers is to involve older adults in the entire design process, from inception through development [55].
Another challenge that older adults face is that apps that solely focus on specific medical problems are potentially not as useful as those that can support patients with multiple medical conditions. If patients have to use different apps for each condition, they face the burden of app overload [56]. Selecting a single app from the estimated 165,000 health-related apps [57] is also a major concern. Currently, patients have few resources to evaluate the apps, other than the limited star rating and consumer reviews. Many new apps are being used with minimal knowledge of their functionality and ability to integrate data into healthcare systems [56], let alone efficacy for improving patient or clinical outcomes. As mHealth apps mature, we will likely see apps being recommended more systematically rather than on an ad hoc basis [56]. Eventually, the most efficacious apps should be able to be judiciously integrated into healthcare management when more appropriate governance structures are in place [56, 58].
Recently, a new methodology for the systematic evaluation of mHealth apps has been proposed [59], including individual app evaluation using three instruments: Mobile Application Rating Scale [60], IMS Institute for Healthcare Informatics functionality score [56], and evidence-based guidelines endorsed by specific medical societies, (i.e., Heart Failure Society of America guidelines for non-pharmacologic management) [10]. Each mHealth app should be rated individually across the specified content and functionality criteria to determine the best-in-class mHealth apps for either general or specific conditions [59]. This is especially important given the current lack of clinical trial data to support mHealth apps.
What Should Providers Tell Patients About Gerontechnology Products?
Currently, healthcare providers can describe mHealth apps to patients; however, to date, there is no high-quality randomized controlled trial data that demonstrates the clinical effectiveness of these apps. In part, this is due to how quickly the technology is changing and that by the time a study has been funded and completed, the technology being tested in nearly obsolete.
With more evidence on the effectiveness of specific apps, healthcare providers will be able to better recommend specific apps and provide clearer clinical guidance. In some cases, healthcare providers will even be able to provide patients with prescriptions for specific mHealth apps, as is the case for diabetes self-management with BlueStar app (Welldoc®).
Challenges for Providers
While the focus of this paper is on the patients who are using these technologies, the reality is that healthcare providers need to endorse these technologies in order for patients to use them. The first major concern for providers is information overload, especially from remote monitoring systems. The reams of data generated by a remote monitor or physical activity tracker are only valuable if synthesized into an elegant visualization or simple dashboard that represents longitudinal data. The second major concern is whether or not there will be any reimbursement to providers for remote monitoring patients. According to Centers for Medicare and Medicaid Services, as of 2016, the Medicare Fee-For-Service Program allows for the billing and payment of medical services for telehealth [61]. In March 2016, Centers for Medicare and Medicaid Services published more detailed answers to frequently asked questions about billing for remote patient monitoring services [62]. The report indicated that remote monitoring activities can count towards the minimum 20 min of time per month for chronic care management services [62].
Conclusions
Patients with heart failure are a heterogeneous patient population, in terms of frailty, ability to self-manage, disease acuity, and functional status. Tablets, smartphones, and remote monitoring systems hold strong potential for supporting outpatient symptom monitoring and self-care management for patients with heart failure; however, they need to be used judiciously. Currently, it is unknown which patients with heart failure will benefit most from these gerontechnologies [31]. In this early phase of adoption, it may be most strategic to focus on active and less frail community-dwelling older adults with heart failure [63] because engaging patients without current access to these gerontechnologies will take a different and perhaps more resource intensive approach.
Highlights for Future Research.
The research agenda for gerontechnologies to support heart failure symptom monitoring and self-care management includes understanding how mHealth tools can be used to improve patient-provider communication and adherence to treatments, as well as answering the questions below:
For patients
Which patients experience the most benefit from using mHealth and remote monitoring devices?
What factors facilitate regular adherence to mHealth technologies?
How can mHealth technologies be “streamlined” to promote ease of use?
For providers
What are the most efficient ways to present information to care providers?
What data points do healthcare providers consider most useful?
How can mHealth technologies be integrated into commercially available electronic health record systems commonly in use?
How can mHealth technologies be integrated into the clinical care of underserved and minority populations to reduced healthcare disparities?
These questions highlight a few of the complex topics that will require additional research, especially given the rapidly changing technology landscape.
Acknowledgments
The authors gratefully acknowledge funding for Dr. Masterson Creber by the National Institutes of Health (NIH)/National Institute of Nursing Research (NINR), K99NR016275, “mHealth for Heart Failure Symptom Monitoring.” The Columbia University School of Nursing also provided post-doctoral funding for Dr. Masterson Creber through NIH/NINR (T32NR007969). Additional mentorship training was provided to Dr. Masterson Creber through the Agency for Healthcare Research and Quality (R01HS021816) at the Department of Biomedical Informatics at Columbia University. Dr. Maurer is supported by a K24 Award from the NIA (AG036778) and Dr. Hickey is supported by a R01 Award from NINR (R01 NR014853: iPhone Helping Evaluate Atrial Fibrillation Rhythm Through Technology (iHEART)).
Footnotes
Conflict of Interest Drs Masterson Creber, Hickey, and Maurer have no conflicts of interests to declare
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by the author.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015 doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–44. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 4.Ross JS, Chen J, Lin Z, et al. Recent national trends in readmission rates after heart failure hospitalization. Circ Heart Fail. 2010;3(1):97–103. doi: 10.1161/CIRCHEARTFAILURE.109.885210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 6.Retrum JH, Boggs J, Hersh A, et al. Patient-identified factors related to heart failure readmissions. Circ Cardiovasc Qual Outcomes. 2013;6(2):171–7. doi: 10.1161/CIRCOUTCOMES.112.967356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mozaffarian D, Benjamin EJ, Go AS, et al. Executive summary: heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):447–54. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 9.Khan RF, Feder S, Goldstein NE, Chaudhry SI. Symptom burden among patients who were hospitalized for heart failure. JAMA Intern Med. 2015;175(10):1713–5. doi: 10.1001/jamainternmed.2015.3871. [DOI] [PubMed] [Google Scholar]
- 10.Lindenfeld J, Albert NM, Boehmer JP, et al. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16(6):e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48(8):1527–37. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 12.Dunlay SM, Chamberlain AM. Multimorbidity in older patients with cardiovascular disease. Curr Cardio Risk Rep. 2016;10(3) doi: 10.1007/s12170-016-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lainscak M, Blue L, Clark AL, et al. Self-care management of heart failure: practical recommendations from the Patient Care Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2011;13(2):115–26. doi: 10.1093/eurjhf/hfq219. [DOI] [PubMed] [Google Scholar]
- 14.van der Wal MH, van Veldhuisen DJ, Veeger NJ, Rutten FH, Jaarsma T. Compliance with non-pharmacological recommendations and outcome in heart failure patients. Eur Heart J. 2010;31(12):1486–93. doi: 10.1093/eurheartj/ehq091. [DOI] [PubMed] [Google Scholar]
- 15.Zan S, Agboola S, Moore SA, Parks KA, Kvedar JC, Jethwani K. Patient engagement with a mobile web-based telemonitoring system for heart failure self-management: a pilot study. JMIR mHealth uHealth. 2015;3(2):e33. doi: 10.2196/mhealth.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mirkovic J, Kaufman DR, Ruland CM. Supporting cancer patients in illness management: usability evaluation of a mobile app. JMIR mHealth uHealth. 2014;2(3):e33. doi: 10.2196/mhealth.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alshurafa N, Eastwood JA, Nyamathi S, et al. Improving compliance in remote healthcare systems through smartphone battery optimization. IEEE J Biomed Health Informatics. 2015;19(1):57–63. doi: 10.1109/JBHI.2014.2329712. [DOI] [PubMed] [Google Scholar]
- 18.Gammon D, Murray E, Franklin VL, et al. Patients’ engagement with “sweet talk” – a text messaging support system for young people with diabetes. J Med Internet Res. 2008;10(2) doi: 10.2196/jmir.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quinn CC, Clough SS, Minor JM, Lender D, Okafor MC, Gruber-Baldini A. WellDoc mobile diabetes management randomized controlled trial: change in clinical and behavioral outcomes and patient and physician satisfaction. Diabetes Technol Ther. 2008;10(3):160–8. doi: 10.1089/dia.2008.0283. [DOI] [PubMed] [Google Scholar]
- 20.Bell AM, Fonda SJ, Walker MS, Schmidt V, Vigersky RA. Mobile phone-based video messages for diabetes self-care support. J Diabetes Sci Technol. 2012;6(2):310–9. doi: 10.1177/193229681200600214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiselev AR, Gridnev VI, Shvartz VA, Posnenkova OM, Dovgalevsky PY. Active ambulatory care management supported by short message services and mobile phone technology in patients with arterial hypertension. J Am Soc Hypertens. 2012;6(5):346–55. doi: 10.1016/j.jash.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Morikawa N, Yamasue K, Tochikubo O, Mizushima S. Effect of salt reduction intervention program using an electronic salt sensor and cellular phone on blood pressure among hypertensive workers. Clin Exp Hypertens. 2011;33(4):216–22. doi: 10.3109/10641963.2011.583966. [DOI] [PubMed] [Google Scholar]
- 23.Liu WT, Wang CH, Lin HC, et al. Efficacy of a cell phone-based exercise programme for COPD. The Eur Respir J. 2008;32(3):651–9. doi: 10.1183/09031936.00104407. [DOI] [PubMed] [Google Scholar]
- 24.Peritz DC, Howard A, Ciocca M, Chung EH. Smartphone ECG aids real time diagnosis of palpitations in the competitive college athlete. J Electrocardiol. 2015;48(5):896–9. doi: 10.1016/j.jelectrocard.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Muhlestein JB. QTC intervals can be assessed with the AliveCor heart monitor in patients on dofetilide for atrial fibrillation. J Electrocardiol. 2015;48(1):10–1. doi: 10.1016/j.jelectrocard.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Haberman ZC, Jahn RT, Bose R, et al. Wireless smartphone ECG enables large-scale screening in diverse populations. J Cardiovasc Electrophysiol. 2015;26(5):520–6. doi: 10.1111/jce.12634. [DOI] [PubMed] [Google Scholar]
- 27.Dharmarajan K, Chaudhry SI. New approaches to reduce readmissions in patients with heart failure. JAMA Intern Med. 2016;176(3):318–20. doi: 10.1001/jamainternmed.2015.7993. [DOI] [PubMed] [Google Scholar]
- 28••.Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363(24):2301–9. doi: 10.1056/NEJMoa1010029. This article is the largest randomized controlled trial to test the efficacy of telemonitoring in heart failure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29••.Ong MK, Romano PS, Edgington S, et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the better effectiveness after transition-heart failure (BEAT-HF) randomized clinical trial. JAMA Intern Med. 2016;176(3):310–8. doi: 10.1001/jamainternmed.2015.7712. This article is another large randomized controlled trial testing the efficacy of remote monitoring and transitional care. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaudhry SI, Barton B, Mattera J, Spertus J, Krumholz HM. Randomized trial of Telemonitoring to Improve Heart Failure Outcomes (Tele-HF): study design. J Card Fail. 2007;13(9):709–14. doi: 10.1016/j.cardfail.2007.06.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koehler F, Winkler S, Schieber M, et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation. 2011;123(17):1873–80. doi: 10.1161/CIRCULATIONAHA.111.018473. [DOI] [PubMed] [Google Scholar]
- 32.Piotrowicz E, Baranowski R, Bilinska M, et al. A new model of home-based telemonitored cardiac rehabilitation in patients with heart failure: effectiveness, quality of life, and adherence. Eur J Heart Fail. 2010;12(2):164–71. doi: 10.1093/eurjhf/hfp181. [DOI] [PubMed] [Google Scholar]
- 33.Scherr D, Kastner P, Kollmann A, et al. Effect of home-based telemonitoring using mobile phone technology on the outcome of heart failure patients after an episode of acute decompensation: randomized controlled trial. J Med Internet Res. 2009;11(3):e34. doi: 10.2196/jmir.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seto E, Leonard KJ, Cafazzo JA, Barnsley J, Masino C, Ross HJ. Mobile phone-based telemonitoring for heart failure management: a randomized controlled trial. J Med Internet Res. 2012;14(1):e31. doi: 10.2196/jmir.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Micera S, Bonato P, Tamura T. Gerontechnology. IEEE Eng Med Biol Mag. 2008;27(4):10–4. doi: 10.1109/MEMB.2008.925213. [DOI] [PubMed] [Google Scholar]
- 36.Graafmans JA, Taipale V. Gerontechnology: a sustainable investment in the future. In: Graafmans JA, Taipale V, Charness N, editors. Gerontechnology: a sustainable investment in the future. Amsterdam: Ios Press; 1998. [PubMed] [Google Scholar]
- 37.Schmitter-Edgecombe M, Seelye A, Cook DJ. Ch. 8: Technologies for health assessment, promotion, and assistance: focus on gerontechnology. In: Randolph JJ, editor. Positive Neuropsychology: Evidence-Based Perspecites on Promoting Cognitive Health. Springer; 2013. [Google Scholar]
- 38.Delello JA, McWhorter RR. Reducing the digital divide: connecting older adults to iPad technology. J Appl Gerontol. 2015 doi: 10.1177/0733464815589985. [DOI] [PubMed] [Google Scholar]
- 39•.Pew Research Center. Smartphone ownership—2013 Update. Pew Research Center’s Internet & American Life Project. 2013 This article reports the prevalence of smartphone ownership among adults in the United States. [Google Scholar]
- 40•.Pew Research Center. Older adults and technology use. 2014 This article provides an overview of how older adults interact with specific mobile devices. [Google Scholar]
- 41.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. J Am Coll Cardiol. 2014;64(21):2246–80. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 42.Hickey KT, Reiffel J, Sciacca RR, et al. The utility of ambulatory electrocardiographic monitoring for detecting silent arrhythmias and clarifying symptom mechanism in an urban elderly population with heart failure and hypertension: clinical implications. J Atr Fibrillation. 2010;1(12):663–74. doi: 10.4022/jafib.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lau JK, Lowres N, Neubeck L, et al. iPhone ECG application for community screening to detect silent atrial fibrillation: a novel technology to prevent stroke. Int J Cardiol. 2013;165(1):193–4. doi: 10.1016/j.ijcard.2013.01.220. [DOI] [PubMed] [Google Scholar]
- 44.Hickey KT, Hauser NR, Valente LE, et al. A single-center randomized, controlled trial investigating the efficacy of a mHealth ECG technology intervention to improve the detection of atrial fibrillation: the iHEART study protocol. BMC Cardiovasc Disord. doi: 10.1186/s12872-016-0327-y. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Worringham C, Rojek A, Stewart I. Development and feasibility of a smartphone, ECG and GPS based system for remotely monitoring exercise in cardiac rehabilitation. PLoS One. 2011;6(2):e14669. doi: 10.1371/journal.pone.0014669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alshurafa N, Xu W, Liu JJ, et al. Designing a robust activity recognition framework for health and exergaming using wearable sensors. IEEE J Biomed Health Informatics. 2014;18(5):1636–46. doi: 10.1109/JBHI.2013.2287504. [DOI] [PubMed] [Google Scholar]
- 47.Mercer K, Giangregorio L, Schneider E, Chilana P, Li M, Grindrod K. Acceptance of commercially available wearable activity trackers among adults aged over 50 and with chronic illness: a mixed-methods evaluation. JMIR mHealth uHealth. 2016;4(1):e7. doi: 10.2196/mhealth.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McMahon SK, Lewis B, Oakes M, Guan W, Wyman JF, Rothman AJ. Older adults’ experiences using a commercially available monitor to self-track their physical activity. JMIR mHealth uHealth. 2016;4(2):e35. doi: 10.2196/mhealth.5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brooks GC, Vittinghoff E, Iyer S, et al. Accuracy and usability of a self-administered 6-minute walk test smartphone application. Circ Heart Fail. 2015;8(5):905–13. doi: 10.1161/CIRCHEARTFAILURE.115.002062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Howell J, Strong BM, Weisenberg J, et al. Maximum daily 6 minutes of activity: an index of functional capacity derived from actigraphy and its application to older adults with heart failure. J Am Geriatr Soc. 2010;58(5):931–6. doi: 10.1111/j.1532-5415.2010.02805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berry R. Older people and the internet: towards a system map of digital exclusion. London: 2011. [Google Scholar]
- 52.Alvseike H, Bronnick K. Feasibility of the iPad as a hub for smart house technology in the elderly; effects of cognition, self-efficacy, and technology experience. J Multidiscip Healthc. 2012;5:299–306. doi: 10.2147/JMDH.S35344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bandura A. Self-efficacy: the exercise of control. New York: WH Freeman; 1997. [Google Scholar]
- 54.Hickey KT, Sciacca RR, Gonzalez P, Castillo C, Frulla A. Assessing health literacy in urban patients with implantable cardioverter defibrillators and pacemakers. J Cardiovasc Nurs. 2015;30(5):428–34. doi: 10.1097/JCN.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 55.Piau A, Campo E, Rumeau P, Vellas B, Nourhashemi F. Aging society and gerontechnology: a solution for an independent living? J Nutr Health Aging. 2014;18(1):97–112. doi: 10.1007/s12603-013-0356-5. [DOI] [PubMed] [Google Scholar]
- 56.IMS Institute for Healthcare Informatics. Patient Apps for improved healthcare from novelty to mainstream. Parsippany, NJ: 2013. [Google Scholar]
- 57.IMS Institute for Healthcare Informatics. Use, evidence and remaining barriers to mainstream acceptance. Sep, 2015. Patient adoption of mHealth. [Google Scholar]
- 58.Charani E, Castro-Sanchez E, Moore LS, Holmes A. Do smartphone applications in healthcare require a governance and legal framework? It depends on the application! BMC Med. 2014;12:29. doi: 10.1186/1741-7015-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Masterson Creber R, Maurer MS, Reading M, Hiraldo G, Hickey KT, Iribarren S. Review and analysis of existing mobile phone applications to support heart failure symptom monitoring and self-care using the Mobile Application Rating Scale. JMIR mHealth uHealth. doi: 10.2196/mhealth.5882. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stoyanov SR, Hides L, Kavanagh DJ, Zelenko O, Tjondronegoro D, Mani M. Mobile app rating scale: a new tool for assessing the quality of health mobile apps. JMIR mHealth uHealth. 2015;3(1):e27. doi: 10.2196/mhealth.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Centers for Medicare and Medicaid Services. 2015 https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/telehealthsrvcsfctsht.pdf.
- 62.Centers for Medicare & Medicaid Services. Frequently Asked Questions about Billing Medicare for Chronic Care Management Services. 2016 https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/Payment_for_CCM_Services_FAQ.pdf.
- 63.Pinto MR, De Medici S, Napoli C. Ergonomics, gerontechnology and well-being in older patients with cardiovascular disease. Int J Cardiol. 2000;72(2):187–8. doi: 10.1016/s0167-5273(99)00156-4. [DOI] [PubMed] [Google Scholar]