Abstract
Background
Drug-resistant tuberculosis (TB) undermines control efforts and its burden is poorly understood in resource-limited settings. We performed a systematic review and meta-analysis to provide an up-to-date summary of the extent of drug-resistant TB in Nigeria.
Methods
We searched PubMed, Scopus, Embase, HINARI, AJOL, the Cochrane library, Web of Science, and Google Scholar for reports published before January 31 2017, that included any resistance, mono-resistance or multidrug resistance to anti-TB drugs in Nigeria. Summary estimates were calculated using random effects models.
Results
We identified 34 anti-TB drug resistance surveys with 8002 adult TB patients consisting of 2982 new and 5020 previously-treated cases. The prevalence rate of any drug resistance among new TB cases was 32.0% (95% CI 24.0–40.0%; 734/2892) and among previously-treated cases, the rate was 53.0% (95% CI 35.0–71.0%; 1467/5020). Furthermore, multidrug resistance among new and previously-treated cases was 6.0% (95% CI 4.0–8.0%;161/2502)and 32.0% (95%CI 20.0–44.0; 357/949), respectively. There was significant heterogeneity between the studies (p<0.001, I2 tests). The prevalence of drug-resistant TB varied according to methods of drug susceptibility testing and geographic region of Nigeria.
Conclusion
The burden of drug-resistant TB in Nigeria is high. We recommend that a national anti-TB drug resistance survey be carried out, and strategies for case detection and programmatic management of drug-resistant TB in Nigeria need to be strengthened.
Background
According to the World Health Organisation (WHO) global report 2016, among 10.4 million incident TB cases worldwide, 3.9% are estimated to have had rifampicin- or multidrug-resistant tuberculosis (MDR/RR-TB) in 2015 [1]. In addition, 21% of previously treated TB cases were estimated to have had MDR/RR-TB in the same year [1]. MDR-TB is caused by strains of M. tuberculosis that is resistant to both isoniazid and rifampicin. Drug-resistant TB (DR-TB) patients require prolonged and expensive treatment using second-line medications that are less effective and more toxic [1]. The acquisition or emergence M. tuberculosis resistance may occur from; previous exposures to quinolones [2], use of inferior regimens [3], poor adherence to anti-TB drug, previous TB treatment [4–5], and high human immunodeficiency virus (HIV) co-infection [6–7]. The diagnosis of drug-resistance require patients to be tested for susceptibility to anti-TB drugs, either by conventional (phenotypic) drug susceptibility testing (DST) or rapid molecular diagnostic (genotypic) methods [1]. The WHO recommends all presumptive TB patients to undergo DST although many countries still lack laboratory capacity to achieve this [1]. Therefore, in most low- and middle-income countries, there may be a high level of under-diagnosis and misdiagnosis of DR-TB. However, with the recent recommendation of rapid molecular diagnostic tests as a first-line TB screening test [1], there is progressive increase in reporting of DR-TB in resource-limited settings [8–9].
Nigeria is one of the countries included among the 30 high burden countries for TB, TB/HIV and DR-TB [1]. According to the WHO, the estimated incidence of TB in Nigeria is 322 per 100 000 population with only 15% of the total burden of the disease in the country being notified in 2015 [1]. The WHO estimates that the proportion of patients with MDR/RR-TB is 4.3% among new cases and 25% among previously-treated cases in Nigeria [1]. With the increasing utilisation of the newer molecular diagnostic techniques for TB diagnosis and the current advocacy for a country-wide roll-out by the Nigeria National TB Programme and other development partners [1, 10], several studies have reported on the rates of DR-TB in different cohorts of TB patients across various settings in Nigeria. However, as most of these studies were based on small sample local or facility-based data, a comprehensive analysis of the burden of DR-TB from different parts of Nigeria is urgently needed. Quantification of a reliable estimate of the extent of DR-TB is crucial to guide intervention policies for programmatic management strategies and for antimicrobial resistance monitoring. We therefore conducted a systematic review and meta-analysis of published data to provide a comprehensive and up-to-date assessment of the burden of DR-TB in Nigeria.
Methods
Data sources and search strategy
We searched the following databases: PubMed/MEDLINE, HINARI, Embase, AJOL, the Cochrane library, Web of Science, Google Scholar (top 800 papers), and Scopus for studies published before January 31 2017, which reported on the prevalence or incidence of DR-TB in Nigeria among patients with new or previously treated TB. Keywords from Medical Subject Headings or titles or abstracts of the studies were searched for with the help of Boolean operators (and, or) without language limitations. Search terms used included “tuberculosis” or “Mycobacterium tuberculosis” and “drug resistance” or “drug susceptibility”, anti-TB resistance, DR-TB, MDR/RR-TB, (isoniazid or rifampicin or ethambutol or streptomycin) resistant TB, and Nigeria. Details of the full search strategy for one of the databases are as shown in S1 Table. Additionally, we reviewed the reference lists of primary studies and review articles.
Inclusion and exclusion criteria
All studies in which prevalence of DR-TB were reported in a given period were included. Also, the included original articles must report on some or all of the following: the prevalence of any resistance, mono-resistance or multidrug resistance to anti-TB drugs. Only studies containing data regarding the proportion of DR-TB among new cases or previously-treated cases were included. Also, included were studies that referenced a standard method for phenotypic or genotypic DST for M. tuberculosis against first-line anti-TB drugs (isoniazid, rifampicin, ethambutol, streptomycin). Studies with the following characteristics were excluded from the analysis: studies on non-tuberculous mycobacterium, articles on extrapulmonary TB or childhood TB; studies reporting on prevalence of an undefined mixture of new and previously-treated patients, and studies not evaluating DST based on first-line anti-TB drugs. Editorials, narrative review articles, case reports, and conference abstracts, as well as duplicate publications were excluded from the analysis.
Data extraction and quality assessments
Two reviewers (KNU and AI) independently screened each title and abstract and resolved discrepancies by consensus. We obtained full texts of citations selected for review, and the reviewers extracted all study data independently, resolving discrepancies by consensus. For all studies, the following data were extracted: first author, year of publication, study setting, study enrolment time, DST method, the number of patients investigated, and drug resistance status. KNU and AI independently assessed the quality of the studies using the Joanna Briggs Institute’s critical appraisal checklist for assessment of quality for studies reporting prevalence data [11].
Operational definitions
We used standard definitions as previously described [1, 8–9]. DR-TB among new TB cases refers to resistance among patients who have never received first-line anti-TB drugs. DR-TB among previously-treated TB cases refers resistance among patients who had previously received first-line anti-TB drugs. Mono-resistance was defined as resistance to only one first-line anti-TB drug. MDR-TB was defined as resistance to at least isoniazid and rifampicin. Any drug resistance was defined as resistance to one or more first-line TB drugs regardless of mono-resistance or MDR. Genotypic DST method is defined as a DST technique which utilised a WHO-certified nucleic acid amplification technology (molecular method) to diagnose the drug-resistant TB i.e., Xpert® MTB/RIF and molecular line probe assays. Phenotypic DST method is defined as a DST technique which utilised a WHO-recognised conventional testing method to diagnose the drug-resistant TB i.e., studies which utilised a liquid culture system, or any of the three solid culture methods (proportion method, resistance ratio method, or absolute concentration method) [1,12].
Statistical analysis
The pooled prevalence and 95% confidence intervals (95% CIs) were calculated using random effects model based on the exact binomial method of Hamza et al fitted using metapropin STATA 13.1 (Stata Corp, College Station, Texas, USA) [13–14]. The χ2 based Q statistic and I2 test were used to assess the between-study heterogeneity using two-sided P-values. Begg rank correlation test and funnel plot was used to assess publication bias using the Comprehensive Meta-Analysis software 2.2 (Biostat Inc, USA) [15–16].
Also, Nigeria is divided into two regions (north and south) with this further subdivided into six geopolitical zones (north-west, north-central, north-east, south-west, south-east and south-south) shown in Fig 1. We carried-out sub-group analyses of the DR-TB prevalence rate based on DST (phenotypic or genotypic) method and geographic region (northern versus southern Nigeria).
Fig 1. Map of Nigeria indicating the geopolitical zones of the country.
[Northern region: North-west, North-central, North-east; Southern region: South-west, South-east, South-south].
Results
Characteristics of studies
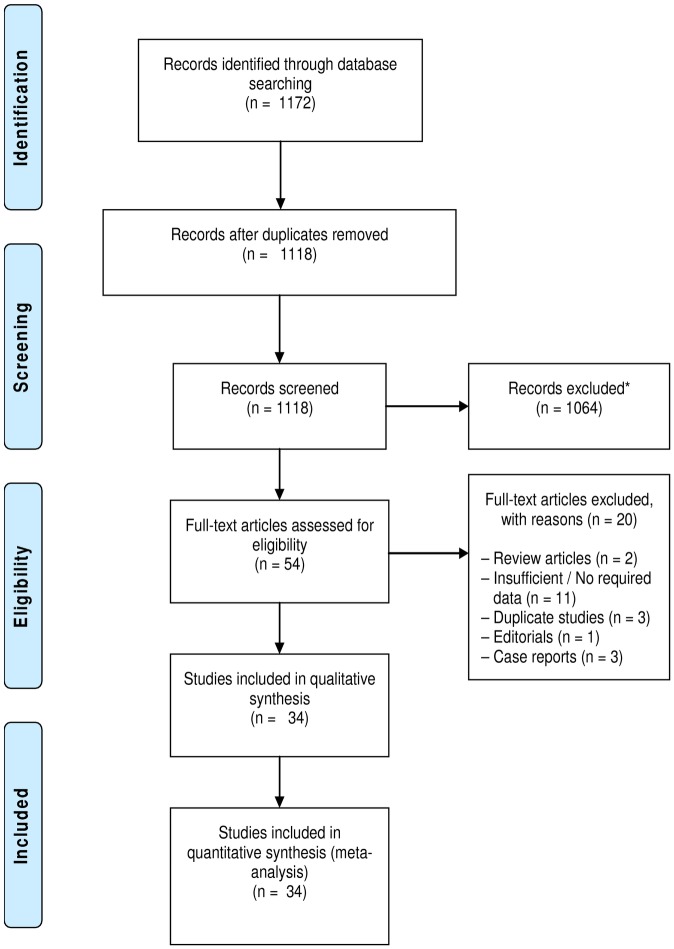
A total of 1172 articles were retrieved by literature search Fig 2. Of these, 1118 articles were retained after duplicates were removed; 1064 of them were excluded due to irrelevance based on their title and abstract, and 54 were retained for full-text evaluation. Finally, after a detailed full-text evaluation, 34 articles published between 1975 and 2016 were included [17–50]. The distribution of the studies and relevant data retrieved for this analysis are summarised in Table 1. Most of the studies were conducted in south-west zone of Nigeria (n = 12) compared with north-central (n = 8), north–west (n = 6), south-east (n = 3), south-south (n = 2) and north-east (n = 1). Overall, 17 studies were from the southern region, 15 were from the northern region and 2 studies were conducted across multiple zones of the country. Regarding DST methods, 23 studies used the phenotypic methods (proportion, absolute concentration and the BACTEC system) and 11 studies used genotypic methods (Xpert MTB/RIF and Genotype MTBDRplus line-probe assays). Of the 34 articles, 11 provided data on new TB cases only, another 11 provided data on retreatment cases only, and 12 provided data on both new and retreatment cases, Table 1. A total of 8002 TB patients consisting of 2982 new and 5020 previously-treated TB cases were analysed.
Fig 2. Flow chart depicting the study selection process.
(*Records excluded due to lack of relevance).
Table 1. Included studies after full-text evaluation.
| First author | Published year | Enrolment time | Geographic zone | Diagnostic method | Total | New cases | Previously-treated cases | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case number | Any | Mono | MDR | Case number | Any | Mono | MDR | ||||||
| Dosunmu | 2008 | 2004–2005 | North-central | Phenotypic | 500 | 500 | 20 | - | 20 | - | - | - | - |
| Lawson | 2010 | 2007 | North-central | Phenotypic | 32 | - | - | - | - | 32 | 14 | 10 | 4 |
| Lawson | 2011 | 2009–2010 | MZ | Phenotypic | 426 | 357 | 259 | 20 | 18 | 69 | 69 | - | 13 |
| Uzoewulu | 2014 | 2009–2011 | South-east | Phenotypic | 180 | 151 | 56 | 23 | 6 | 29 | 27 | 11 | 8 |
| Nwachukwu | 2016 | 2013–2014 | South-east | Genotypic | 389 | 389 | 12 | - | - | - | - | - | - |
| Pokam | 2013 | 2008–2009 | South-south | Phenotypic | 58 | 58 | 35 | 13 | 6 | - | - | - | - |
| Otu | 2013 | 2011–2012 | South-south | Phenotypic | 100 | 100 | 42 | 17 | 4 | - | - | - | - |
| Aghaji | 2010 | 2003–2005 | South-east | Phenotypic | 82 | - | - | - | - | 82 | 81 | 1 | 59 |
| Gidado | 2015 | 2011–2013 | MZ | Genotypic | 3669 | - | - | - | - | 3669 | 815 | - | - |
| Halilu | 2013 | 2011–2012 | North-east | Genotypic | 300 | - | - | - | - | 300 | 22 | - | - |
| Aliyu | 2013 | 2010–2011 | North-west | Genotypic | 375 | 324 | 15 | 12 | 3 | 51 | 8 | 6 | 2 |
| Fawcett | 1975 | 1973 | North-west | Phenotypic | 61 | 61 | 7 | 6 | - | - | - | - | - |
| Rikoto | 2015 | 2013–2014 | North-west | Phenotypic | 81 | 38 | 15 | 5 | 10 | 43 | 43 | 11 | 32 |
| Kolo | 1991 | 1987–1989 | North-west | Phenotypic | 86 | 75 | 41 | 5 | 0 | 11 | 8 | 5 | 1 |
| Adamu | 2015 | 2013–2014 | North-west | Genotypic | 339 | 298 | 11 | 2 | 9 | 41 | 32 | 5 | 27 |
| Rasaki | 2015 | 2013 | North-west | Genotypic | 54 | - | - | - | - | 54 | 10 | - | - |
| Nwofor | 2015 | 2012–2013 | North-central | Phenotypic | 97 | - | - | - | - | 97 | 14 | 8 | 6 |
| Idigbe | 1992 | 1987–1990 | South-west | Phenotypic | 96 | - | - | - | - | 96 | 54 | 33 | - |
| Egbe | 2016 | 2015 | North-central | Genotypic | 91 | 91 | 6 | - | - | - | - | - | - |
| Daniel | 2011 | 2007–2009 | South-west | Phenotypic | 67 | 23 | 3 | 3 | 0 | 44 | 38 | 4 | 34 |
| Oluwaseun | 2013 | 2011 | South-west | Phenotypic | 69 | 69 | 24 | 9 | 9 | - | - | - | - |
| Okodua | 2012 | 2008–2010 | South-west | Phenotypic | 103 | 103 | 45 | 17 | 18 | - | - | - | - |
| Bello | 2014 | 2013 - | South-west | Genotypic | 48 | - | - | - | - | 48 | 9 | - | - |
| Kehinde | 2012 | 2011 | South-west | Genotypic | 24 | - | - | - | - | 24 | 5 | 3 | 1 |
| Kehinde | 2013 | 2011 | South-west | Genotypic | 6 | 6 | 3 | 1 | 2 | - | - | - | - |
| Kehinde | 2007 | 2005–2006 | South-west | Phenotypic | 56 | 56 | 30 | 0 | 30 | - | - | - | - |
| Gehre | 2016 | 2009–2013 | South-west | Phenotypic | 173 | 41 | 19 | 8 | 9 | 132 | 96 | 15 | 75 |
| Olusoji | 2011 | 2007–2009 | South-west | Phenotypic | 88 | 23 | 4 | 2 | 0 | 65 | 51 | 5 | 42 |
| Eltayeb | 2011 | 2007–2010 | South-west | Phenotypic | 82 | - | - | - | - | 82 | 52 | - | 46 |
| Sogaolu | 2012 | 2008–2011 | South-west | Phenotypic | 82 | 68 | 23 | 10 | 4 | 14 | 3 | 2 | 0 |
| Ani | 2009 | 2006–2007 | North-central | Phenotypic | 61 | 50 | 12 | 9 | 2 | 11 | 6 | 3 | 2 |
| Mawak | 2006 | 1997–2000 | North-central | Phenotypic | 35 | 35 | 12 | 8 | 0 | - | - | - | - |
| Ukaegbu | 2016 | 2013 | North-central | Genotypic | 83 | 66 | 40 | 29 | 11 | 17 | 6 | 5 | 1 |
| Ukoli | 2012 | 2008–2010 | North-central | Phenotypic | 9 | - | - | - | - | 9 | - | - | 4 |
MZ = multiple zones; (–) indicates not applicable Any = any resistance; Mono = mono-resistance; MDR = multidrug resistance
Quality assessment
Across the 10 quality domains evaluated, majority of the studies met five or more of the quality criteria. Most of the studies (n = 19) met 7 to 10 of the quality criteria assessed, and others (n = 15) met 5 to 6 of the quality criteria assessed for prevalence studies, S2 Table. The most common quality criteria failed by the studies were: inadequate sample size, poor statistical analytical strategy, not evaluating for confounders and non-reporting of results for sub-groups S2 Table.
Anti-tuberculosis resistance among new TB patients
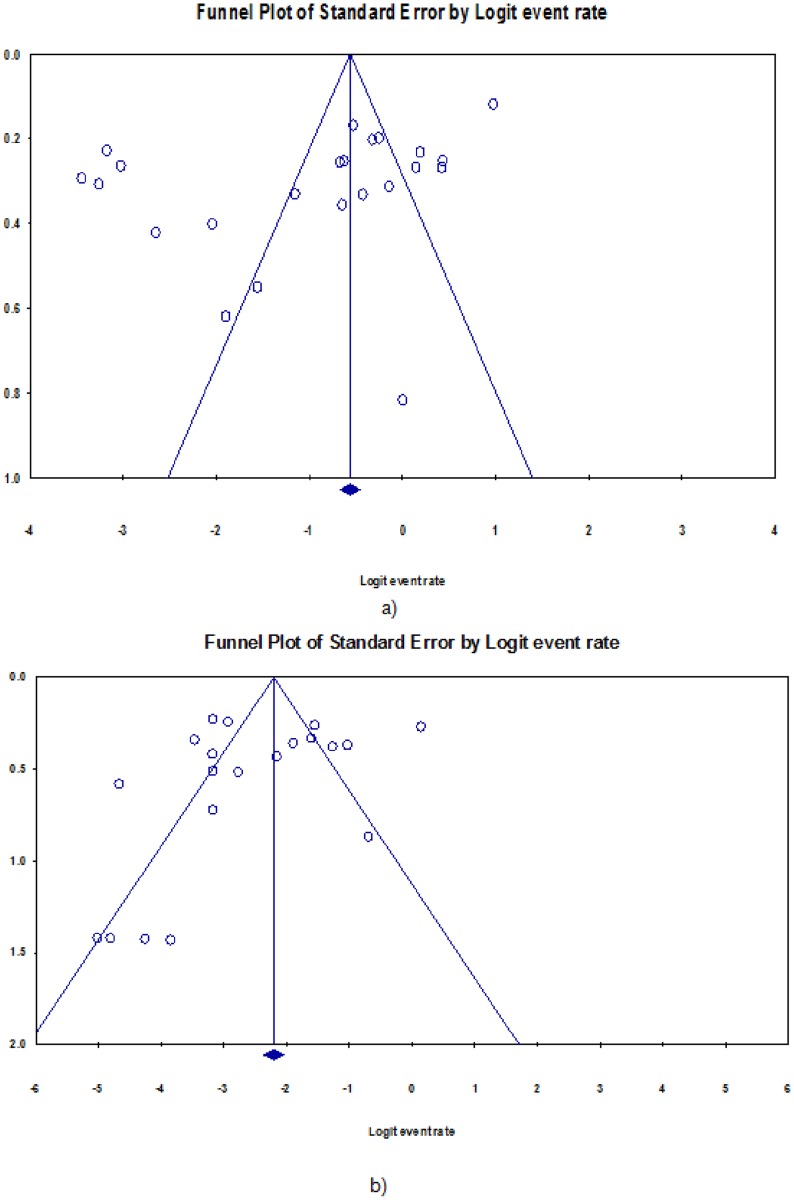
Table 2 presents the pooled analysis of the burden of DR-TB among newly diagnosed TB patients in Nigeria. The prevalence of any drug resistance among new cases was 32.0% (95% CI 24.0–40.0%; 734/2892). However, evident heterogeneity was observed (P <0 .001). Fig 3 shows the forest plot of the meta-analysis of any anti-TB resistance in new TB cases. Also, as shown in Fig 4, little evidence for publication bias was observed (Begg rank correlation analysis P = 0.101). There was a wide variation in the rate of any resistance among new TB cases between studies utilising phenotypic and genotypic methods of DST (37.0 vs. 12.0%) respectively; and the difference was significant (χ2 = 8.56, P < 0.001). In addition, pooled rates for the northern region (21.0%) were lower compared to the southern Nigeria (36.0%); but the between group difference was not significant (χ2 = 3.08, P = 0.08). The rate of anti-TB mono-resistance among new patients was 13.0% (95% CI 10.0–17.0%; 199/2002); this rate varied with region and DST methods, Table 2. Overall, the pooled prevalence of MDR-TB among new TB patients in Nigeria was 6.0% (95% CI 4.0–8.0%;161/2502), there was little variation according to DST method—occurring in 4.0% and 7.0% of patients diagnosed using genotypic and phenotypic methods, respectively; (χ2 = 1.45, P = 0.23). Also, we observed little evidence for publication bias (Begg rank correlation analysis P = 0.601) and Fig 4. The pooled prevalence of MDR-TB was 3.0% (95% CI 1.0–5.0; 55/1447) in northern and 12.0% (95% CI, 7.0–18.0; 88/698) in southern Nigeria; (χ2 = 9.59, P < 0.001). S1 Fig shows the forest plot of the meta-analysis of MDR-TB in new TB cases in Nigeria.
Table 2. Pooled event rates of drug resistant tuberculosis among newly-diagnosed TB patients, Nigeria.
| Variables | Sub-group | Pooled event rate % (95% CI) | n/N | No. of Studies | Heterogeneity I2 (P -value) |
|---|---|---|---|---|---|
| Any drug resistance | Total | 32.0 (24.0–40.0) | 734/2892 | 23 | 98.3 (<0.001) |
| Stratified by DST | |||||
| Genotypic | 12.0 (6.0–18.0) | 87/1174 | 6 | 94.8 (<0.001) | |
| Phenotypic | 37.0 (22.0–52.0) | 647/1808 | 17 | 98.4 (<0.001) | |
| Stratified by regiona | |||||
| North | 21.0 (15.0–28.0) | 179/1538 | 10 | 95.6 (<0.001) | |
| South | 36.0 (21.0–51.0) | 296/1087 | 12 | 97.0 (<0.001) | |
| Mono-drug resistance | Total | 13.0 (10.0–17.0) | 199/2002 | 20 | 89.9 (<0.001) |
| Stratified by DST | |||||
| Genotypic | 11.0 (4.0–18.0) | 44/694 | 4 | 94.7 (<0.001) | |
| Phenotypic | 13.0 (10.0–17.0) | 155/1308 | 16 | 64.6 (0.001) | |
| Stratified by regiona | |||||
| North | 12.0 (0.07–0.17) | 76/947 | 8 | 90.0 (<0.001) | |
| South | 16.0 (13.0–18.0) | 103/698 | 11 | 0 (0.90) | |
| Multidrug resistance | Total | 6.0 (4.0–8.0) | 161/2502 | 21 | 86.1 (<0.001) |
| Stratified by DST | |||||
| Genotypic | 4.0 (0.0–8.0) | 25/694 | 4 | 82.5 (<0.001) | |
| Phenotypic | 7.0 (4.0–10.0) | 136/1808 | 17 | 86.2 (<0.001) | |
| Stratified by regiona | |||||
| North | 3.0 (1.0–5.0) | 55/1447 | 9 | 77.5 (<0.001) | |
| South | 12.0 (7.0–18.0) | 88/698 | 11 | 87.2 (<0.001) |
DST = drug susceptibility testing method; TB = tuberculosis;
a = studies from multiple regions excluded
Fig 3. Forest plot of the meta-analysis on any drug resistance in new TB cases.
[CI: confidence interval].
Fig 4. Funnel plot of the meta-analysis on prevalence of: a) any drug resistance, b) multidrug resistance among newly-diagnosed TB patients, Nigeria.
Anti-tuberculosis resistance among previously-treated TB patients
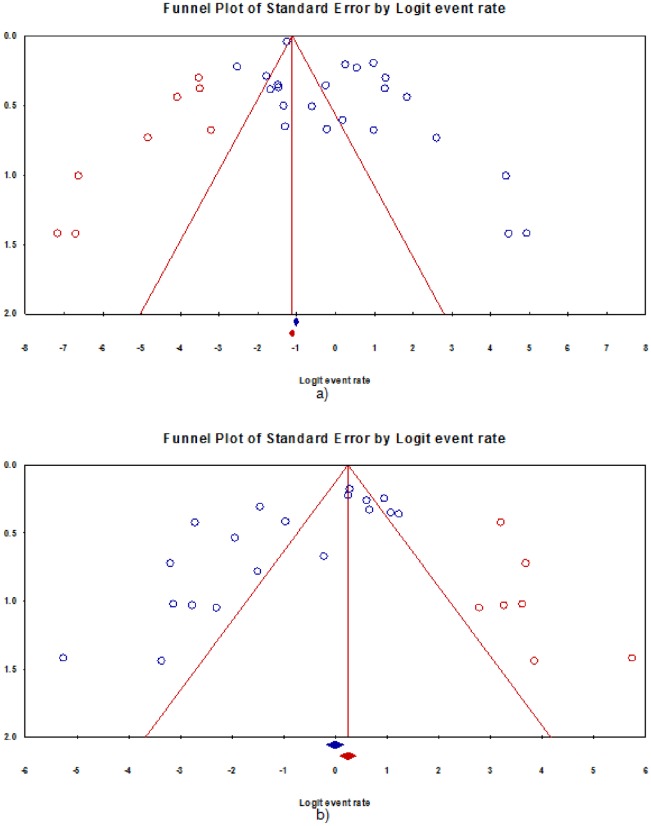
The pooled analyses of the burden of DR-TB among previously-treated TB patients in Nigeria are as shown in Table 3. The rate of any resistance among previously-treated TB patients was 53.0% (95% CI 35.0–71.0%; 1467/5020). Fig 5 shows the forest plot of the meta-analysis of any anti-tuberculosis resistance in previously-treated TB patients. There was no statistical evidence for publication bias (Begg rank correlation analysis P = 0.37); however analysis of the funnel plot Fig 6 suggest that there is publication bias (Duval and Tweedie’s trim and fill indicate that there might be eight missing studies to the left of the mean). There was significant heterogeneity between studies (p<0.001). In addition, there was a wide variation in the rate of any resistance among previously-treated TB cases between studies utilising phenotypic and genotypic methods of DST (62.0 vs 26.0%) respectively (χ2 = 11.8, P <0.001). Furthermore, pooled rates for northern region (36.0%) was significantly lower compared with 62.0% observed among previously-treated TB patients in Southern Nigeria (χ2 = 4.76, P = 0.03). Overall the rate of mono-resistance to anti-TB drugs among previously-treated TB patients was 17.0% (95% CI 11.0–23.0; 127/789). This did not substantially vary across region and DST categories, Table 3. The pooled prevalence of MDR-TB among previously treated TB patients was 32.0% (95%CI 20.0–44.0; 357/949); there was some variation in the rate according to DST method—occurring in 19.0% and 36.0% of patients diagnosed using genotypic and phenotypic methods, respectively; (χ2 = 1.71, P = 0.19). Some evidence of publication bias was observed (Begg rank correlation analysis P = 0.06), and analysis of the funnel plot, Fig 6 suggest that there might be some publication bias (Duval and Tweedie’s trim and fill indicate that there might be seven missing studies to the right of the mean). Also, the rate of MDR-TB among previously-treated TB patients in Nigeria was 26.0% in the northern and 40.0% in the southern region (χ2 = 1.02, P = 0.31). S2 Fig shows the forest plot of the meta-analysis of MDR-TB in previously-treated TB cases in Nigeria.
Table 3. Pooled event rates of drug resistant tuberculosis among previously-treated TB patients, Nigeria.
| Variables | Sub-group | Pooled event rate % (95% CI) | n/N | No. of Studies | Heterogeneity I2 (P -value) |
|---|---|---|---|---|---|
| Any drug resistance | Total | 53.0 (35.0–71.0) | 1467/5020 | 23 | 99.5 (<0.001) |
| Stratified by DST | |||||
| Genotypic | 26.0 (16.0–36.0) | 907/4204 | 8 | 95.7 (0.001) | |
| Phenotypic | 62.0 (44.0–80.0) | 560/816 | 15 | 98.2 (0.001) | |
| Stratified by regiona | |||||
| North | 36.0 (22.0–51.0) | 167/666 | 11 | 94.7 (0.001) | |
| South | 62.0 (44.0–80.0) | 416/616 | 10 | 97.8 (0.001) | |
| Monodrug resistance | Total | 17.0 (11.0–23.0) | 127/789 | 16 | 85.3% (0.001) |
| Stratified by DST | |||||
| Genotypic | 13.0 (8.0–19.0) | 19/133 | 4 | 0.0 (0.51) | |
| Phenotypic | 18.0 (11.0–25.0) | 108/656 | 12 | 88.5 (<0.001) | |
| Stratified by regiona | |||||
| North | 19.0 (12.0–27.0) | 53/303 | 8 | 63.9 (0.01) | |
| South | 15.0 (0.07–0.23) | 74/486 | 8 | 89.8 (<0.001) | |
| Multidrug resistance | Total | 32.0 (20.0–44.0) | 357/949 | 19 | 97.9 (<0.001) |
| Stratified by DST method | |||||
| Genotypic | 19.0 (1.0–39.0) | 31/133 | 4 | 95.3 (<0.001) | |
| Phenotypic | 36.0 (21.0–51.0) | 326/816 | 15 | 98.3 (<0.001) | |
| Stratified by regiona | |||||
| North | 26.0 (10.0–41.0) | 79/312 | 9 | 95.0 (0.001) | |
| South | 40.0 (17.0–63.0) | 265/568 | 9 | 98.8 (0.001) |
DST = drug susceptibility testing method; TB = tuberculosis;
a = studies from multiple regions excluded
Fig 5. Forest plot of the meta-analysis on any drug resistance in previously-treated cases.
[CI: confidence interval].
Fig 6. Funnel plot of the meta-analysis on prevalence of: a) any drug resistance, b) multidrug resistance among previously-treated TB patients, Nigeria.
Discussion
Our analyses showed that 32.0% of newly diagnosed cases and 53.0% of previously-treated TB patients from different settings in Nigeria were resistant to at least one anti-TB medication. Furthermore, we found that the burden of MDR-TB was high—occurring in 6.0% of new and 32.0% of previously-treated TB patients. In addition, we found that the pooled burden of DR-TB across new and previously-treated cases varied substantially across geographic regions of the country and the DST methods used.
In this study, we found that almost a third of newly-diagnosed TB patients were resistant to at least one first-line anti-TB drugs. This suggests that there is a high rate of primary drug resistance among treatment-naive individuals diagnosed for the first time with TB. The existence of high rates of DR-TB among treatment-naïve individuals may be a reflection of active transmission of DR-TB in the community from the infectious DR-TB patients who are not on treatment. The prevalence of any DR-TB observed in this study is similar to the recent comprehensive estimates from Iran, China and Ethiopia; but lower than rates reported from Burundi and Portugal [7–8, 51–53]. In addition, the 6.0% prevalence of MDR-TB among new patients observed in this study is above the current WHO estimates of 4.3% (3.2–5.4%) for Nigeria [1]. This suggests that the burden of MDR-TB among new cases may be grossly underestimated and greater programmatic strategies are needed to detect and treat them. Since 2016, the NTP has adopted an algorithm positioning Xpert MTB/RIF as the initial diagnostic test for all persons with signs and symptoms of pulmonary TB [1]. It is expected that as more cases of DR-TB cases are detected and treated there will be a reduction in community transmission of primary anti-TB drug resistance in the country.
Also, we found that over half of previously-treated TB patients in Nigeria were resistant to at least one first-line anti-TB drug, and overall 32.0% of this group of patients had MDR-TB. This indicates that there is a high level of acquired resistance to anti-TB medications in the country. The pooled prevalence of any drug resistance among previously-treated TB patients observed fell within the range in Ethiopia (11.1 to 72.9%) and was similar to the rate reported by a review in China (49.8%), but lower than the rate reported from Iran (65.6%) [8–9, 51]. This indicates that in low- and middle-income countries there may be high rates of acquired resistance to anti-TB medications. The most common determinant of the occurrence of this drug resistance is the failure of the appropriate treatment of TB patients. Programmatically this could be from improper prescription of anti-TB treatment regimens, inadequate drug supply, poor quality of drugs, high default and treatment failure rates [3–6, 54–56]. In addition, once selected, drug resistant strains of M. tuberculosis may be transmitted in the community. From our review, we cannot determine the drivers of drug resistant TB in Nigeria hence the need for further studies. In our meta-analysis, 32.0% of previously-treated cases had MDR-TB. The rate of MDR-TB observed among previously-treated cases was higher than the global average (20.5%) and the current WHO estimates for Nigeria (25%) (19–31%) [1]. This suggests that the NTP need to strengthen the management of drug susceptible TB in order to bridge the gaps identified; and conduct a nationwide survey of DR-TB in Nigeria to provide a more accurate estimate of the burden, to inform programmatic management of DR-TB.
In this study, stratified analyses were performed according to the geographic region and DST method used. Except two studies that collected data from multiple regions of Nigeria, most of the included studies were conducted either in the northern or southern region of Nigeria. Our analyses for both new and previously-treated TB patients indicate that there is a distinct pattern in the rates of DR-TB in Nigeria—overall the rates were lower in the northern compared to the southern part of the country. For example, among new patients, the rate of any resistance in the northern versus southern part of Nigeria was 21.0% and 36.0%, respectively. Similarly, among previously treated TB patients the rates of any resistance in the northern versus southern part of Nigeria was 36.0% and 62.0%, respectively. This north-south disparity was also observed in the rates of MDR-TB among new and previously-treated TB cases. Although the regional differences between the estimates for most of the sub-groups in the northern and southern region of Nigeria did not reach statistical significance (probably because of the small sample size in most of the studies), the observed differences may be because most early studies on DR-TB were conducted in southern Nigeria where the only TB national reference laboratory available then was located. At least one TB reference laboratory is now available per zone in the country; these findings suggest a need to strengthen the detection and treatment of DR-TB cases in all parts of the country. Also, we observed a variation in the rates of DR-TB according to DST technique with studies utilising genotypic methods detecting fewer rates of resistance compared with those that used phenotypic methods. This may be because current genotypic methods limit their anti-TB drug resistance screening to one or two anti-TB drugs only, and again most of the earlier studies included utilised phenotypic methods (23 phenotypic verses 11 genotypic).
Our study had several strengths. In total we identified 34 anti-TB drug resistance surveys, which allowed us to pool results from 8002 patients with TB who underwent DST for possible detection of resistance. There are some limitations, however. First, our analyses may not fully represent the prevalence of drug resistance in TB in Nigeria because the magnitude of drug resistance has not yet been fully investigated in some parts of the country particularly the north-eastern zone (1 study only) which has been facing security challenges from the unpredictable Boko Haram terrorist group in the last decade. Second, due to unavailability of data from the primary studies, the potential effect of age, sex, ethnicity, socioeconomic status and life style of the patients on DR-TB prevalence could not be analysed. Third, although Nigeria has a high burden of TB-HIV co-infection [1, 57]; due to unavailability of data, we did not evaluate for the impact of HIV on DR-TB in Nigeria. Two recent reviews indicate that HIV is not a driver of DR-TB in Sub-Saharan Africa [58–59]. Fourth, due to inadequate number of studies, we are unable to conduct a meta-regression analysis to evaluate for statistical differences in prevalence of drug-resistant TB in Nigeria between and within region and DST methods. Finally, although our analyses indicated some evidence for its existence, potential publication bias could not be completely excluded.
In conclusion, our systematic review and meta- analysis showed high levels of MDR-TB more than the WHO estimates for Nigeria. This study has important policy implications. Due to the high rate of anti-TB drug resistance observed, this study affirms the Nigeria National Tuberculosis Programme’s decision to switch to molecular DST screening test as the initial diagnostic test for all people with signs and symptoms of pulmonary TB. Furthermore, it indicates the need to strengthen strategies for the detection and programmatic management of drug-susceptible and drug-resistant TB in Nigeria, as well as sustained monitoring of anti-TB drug resistance in the country. It is our recommendation that a national anti-TB drug resistance survey be carried out in all geopolitical regions of Nigeria to determine the actual prevalence of DR-TB using the existing available genotypic DST methods. This survey should also assess the potential effects of social and economic determinants (e.g., age, sex, place of residence, socio-economic status, literacy, family income) on the prevalence of DR-TB in Nigeria.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOC)
(TIF)
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.World Health Organisation. Global Tuberculosis Control: WHO report 2016. Geneva, Switzerland: World Health Organisation, 2016.
- 2.Deutschendorf C, Goldani LZ, Pires dos Santos R. Previous use of quinolones: a surrogate marker for first line anti-tuberculosis drugs resistance in HIVinfected patients? Braz J Infect Dis. 2012; 16:142–145. [DOI] [PubMed] [Google Scholar]
- 3.van der Werf MJ, Langendam MW, Huitric E, Manissero D. Multidrug resistance after inappropriate tuberculosis treatment: a metaanalysis. Eur Respir J. 2012; 39: 1511–1519. doi: 10.1183/09031936.00125711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao P, Li XJ, Zhang SF, Wang XS, Liu CY. Social behavior risk factors for drug resistance tuberculosis in main land china: a meta-analysis. J Int Med Res. 2012; 40:436–445. doi: 10.1177/147323001204000205 [DOI] [PubMed] [Google Scholar]
- 5.Blöndal K. Barriers to reaching the targets for tuberculosis control: multidrug-resistant tuberculosis. Bull World Health Organ. 2007; 85: 387394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jindani AN, Enarson DA. Two 8-month regimens of chemotherapy for treatment of newly-diagnosed tuberculosis: international multicentre randomised trial. Lancet. 2004;8:1244–1251 [DOI] [PubMed] [Google Scholar]
- 7.Suchindran S, Brouwer ES, Van Rie A. Is HIV infection a risk factor for multi-drug resistant tuberculosis? a systematic review. PLoS One. 2009;4:e5561 doi: 10.1371/journal.pone.0005561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan Q, Chen Z, Chen C,Zhang Z, Lu Z, Yang Y, et al. The Prevalence of drug resistant tuberculosis in mainland China:an updated systematic review and meta-analysis. PLoS One. 2016;11:e0148041 doi: 10.1371/journal.pone.0148041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasiri MJ, Dabiri H, Darban-Sarokhalil D, Rezadehbashi M, Zamani S. Prevalence of drug-resistant tuberculosis in Iran: systematic review and meta-analysis. Am J Infect Control. 2014;42: 1212–1218 doi: 10.1016/j.ajic.2014.07.017 [DOI] [PubMed] [Google Scholar]
- 10.Federal Ministry of Health. National Tuberculosis, Leprosy and Buruli ulcer management and control guidelines. 6th Edn Federal Ministry of Health, Abuja, September 2015 [Google Scholar]
- 11.The Joanna Briggs Institute. Joanna Briggs Institute Reviewers’ Manual. Adelaide, SA, Australia: The Joanna Briggs Institute; 2014. [Google Scholar]
- 12.World Health Organization; Guidelines for surveillance of drug resistance in tuberculosis–5th edition Geneva, World Health Organization, 2015. [Google Scholar]
- 13.Hamza TH, van Houwelingen HC, Stijnen T. The binomial distribution of meta-analysis was preferred to model within-study variability. J Clin Epidemiol. 2008;61:41–51. doi: 10.1016/j.jclinepi.2007.03.016 [DOI] [PubMed] [Google Scholar]
- 14.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39 doi: 10.1186/2049-3258-72-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50:1088–1101. [PubMed] [Google Scholar]
- 16.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000; 56:455–63. [DOI] [PubMed] [Google Scholar]
- 17.Dosunmu EA, Osagie K, Shuaib A, Lawson L. Multidrug-resistant tuberculosis at the National Hospital, Abuja, Nigeria. Afr J Respir Med. 2008; 4: 22–23. [Google Scholar]
- 18.Lawson L, Habib AG, Okobi MI, Idiong D, Olajide I, Emenyonu N, et al. Pilot study on multidrug resistant tuberculosis in Nigeria. Ann Afr Med. 2010; 9:184–187. doi: 10.4103/1596-3519.68355 [DOI] [PubMed] [Google Scholar]
- 19.Lawson L, Yassin MA, Abdurrahman ST, Parry CM, Dacombe R, Sogaolu OM, et al. Resistance to first-line tuberculosis drugs in three cities of Nigeria. Trop Med Int Health. 2011; 16:974–980. doi: 10.1111/j.1365-3156.2011.02792.x [DOI] [PubMed] [Google Scholar]
- 20.Uzoewulu NG, Ibeh IN, Lawson L, Goyal M, Umenyonu N, Ofiaeli RO,et al. Drug resistant Mycobacterium tuberculosis in tertiary hospital South East, Nigeria. J Med Microb Diagn. 2014; 3:2 [Google Scholar]
- 21.Nwachukwu NO, Onyeagba RA, Nwaugo VO, Ononiwu HA, Okafor DC. Diagnostic accuracy of Xpert MTB/RIF assay in diagnosis of pulmonary tuberculosis. J Infec Dis Treat. 2016; 2:1 [Google Scholar]
- 22.Pokam BT, Asuquo AE, Abia-Bassey LN, Idasa MB, Umoh NO, Eko FO,et al. Multidrug resistance and demography of newly diagnosed tuberculosis patients in Cross River State, Nigeria. Int J Mycobacteriol. 2013; 2:89–93. doi: 10.1016/j.ijmyco.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 23.Otu A, Umoh V, Habib A, Ameh S, Lawson L, Ansa V. Drug resistance among pulmonary tuberculosis patients in Calabar, Nigeria. Pulm Med. 2013; 2013:235190 doi: 10.1155/2013/235190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aghaji MN, Nwakoby BAN. Drug-resistance in chronic tuberculosis cases in Southern Nigeria. Niger J Clin Pract. 2010;13:58–63 [Google Scholar]
- 25.Mustapha G, Jumoke O, Nwadike P, Emeka E, Akang G, Eneogu R,et al. Assessment of Gene-xpert MTB RIF program implementation and the challenges for enhanced tuberculosis diagnosis in Nigeria. SAARC J Tuberc Lung Dis HIV/AIDS. 2015. XII(2): 1–7 [Google Scholar]
- 26.Halilu TB, Bala Z, Sado F, Yerima BI. Multi-drug resistance tuberculosis (MDR-TB) survey in North East Nigeria. J Pharm Cosmet Sci. 2013; 1:45–52 [Google Scholar]
- 27.Aliyu G, El-Kamary SS, Abimiku A, Ezati N, Mosunmola I, Hungerford L, et al. Mycobacterial etiology of pulmonary tuberculosis and association with HIV infection and multidrug resistance in northern Nigeria. Tuberc Res Treat. 2013;2013:650561 doi: 10.1155/2013/650561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fawcett IW, Watkins BJ. Initial resistance of Mycobacterium tuberculosis in Northern Nigeria. Tubercle. 1975;57:71–73 [DOI] [PubMed] [Google Scholar]
- 29.Rikoto JA. Pattern of first-line anti-tuberculosis drug resistance and associated factors in patients attending national tuberculosis and leprosy training centre and referral hospital Zaria. 2015 PhD thesis: Zaria, Nigeria: Ahmadu Bello University.
- 30.Kolo I. Bacteriological and drug sensitivity studies on Mycobacteria isolated from tuberculosis patients and their close contacts in ABUTH, Zaria, Nigeria. 1991; PhD Thesis, Zaria, Nigeria.
- 31.Adamu AU, Hafiz TR. Multi-drug resistant tuberculosis pattern in Kano metropolis, Nigeria. J Am Sci. 2015;11:293–296 [Google Scholar]
- 32.Rasaki SO, Ajibola AA, Musa SA, Moradeyo AK, Odeigah LO, Abdullateef SG,et al. Rifampicin resistant tuberculosis in a secondary health institution in Nigeria, West Africa. J Infect Dis Ther. 2014;2:3 [Google Scholar]
- 33.Nwofor AC, Nyamngee A, Nwabuisi C, Iwakun M, Gidado M, Mensah C, et al. Performance of genotype MTBDRplus in the detection of resistance to rifampicin and isoniazid among clinical mycobacteria isolates in Ilorin, Nigeria. Curr HIV Res. 2015; 13: 308–314 [DOI] [PubMed] [Google Scholar]
- 34.Idigbe EO, Duque JP, John EK, Annam O. Resistance to antituberculosis drugs in treated patients in Lagos, Nigeria. J Trop Med Hyg. 1992;95:18691. [PubMed] [Google Scholar]
- 35.Egbe K, Ike AC, Aleruchi C. Prevalence of tuberculosis and rifampicin resistance among patients seeking medical care in Nasarawa State, north central Nigeria. Sci J Public Health. 2016;4:214–218 [Google Scholar]
- 36.Daniel O, Osman E, Bakare R, Adebiyi P, Ige O, Ogiri S, et al. Ofloxacin resistance among Mycobacterium tuberculosis isolates in two states of south-west Nigeria. Afr J Respir Med. 2011; 6:18–20 [Google Scholar]
- 37.Oluwaseun E, Akinniyi AP, Afolabi O. Primary multi-drug resistant tuberculosis among HIV seropositive and seronegative patients in Abeokuta, southwestern Nigeria. Am J Res Comm. 2013;1:224–237 [Google Scholar]
- 38.Okodua M, Ihongbe J, Esumeh F. Pulmonary tuberculosis and resistance pattern to first line antituberculosis drugs in a city of western Nigeria. Int J Basic Appl Innov Res. 2012;1: 48–56 [Google Scholar]
- 39.Bello LA, Shittu MO, Shittu BT, Oluremi AS, Akinnuroju ON, Adekola SA. Rifampicin-monoresistant Mycobacterium tuberculosis among the patients visiting chest clinic, state specialist hospital, Akure, Nigeria. Int J Res Med Sci. 2014;2:1134–1137 [Google Scholar]
- 40.Kehinde AO, Adetoye AE. Diagnosis of pulmonary tuberculosis using genotype MTBDRPLUS assay in three local government primary health centres of Osun State, Nigeria- a pilot study. J Med Microb Diagn. 2012;S3:001. [Google Scholar]
- 41.Kehinde AO, Adebiyi EO. Molecular diagnosis of MDR-TB using GenoType MTBDRplus 96 assay in Ibadan, Nigeria. Niger J Physiol Sci. 2013; 28:187–191 [PubMed] [Google Scholar]
- 42.Kehinde AO, Obaseki FA, Ishola OC, Ibrahim KD. Multidrug resistance to Mycobacterium tuberculosis in a tertiary hospital J Natl Med Assoc. 2007;99:1185–9 [PMC free article] [PubMed] [Google Scholar]
- 43.Gehre F, Otu J, Kendall L, Forson A, Kwara A, Kudzawu S,et al. The emerging threat of pre-extensively drug-resistant tuberculosis in West Africa: preparing for large-scale tuberculosis research and drug resistance surveillance. BMC Med. 2016;14:160 doi: 10.1186/s12916-016-0704-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olusoji D, Eltayeb O. Prevalence and risk factors associated with drug resistant TB in Southwest, Nigeria. Asian Pac J Trop Med. 2011; 4:148–151. doi: 10.1016/S1995-7645(11)60057-6 [DOI] [PubMed] [Google Scholar]
- 45.Eltayeb O, Daniel O, Ogiri S, Awe A, Obasanya O, Adebiyi E,et al. Resistance of Mycobacterium tuberculosis to first and second line anti tuberculosis drugs in South West, Nigeria. J Pulmon Resp Med. S6:001. [Google Scholar]
- 46.Sogaolu OM, Ige OM, Lawson L, Akinyemi J, Lawal O. Pattern of resistance to first line antituberculosis drugs in Ibadan, Nigeria—preliminary observations. Am J Respir Crit Care Med. 2012;185:A3257 [Google Scholar]
- 47.Ani AE, Idoko J, Dalyop YB, Pitmang SL. Drug resistance profile of Mycobacterium tuberculosis isolates from pulmonary tuberculosis patients in Jos, Nigeria. Trans R Soc Trop Med Hyg. 2009;103:67–71. doi: 10.1016/j.trstmh.2008.08.004 [DOI] [PubMed] [Google Scholar]
- 48.Mawak JD, Gomwalk NE, Bello CSS, Kandakai-Olukemi YT. Drug susceptibility pattern of mycobacterium tuberculosis among, pulmonary tuberculosis patients in Jos, Nigeria. Nig J Exp Appl Biol. 2006; 7: 128–133 [Google Scholar]
- 49.Ukaegbu CO, Ani A, Nnachi AU. Molecular detection of mycobacterium tuberculosis complex by genotype®MTBDRPlus from patients attending Bingham University Teaching Hospital, Jos, Nigeria. Br J Pharm Res. 2016; 9: 1–11 [Google Scholar]
- 50.Ukoli CO, Akanbi MO, Adiukwu CV, Amusa GA, Akanbi FO. Diagnosing tuberculosis in resource limited settings: experience from a referral TB clinic in North Central Nigeria. Jos J Med. 2012;6:26–27 [Google Scholar]
- 51.Weldegebreal S, Mebrahtu T. Anti-tuberculosis drug resistance in Ethiopia: systematic review. Int J Tuberc Lung Dis. 2017;21:18–22 doi: 10.5588/ijtld.16.0286 [DOI] [PubMed] [Google Scholar]
- 52.Sanders M, Van Deun A, Ntakirutimana D, Masabo JP, Rukundo J, Rigouts L, et al. Rifampicin mono-resistant Mycobacterium tuberculosis in Bujumbura, Burundi: results of a drug resistance survey. Int J Tuberc Lung Dis. 2006; 10: 178–183. [PubMed] [Google Scholar]
- 53.Antunes ML, Aleixo-Dias J, Antunes AF, Pereira MF, Raymundo E, Rodrigues MF. Anti-tuberculosis drug resistance in Portugal. Int J Tuberc Lung Dis. 2000; 4: 223–231. [PubMed] [Google Scholar]
- 54.Van der Werf MJ, Langendam MW, Huitric E, Manissero D. Multidrug resistance after inappropriate tuberculosis treatment: a meta-analysis. Eur Respir J. 2012; 39: 1511–1519. doi: 10.1183/09031936.00125711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Umubyeyi AN, Shamputa IC, Rigouts L, Dediste A, Karita E, Struelens MJ, et al. Molecular investigation of recurrent tuberculosis in patients from Rwanda. Int J Tuberc Lung Dis. 2007; 11: 860–867. [PubMed] [Google Scholar]
- 56.Alobu I, Oshi SN, Oshi DC, Ukwaja KN. Risk factors of treatment default and death among tuberculosis patients in a resource-limited setting. Asian Pac J Trop Med. 2014;7:977–984 doi: 10.1016/S1995-7645(14)60172-3 [DOI] [PubMed] [Google Scholar]
- 57.Ifebunandu NA, Ukwaja KN, Obi SN. Treatment outcome of HIV-associated tuberculosis in a resource-poor setting. Trop Doct. 2012;42:74–76. doi: 10.1258/td.2011.110421 [DOI] [PubMed] [Google Scholar]
- 58.Lukoye D, Ssengooba W, Musisi K, Kasule GW, Cobelens FG, Joloba M,et al. Variation and risk factors of drug resistant tuberculosis in sub-Saharan Africa: a systematic review and meta-analysis. BMC Public Health. 2015;15:291 doi: 10.1186/s12889-015-1614-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berhan A, Berhan Y, Yizengaw D. A meta-analysis of drug resistant tuberculosis in Sub-Saharan Africa: how strongly associated with previous treatment and HIV co-infection? Ethiop J Health Sci. 2013;23:271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOC)
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.