Abstract
Introduction
Stillbirth has a long-lasting impact on parents and families. This study examined socio-economic predictors associated with stillbirth in Nepal for the year 2001, 2006 and 2011.
Methods
The Nepalese Demographic and Health Survey (NDHS) data for the period (2001–2011) were pooled to estimate socio-economic predictors associated with stillbirths in Nepal using binomial logistic regression while taking clustering and sampling weights into account.
Results
A total of 18,386 pregnancies of at least 28 weeks gestation were identified. Of these pregnancies, 335 stillbirths were reported. Stillbirth increased significantly among women that lived in the hills ecological zones (aRR 1.38, 95% CI 1.02, 1.87) or in the mountains ecological zones (aRR 1.71, 95% CI 1.10, 2.66). Women with no schooling (aRR 1.72, 95% CI 1.10, 2.69), women with primary education (aRR 1.81, 95% CI 1.11, 2.97); open defecation (aRR 1.48, 95% CI 1.00, 2.18), and those whose major occupation was agriculture (aRR 1.80, 95% CI 1.16, 2.78) are more likely to report higher stillbirth.
Conclusions
Low levels of education, ecological zones and open defecation were found to be strong predictors of stillbirth. Access to antenatal care services and skilled birth attendants for women in the mountainous and hilly ecological zones of Nepal is needed to further reduce stillbirth and improved services should also focus on women with low levels of education.
Introduction
Stillbirth refers to the birth of a baby with no signs of life at or after 28 weeks' gestation[1]. Globally, stillbirth is a major public health problem, with more than 2.7 million stillbirths occurring annually; of these, 98% are from developing countries[2]. Sub-Sahara Africa and South Asia account for the highest numbers of stillbirth[3]. The long-lasting impact of stillbirth remains a large burden for parents, families, policy makers and public health practitioners[4]. Evidence has shown that stillbirth is associated with physical and psychological morbidity, and remains a significant source of cost for the affected family and community [2, 5, 6]. Despite the huge burden of stillbirth on families and global health, progress made in low-middle-income countries to reduce stillbirth is considerably slower than the decline in child mortality[3].
Stillbirth rates vary within and between countries; with economically disadvantaged communities having higher rates compared to their economically well-off counterparts [3, 7]. In developing countries, the major risk factors for stillbirth include advanced maternal age, maternal educational status, infections, fetal development, environmental hazards, diabetes, malaria and umbilical cord complications [8–11]. Recent studies from developed countries (such as the United Kingdom and Sweden) have also reported that psychological issues are associated with higher stillbirth rates [12, 13].
The major causes and predictors of stillbirth in South Asia are not well understood because of the huge variation in data availability and quality that underestimates the true number of stillbirths[5]. Recent case-control studies [14, 15] conducted in Nepal found that stillbirth is associated with older maternal age, lower level of maternal education, coming from the poorest households, inadequate antenatal care and antepartum haemorrhage. Similarly, a verbal autopsy study conducted in Nepal revealed that obstetric complications which included prolonged labour, antepartum haemorrhage and pregnancy induced hypertension were associated with stillbirth[16]. A community-based study from a rural area of Nepal found that a history of prior child loss, maternal age above 30 years and low socio-economic status were associated with higher stillbirth rates[17].
The 2011 Lancet series on stillbirths suggested that for better estimation and intervention, the epidemiology of stillbirths should be at a country level instead of at the regional level because of the regional variations[4]. A major limitation of these Nepalese studies is that the findings cannot be used to inform initiatives and policy responses at the national level because the samples do not represent geographically diverse population across the country. Hence, the aim of this study was to provide nationally representative evidence on the socio-economic predictors associated with stillbirths in Nepal, using pooled data from the 2001, 2006 and 2011 Nepal Demographic and Health Surveys (NDHS). Findings from this study would enable public health professionals to inform different policies and programmes to reduce stillbirth, with subsequent improvements in maternal and newborn outcomes in Nepal.
Methods
Data sources
The NDHS is nationally representative, collected by the Nepalese Ministry of Health and Population, in collaboration with New ERA and ICF International, USA using a multi-stage cluster sampling design. Data on fertility, mortality, family planning, and important aspects of nutrition, health, and health services were collected for the years 2001, 2006 and 2011 using standard model questionnaires designed for, and widely used in developing countries[18–20]. For the 2001 NDHS, 8726 women aged 15–49 were interviewed, of these 7089 pregnancies were 7+ months’ gestation. Similarly in the 2006 NDHS, 10,793 women aged 15–49 years were interviewed. Of these, 5921 pregnancies were 7+ months’ gestation. In the 2011 NDHS, 12,674 women aged 15–49 years were interviewed; of which 5376 reported pregnancies 7+ months’ gestation. A total sample of 18,386 pregnancies 7+ months’ gestation five year prior each survey was included in the final analysis. For the year 2006 and 2011, pregnancies were identified using calendar information such as pregnancy outcomes and duration of pregnancy; whereas for the year 2001, pregnancies were identified using information such as pregnancy history index, and outcome and duration of pregnancy. Further detail of the survey methodology, sampling procedure, and questionnaires are reported elsewhere[18–20]. In all surveys, the response was more than 98%.
Study outcome
The outcome variable was stillbirth, defined as the birth of a baby with no signs of life at or after 28 weeks' gestation[1]. The outcome was recorded as a binary variable in the data set coded as 1 for ‘stillbirth’ and 0 for ‘Alive at birth’. Information on stillbirth was obtained using reproductive calendar (for 2006 and 2011 NDHS); and pregnancy history and outcome of pregnancies (for 2001 NDHS).
Exploratory variables
The exploratory variables selected for this study were based on previous studies from developing countries [8, 14, 16, 21–23] and the information available in the pooled data sets.
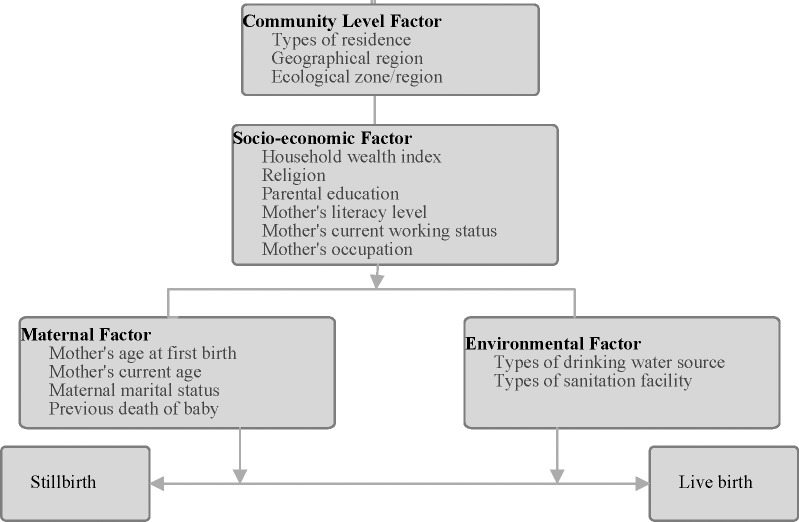
Fig 1 presented the modified Mosley and Chen [24] conceptual framework which comprise four groups of variables used in this study: community level factors, socio-economic level factors, maternal factor and environmental factors. The community level factors assessed included ecological zone (terai, hill and mountain), Geographical region (Eastern, Central, Western, Mid-Western and Far-Western) and place of residence (rural or urban). The socio-economic level factors considered were maternal education, literacy level, occupation (categorised as not working or working in agricultural or working in non-agricultural sector), paternal education, mother’s current work status and household wealth index. The household wealth index measures the economic status of the household. As a measure of household wealth index, we pooled the wealth index factor scores in each of the three original Individual Recode data files as calculated by original DHS. The pooled original household wealth index factor scores were then categorised into three: the bottom, 40% of households was referred to as poor households, the next 40% as the middle households and the top 20% as rich households[25].
Fig 1. Conceptual framework of socio-economic predictors of stillbirth in Nepal.
Maternal factors encompass maternal age at first birth, previous death of a baby, mother’s current age and maternal marital status. We also considered environmental factors consisting of drinking water source and types of sanitation facility for each household classified based on the WHO and UNICEF Joint Monitoring Program (JMP) guidelines[26]. Based on JMP guidelines, we categorized sources of drinking water as: piped water on premises (piped water system into dwelling), other improved drinking water sources (neighbours tap or tubewell, tubewell or borehole in yard, stone tap, protected well and rainwater), unimproved water sources (unprotected well in house, unprotected public or neighbour’s well, unprotected spring, bottled water and water from tanker or truck) and surface drinking water sources (river, stream, pond, lake, dam, canal, or irrigation water). Similarly, we categorized types of sanitation facility as improved and unimproved. Improved sanitation facilities included (households with flush toilet, ventilated or improved pit latrine, pit latrine with slab and composting toilet). Unimproved sanitation facilities were traditional pit toilet, pit latrine without slab or open pit and bucket toilet and open defecation (bush or field for defecation).
Statistical analysis
Frequency tabulations were first conducted to describe the frequency and relative frequency of all potential confounding factors. This was followed by calculating the stillbirth rate and 95% confidence interval, using ‘the number of stillbirths divided by the number of live births multiplied by 1,000’.
Generalized linear latent and mixed models (GLLAM) with the log link and binomial family[27] that adjusted for cluster and survey weights were used to identify those socio-economic predictors associated with stillbirth. A staged modelling technique[28] was adopted. Community-level factors were first entered into the baseline multivariable model with manual backward elimination process to keep statistically significant variables with p-value <0.05 (model 1). Second, socio-economic factors were added into community-level factors associated with outcome variable and those factors with p-values < 0.05 were retained after backward elimination process was conducted (model 2). Third, maternal factors were added into model 2. After applying similar approach as above, variables with p-values < 0.05 were retained in the next model (model 3). In the final stage, environmental factors were assessed with a list of significant variables from model 3. Variables with p-values < 0.05 were retained in the final model (model 4). Only those factors significantly associated with stillbirth at a 5% significance level in the final model were reported in the study. In the final model, collinearity was tested and reported. The analysis was restricted to five years preceding each of the survey.
A total of 57 missing values were excluded from the multivariate analysis, and GLLAM estimates were translated to relative risk and 95% confidence interval. All analyses were performed using STATA statistical software, version 14.1 (Stata Corporation, College Station, TX, USA) with ‘Svy’ commands to allow for adjustments for sampling weights and cluster sampling design.
Ethics
The DHS project obtained ethical approval from the Nepal Health Research Council-Kathmandu. The first author communicated with MEASURE DHS/ ICF International and permission was granted to download and use the data for his doctoral dissertation with the School of Science and Health at Western Sydney University, Australia.
Results
Basic characteristics of the study participants
The majority of mothers who reported higher rates of stillbirth were from rural and mountainous areas, poor households, parents with low levels of education, households with unimproved sources of drinking water and unimproved toilet facilities (sanitation) (Table 1). We also noted that mothers whose major occupation was agriculture had more stillbirths compared to those mothers who worked in non-agricultural sectors.
Table 1. Characteristics of study population as weighted counts and stillbirth, with rates with 95% confidence interval in Nepal: 2001, 2006 and 2011 (N = 18249).
| Study variables | N | Stillbirth (n) | Rate (95% CI) | |
|---|---|---|---|---|
| Type of residence | ||||
| Urban | 1656 | 27 | 17(10.3 to 22.8) | |
| Rural | 16593 | 308 | 19(16.8 to 21.0) | |
| Ecological zone | ||||
| Terai | 9358 | 154 | 17(14.1 to 19.4) | |
| Hill | 7405 | 141 | 19(16.2 to 22.6) | |
| Mountain | 1487 | 41 | 28(19.7 to 37.1) | |
| Geographical region | ||||
| Eastern | 4154 | 75 | 18(14.2 to 22.5) | |
| Central | 5936 | 98 | 17(13.5 to 20.1) | |
| Western | 3363 | 62 | 19(14.1 to 23.5) | |
| Mid-western | 2596 | 53 | 21(15.2 to 26.5) | |
| Far-western | 2201 | 47 | 22(15.6 to 28.1) | |
| Wealth index | ||||
| Rich | 3615 | 48 | 13(9.5 to 17.0) | |
| Middle | 7595 | 163 | 21(18.2 to 24.8) | |
| Poor | 7040 | 124 | 18(14.5 to 20.7) | |
| Religion | ||||
| Hindu | 15288 | 16 | 12(6.0 to 17.4) | |
| Buddhist | 1385 | 288 | 19(17.0 to 21.4) | |
| Others | 1576 | 32 | 21(13.5 to 27.9) | |
| Mother education | ||||
| Secondary or higher | 3833 | 46 | 12(8.6 to 15.7) | |
| Primary | 3122 | 63 | 21(15.5 to 25.7) | |
| No education | 11295 | 227 | 21(17.8 to 23.2) | |
| Mother's literacy level (N = 18228) | ||||
| Can read | 8096 | 126 | 16(13.0 to 18.6) | |
| Cannot read | 10133 | 210 | 21(18.3 to 24.0) | |
| Father's education | ||||
| Secondary or higher | 3386 | 40 | 12(8.2 to 15.7) | |
| Primary | 6463 | 113 | 18(14.5 to 21.1) | |
| No schooling | 8400 | 182 | 22(18.9 to 25.4) | |
| Mother current working status | ||||
| Not working | 5470 | 72 | 13(10.3 to16.4) | |
| Currently Working | 12779 | 263 | 21(18.5 to 23.6) | |
| Mother occupation (N = 18247) | ||||
| Not working | 3834 | 46 | 12(8.6 to 15.7) | |
| Agriculture | 12849 | 271 | 22(19.0 to 24.1) | |
| Non- agriculture | 1565 | 18 | 12(6.3 to 17.0) | |
| Mother’s age at first birth in years (N = 18191) | ||||
| <18 | 8043 | 106 | 13(10.7 to 19.0) | |
| 19–24 | 9036 | 148 | 16(13.7 to19.0) | |
| 25+ | 1112 | 23 | 21(12.2 to29.1) | |
| Mother current age | ||||
| 20–29 | 11643 | 201 | 18(15.1 to 20.0) | |
| <20 | 1198 | 22 | 19(10.9 to 26.5) | |
| 30–39 | 4527 | 94 | 21(16.9 to 25.5) | |
| 40–49 | 882 | 17 | 20(10.3 to 29.0) | |
| Maternal marital status | ||||
| Currently married | 18069 | 332 | 19(16.7 to 20.7) | |
| Not currently married | 180 | 4 | 23(0.5 to 45.0) | |
| Previous death of baby | ||||
| No | 13457 | 254 | 19(16.9 to 21.6) | |
| Yes | 4793 | 82 | 17(13.6 to 21.2) | |
| Types of drinking water source (N = 17092) | ||||
| Piped water on premises | 1843 | 26 | 14(8.7 to 19.5) | |
| Other improved drinking water sources | 12012 | 205 | 17(14.7 t019.4) | |
| Unimproved drinking water sources | 1203 | 26 | 22(13.3 to29.9) | |
| Surface drinking water sources | 2035 | 53 | 26(19.0 to33.1) | |
| Types of sanitation facility (N = 17093) | ||||
| Improved sanitation facilities | 4337 | 50 | 12(8.3 to14.7) | |
| Unimproved sanitation facilities | 1859 | 36 | 19(13.0 to 25.7) | |
| Open defecation | 10898 | 224 | 21(17.9 to23.2) | |
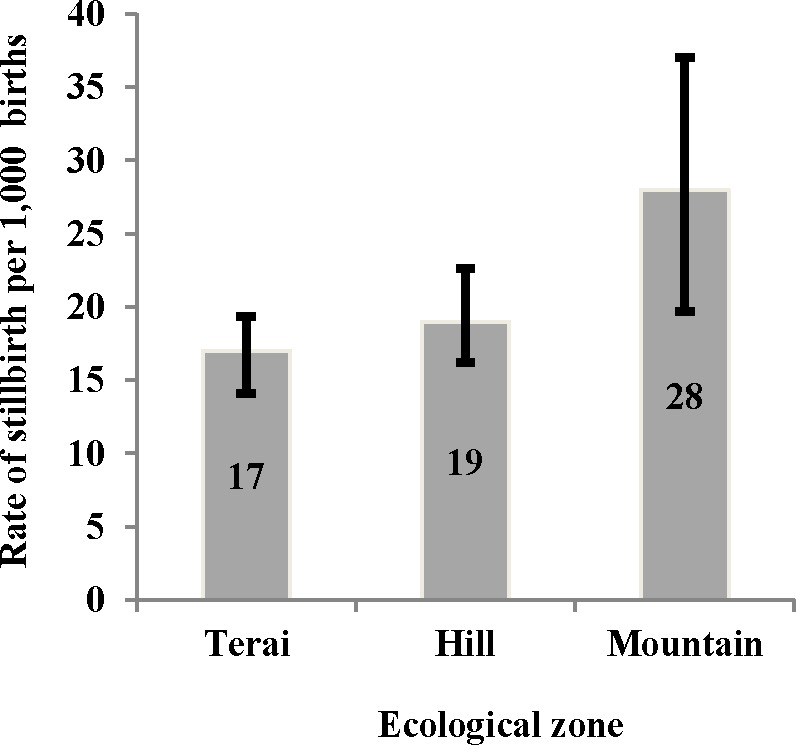
The prevalence of stillbirth across three ecological zones indicates that the rate was 28 per 1000 amongst mothers who resided in the mountains whereas this rate was 17 per 1000 in the terai, and 19 per 1000 in the hills (Fig 2).
Fig 2. Pooled stillbirth rate by ecological zone in Nepal, 2001–2011.
Predictors of stillbirth
The univariate analyses revealed that ecological zone (mountains or hills); religion (Muslim, Christian and others); mother’s literacy (illiterate); parental level of education (primary education or no schooling), currently not working mothers, mother’s whose major occupation was agriculture, mother’s age (25 years and above at the time of the first birth), types of drinking water source (surface drinking water sources) and types of sanitation facility (unimproved sanitation facility or open defecation) were all significantly associated with higher stillbirth (Table 2).
Table 2. Crude and adjusted Relative Risk (RR) for socio-demographic predictors of stillbirths in Nepal, 2001–2011 (N = 18249).
| Study variables | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| RR (95% CI) | P-value | RR (95% CI) | P-value | |
| Type of residence | ||||
| Urban | Reference | |||
| Rural | 1.07 (0.71, 1.61) | 0.738 | ||
| Ecological zone | ||||
| Terai | Reference | Reference | ||
| Hill | 1.17(0.90, 1.51) | 0.241 | 1.37(1.02, 1.84) | 0.036 |
| Mountain | 1.71(1.16, 2.52) | 0.006 | 1.68(1.09, 2.59) | 0.018 |
| Geographical region | ||||
| Eastern | Reference | |||
| Central | 0.88(0.63, 1.25) | 0.478 | ||
| Western | 1.01(0.69, 1.48) | 0.956 | ||
| Mid-western | 1.11(0.74, 1.65) | 0.621 | ||
| Far-western | 1.17(0.77, 1.77) | 0.459 | ||
| Wealth index | ||||
| Rich | Reference | |||
| Middle | 1.56(1.12, 2.17) | 0.009 | ||
| Poor | 1.28(0.91, 1.80) | 0.156 | ||
| Religion | ||||
| Buddhist | Reference | Reference | ||
| Hindu | 1.64(0.97, 2.76) | 0.062 | 2.19(1.19, 4.04) | 0.012 |
| Others including Muslim and Christian | 1.87(1.00, 3.50) | 0.05 | 2.68(1.29, 5.58) | 0.008 |
| Mother education | ||||
| Secondary or higher | Reference | Reference | ||
| Primary | 1.67(1.14, 2.45) | 0.009 | 1.80(1.10, 2.93) | 0.019 |
| No schooling | 1.65(1.20, 2.28) | 0.002 | 1.71(1.09, 2.66) | 0.019 |
| Mother's literacy level | ||||
| Can read part or whole of the sentence | Reference | |||
| Cannot read | 1.31(1.05, 1.65) | 0.018 | ||
| Father's education | ||||
| Secondary or higher | Reference | |||
| Primary | 1.47(1.02, 2.12) | 0.036 | ||
| No schooling | 1.83(1.30, 2.59) | 0.001 | ||
| Mother current working status | ||||
| Not working | Reference | |||
| Currently working | 1.54(1.18, 2.01) | 0.002 | ||
| Mother occupation | ||||
| Not working | Reference | Reference | ||
| Agriculture | 1.70(1.24, 2.34) | 0.001 | 1.78(1.16, 2.75) | 0.009 |
| Non- Agriculture | 0.99(0.57, 1.70) | 0.964 | 1.38(0.72, 2.63) | 0.328 |
| Mother’s age at first birth | ||||
| <18 | Reference | Reference | ||
| 19–24 | 1.23(0.95, 1.58) | 0.109 | 1.20(0.93, 1.56) | 0.186 |
| >25 | 1.59(1.00, 2.50) | 0.046 | 1.77(1.12, 2.82) | 0.015 |
| Mother current age | ||||
| <20 | Reference | |||
| 20–29 | 1.10(0.71, 1.70) | 0.675 | ||
| 30–39 | 1.18(0.92, 1.51) | 0.180 | ||
| 40–49 | 1.15(0.70, 1.88) | 0.590 | ||
| Maternal marital status | ||||
| Currently married | Reference | |||
| Not currently married | 1.20(0.44, 3.28) | 0.720 | ||
| Previous death of baby | ||||
| No | Reference | |||
| Yes | 0.88(0.69, 1.14) | 0.332 | ||
| Types of drinking water source | ||||
| Piped water on premises | Reference | |||
| Other improved drinking water sources | 1,21(0.80, 1.84) | 0.371 | ||
| Unimproved drinking water sources | 1.53(0.88, 2.65) | 0.135 | ||
| Surface drinking water sources | 1.81(1.12, 2.94) | 0.016 | ||
| Types of sanitation facility | ||||
| Improved sanitation facilities | Reference | Reference | ||
| Unimproved sanitation facilities | 1.57(1.01, 2.43) | 0.044 | 1.10(0.67, 1.82) | 0.697 |
| Open defecation | 1.76(1.29, 2.41) | <0.001 | 1.47(1.00, 2.16) | 0.049 |
Multivariable analysis revealed that factors associated with stillbirth were mothers in the age bracket (>25years), mothers who lived in mountains or hills, mothers whose religion was Hindu, Muslim, Christian and others, mothers who had no schooling or only primary level of education. Further we found that mothers whose major occupation was agriculture and those who used open defecation reported higher stillbirth.
In the final model, we removed maternal education level and replaced it with father’s education level; the result indicated that stillbirth increased significantly among fathers with no schooling (aRR 1.71, 95% CI 1.10, 2.64).
Discussion
This study reports the predictors associated with stillbirths in Nepal by using pooling the three most recent Nepal demographic and Health survey and found that maternal age (25 years and over), low levels of education, sanitation and ecological zones were predictors for stillbirth. Additionally, when mother education was replaced by father education in the final model, father with no education reported significantly higher stillbirth. This current study provides an evidence-base that could be used to inform the design of effective interventions, policies and programmes aimed at health professionals and individuals recognising stillbirths.
Primiparity is an established risk factor for stillbirth in both high and low income countries[29], and our results also found this association. This study demonstrated that mothers aged 25 years and above at the time of their first birth were more likely to experience stillbirth. This finding was supported by case-control studies conducted in Nepal, Bangladesh and Canada, which indicated that older mothers (35 years and above) significantly reported higher stillbirth than younger mothers [14, 22, 30]. Similarly, a hospital-based study conducted in Nigeria also revealed that mothers aged 35 years or older were significantly more likely to report higher rate of stillbirths[31]. Studies conducted in high-income countries showed a significant relationship between stillbirth and maternal age [7, 32–35]. Higher stillbirth rate in older women has been attributed to the increase likelihood of congenital anomalies, chronic hypertension, placenta praevia, uterine rupture, and breech deliveries in older mothers which may contribute to an increased fetal death [36–39]. Studies have also shown that advanced maternal age has been associated with an increased risk of abnormal chromosomes, and or decreasing uterine and hormonal function[40, 41].
Parental education is considered as one of the important determinants of health. Previous studies from Pakistan and Bangladesh reported that education could increase the uptake of health service utilization [42, 43] with subsequent reduction in stillbirth. This study found that women with only primary level of education or no schooling had higher risk of stillbirth compared to those who had secondary or higher levels of education. There are very few studies from developing countries that have examined the relationship between maternal education and stillbirth. A study conducted in a province of Thailand revealed women who had low levels of education were at a higher risk of having stillbirths[44]. This finding is also consistent with previous studies from Canada and Denmark, which found that lower level of maternal education was associated with higher risk of stillbirths [45–47]. Plausible reasons for this finding may be that educated mothers are more likely to practice healthy behaviours, including health seeking, which may contribute to reducing their risk of stillbirth compared to mothers with no education. Likewise, fathers with no schooling were also associated with higher risk of stillbirth.
Our study demonstrated that the risk of stillbirth was significantly higher among women who worked in an agricultural sector, similar to a finding from a hospital-based case-control study conducted in the Nguyen province of Vietnam[44]. Our finding of higher risk of stillbirth among mothers residing in the high altitude mountains or hills was similar to a retrospective births record obtained from four regional centres in Peru, which indicated that after controlling for potential confounding factors, mothers who lived in high altitude (greater than 3000 meters) were significantly more likely to report higher stillbirths than those mothers that lived in low altitude [48]. Whether our finding is related to altitude or access to antenatal and birth service is unknown due to the limitations of the DHS data. However, literature has shown that management of pregnancy complications through quality antenatal care[49] and provision of skilled birth attendance around labour time[4] help to prevent stillbirth. Based on these evidences, it can be argued that the focused antenatal care as well as targeted skilled birth attendance for women residing in the mountainous region would help to reduce the number of stillbirth.
Our study also found an association between stillbirth and mothers religious affiliation. Mothers whose religion was Hindu and others including Muslim and Christian reported significantly higher stillbirth compared to those mothers whose religion was Buddhist. Analysis [50] conducted in India using the National Family Health Survey (NFH) reported differences in child mortality based on religious affiliation. In Nepal access and utilization of birthing services differs by religious affiliation and this may contribute to the increased stillbirth in some religious groups[51].
Unimproved water and sanitation contributes 0.9% to the global Disability Adjusted Live Years [52]. It is not surprising that women who reported open defecation were at greater risk of stillbirth compared to mothers who reported improved sanitation facilities; similar with the finding from a population-based prospective cohort study conducted in India that revealed open defecation among pregnant women was associated with adverse pregnancy outcomes[53].
The study has a number of strengths. Firstly, the analysis was based on nationally representative data (NDHS); thus, estimates from this study are generalizable to the Nepalese population and can inform national policies and initiatives in Nepal. Secondly, the response to the surveys was high (>98%), reducing a likely chance of selection bias from the observed findings. Thirdly, measurement bias is unlikely to affect the observed results as the data were collected using a standardised questionnaire developed for developing countries including Nepal[18–20]. It is however retrospective data, and there may be some bias in reporting stillbirth. Despite these advantages, this study is limited in a number of ways. Firstly, the diagnosis of stillbirth was based on self-report from mother and is subject to recall and misclassification bias. Secondly, formal verbal autopsies were not conducted on stillbirths. Finally, no information on health services factors or other factors such as tobacco, gestational diabetes and genetic abnormality that may have been associated with stillbirth were included in the NDHS data.
Policy implications
To close the equity gaps, community-based interventions need to be formulated and implemented in order to improve maternal and child health in Nepal. At the individual level intervention, uptake and quality of antenatal care should be encouraged among mothers from low socio-economic group and those mothers from hilly and mountainous ecological zones. At the community level intervention, increase awareness and access to basic and emergency obstetric care to women from hilly and mountainous ecological zones. These interventions will improve prevention strategies that could have massive and far-reaching improvement on Nepalese mothers and children in order for the country to accelerate progress towards achievement of ending preventable stillbirths by 2035[54].
Conclusions
Our findings suggest that antenatal care service should be targeted to women from low socioeconomic status and those who lived in the mountainous ecological zone in order for Nepal to further reduce the rate of stillbirth to a target of 12 stillbirths per 1000 births by the year 2030.
Acknowledgments
The authors are grateful to Measure DHS, ICF International, Rockville, Marylands, USA for providing the data for this analysis. CRG was supported by National Health and Medical Research Council (of Australia) Career Development Fellowship #1087062, AR is supported by an Australian Research Council Future fellowship FT110100345, AC is supported by an Australian Postgraduate Award funded through the Australian Commonwealth Government, and MKN is supported by Early Career Researcher Postgraduate Scholarship, Sydney Medical School. The first author gratefully acknowledges the comments made on first draft by Dr. Amit Arora from Western Sydney University.
Data Availability
The data was obtained from http://www.dhsprogram.com/data/available-datasets.cfm.
Funding Statement
CRG was supported by National Health and Medical Research Council (of Australia) Career Development Fellowship #1087062, AR is supported by an Australian Research Council Future fellowship FT110100345, AC is supported by an Australian Postgraduate Award funded through the Australian Commonwealth Government, and MKN is supported by Early Career Researcher Postgraduate Scholarship, Sydney Medical School. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World health Organization. Maternal, newborn, child and adolescent health, 2015. Available from: http://www.who.int/maternal_child_adolescent/epidemiology/stillbirth/en/.
- 2.De Bernis L, Kinney MV, Stones W, Ten Hoope-Bender P, Vivio D, Leisher SH, et al. Stillbirths: ending preventable deaths by 2030. The Lancet. 2016. January 18; 387: 703–716. [DOI] [PubMed] [Google Scholar]
- 3.Lawn JE, Blencowe H, Waiswa P, Amouzou A, Mathers C, Hogan D, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. The Lancet. 2016. January 18; 387: 587–603. [DOI] [PubMed] [Google Scholar]
- 4.Lawn JE, Blencowe H, Pattinson R, Cousens S, Kumar R, Ibiebele I, et al. Stillbirths: Where? When? Why? How to make the data count? The Lancet. 2011. April 14; 377: 1448–1463. [DOI] [PubMed] [Google Scholar]
- 5.Cousens S, Blencowe H, Stanton C, Chou D, Ahmed S, Steinhardt L, et al. National, regional, and worldwide estimates of stillbirth rates in 2009 with trends since 1995: a systematic analysis. The Lancet. 2011. April 14; 377:1319–1330. [DOI] [PubMed] [Google Scholar]
- 6.Mullan Z, Horton R. Bringing stillbirths out of the shadows. The Lancet. 2011. April 14; 377: 1291–1292. [DOI] [PubMed] [Google Scholar]
- 7.Gordon A, Raynes-Greenow C, McGeechan K, Morris J, Jeffery H. Risk factors for antepartum stillbirth and the influence of maternal age in New South Wales Australia: a population based study. BMC pregnancy and childbirth. 2013; 13:12 doi: 10.1186/1471-2393-13-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClure EM, Pasha O, Goudar SS, Chomba E, Garces NA Tshefu A, et al. Epidemiology of stillbirth in low-middle income countries: a Global Network Study. Acta obstetricia et gynecologica Scandinavica. 2011; 90: 1379–1385. doi: 10.1111/j.1600-0412.2011.01275.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McClure EM, Nalubamba-Phiri M, Goldenberg RL. Stillbirth in developing countries. International Journal of Gynecology & Obstetrics. 2006. May 30; 94: 82–90. [DOI] [PubMed] [Google Scholar]
- 10.Goldenberg RL, McClure EM, Saleem S, Reddy UM. Infection-related stillbirths. The Lancet. 2010. March 10; 375: 1482–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aminu M, Unkels R, Mdegela M, Utz B, Adaji S, Den Broke N. Causes of and factors associated with stillbirth in low‐and middle‐income countries: a systematic literature review. An International Journal of Obstetrics & Gynaecology. 2014. September 18; 121: 141–153. [DOI] [PubMed] [Google Scholar]
- 12.Turton P, Evans C, Hughes P. Long-term psychosocial sequelae of stillbirth: Phase II of a nested case-control cohort study. Archives of women's mental health. 2009. January 10; 12: 35–41. doi: 10.1007/s00737-008-0040-7 [DOI] [PubMed] [Google Scholar]
- 13.Radestad I, Steineck G, Nordin C, Sjogren B. Psychological complications after stillbirth—influence of memories and immediate management: population based study. BMJ. 1996. June 15; 312: 1505–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashish KC, Nelin V, Wrammert J, Ewald U, Vitrakoti R, Baral GN, et al. Risk factors for antepartum stillbirth: a case-control study in Nepal. BMC Pregnancy Childbirth. 2015. July 5; 15: 146 doi: 10.1186/s12884-015-0567-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashish KC, Wrammert J, Ewald U, Clark RB, Gautam J, Baral G, et al. Incidence of intrapartum stillbirth and associated risk factors in tertiary care setting of Nepal: a case-control study in Nepal. Reproductive Health. 2016. August 31; 13:1 doi: 10.1186/s12978-016-0226-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manandhar SR, Ojha A, Manandhar DS, Shrestha B, Shrestha D, Saville N, et al. Causes of stillbirths and neonatal deaths in Dhanusha district, Nepal: a verbal autopsy study. University Medical Journal. 2010; 8: 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee AC, Mullany LC, Tielsch JM, Katz J, Khatry SK, LeClerg SC, et al. Community-based stillbirth rates and risk factors in rural Sarlahi, Nepal. International Journal of Gynecology & Obstetrics. 2011; 113: 199–204. [DOI] [PubMed] [Google Scholar]
- 18.Ministry of Health [Nepal], New ERA, and ORC Macro. 2002. Nepal Demographic and Health Survey 2001 Calverton, Maryland, USA: Family Health Division, Ministry of Health; New ERA; and ORC Macro. [Google Scholar]
- 19.Ministry of Health and Population (MOHP) [Nepal], New ERA, and Macro International Inc. 2007. Nepal Demographic and Health Survey 2006 Kathmandu, Nepal: Ministry of Health and Population, New ERA, and Macro International Inc. [Google Scholar]
- 20.Ministry of Health and Population (MOHP) [Nepal], New ERA, and ICF International Inc. 2012. Nepal Demographic and Health Survey 2011 Kathmandu, Nepal: Ministry of Health and Population, New ERA, and ICF International, Calverton, Maryland. [Google Scholar]
- 21.Cherry N, Shaikh K, McDonald C, Chowdhury Z. Stillbirth in rural Bangladesh: arsenic exposure and other etiological factors: a report from Gonoshasthaya Kendra. Bulletin of the World Health Organization. 2008. January 25; 86: 172–177. doi: 10.2471/BLT.07.043083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nahar S, Rahman A, Nasreen HE. Factors influencing stillbirth in Bangladesh: a case-control study. Paediatric Perinatal Epidemiology. 2013; 27: 158–164. doi: 10.1111/ppe.12026 [DOI] [PubMed] [Google Scholar]
- 23.Kumar R, Singhi S. Risk factors for stillbirths in a rural community. Indian Journal of Paediatrics. 1992. July; 59: 455–461. [DOI] [PubMed] [Google Scholar]
- 24.Mosley WH, Chen LC. An analytical framework for the study of child survival in developing countries. Population and development review. 1984. 10: 25–45. [PMC free article] [PubMed] [Google Scholar]
- 25.UNICEF. Childhood under threat: the state of the world's children. 2004
- 26.World Health Organization. Progress on sanitation and drinking water: 2015 update and MDG Assessment. World Health Organization, Geneva, Switzerland; 2015 [Google Scholar]
- 27.Skrondal A, Rabe-Hesketh S. Generalized latent variable modeling: Multilevel, longitudinal, and structural equation models, Crc Press; 2004. [Google Scholar]
- 28.Victora CG, Huttly SR, Fuchs SC, Olinto MT. The role of conceptual frameworks in epidemiological analysis: a hierarchical approach. International journal of epidemiology. 1997. February 1; 26: 224–227. [DOI] [PubMed] [Google Scholar]
- 29.Flenady V, Koopmans L, Middleton P, Froen JF, Smith GC, Gibbons K, et al. Major risk factors for stillbirth in high income countries: a systematic review and meta-analysis. The lancet. 2011. April 14; 377: 1331–1340. [DOI] [PubMed] [Google Scholar]
- 30.Dodds L, King WD, Fell DB, Armson BA, Allen A Nirmod K. Stillbirth risk factors according to timing of exposure. Annals of epidemiology. 2006. August; 16: 607–613. doi: 10.1016/j.annepidem.2005.11.006 [DOI] [PubMed] [Google Scholar]
- 31.Suleiman BM, Ibrahim HM, Abdulkarim N. Determinants of stillbirths in katsina, Nigeria: a hospital-based study. Paediatric reports. 2015; 7: 5615 doi: 10.4081/pr.2015.5615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naeye RL. Maternal Age, Obstetric Complications, and the Outcome of Pregnancy. Obstetrics & Gynecology. 1983. February; 61: 210–216. [PubMed] [Google Scholar]
- 33.Forman MR, Meirik O, Berendes HW. Delayed childbearing in Sweden. Jama. 1984. December 14; 252: 3135–3139. [PubMed] [Google Scholar]
- 34.Leyland AH, Boddy FA. Maternal age and outcome of pregnancy. New England Journal Medicine. 1990. August 9; 323: 413–416. [DOI] [PubMed] [Google Scholar]
- 35.Cnattingius S, Forman MR, Berendes HW, Isotalo L. Delayed childbearing and risk of adverse perinatal outcome: a population-based study. Jama. 1992. August 19; 268: 886–890. [PubMed] [Google Scholar]
- 36.Biggs J. Pregnancy at 40 years and over. Obstetrical & Gynecological Survey. 1973. October 1; 28: 710–711. [Google Scholar]
- 37.Booth RT, Williams GL. Elderly primigravidae. An International Journal of Obstetrics & Gynaecology. 1964. April 1; 71: 249–254. [DOI] [PubMed] [Google Scholar]
- 38.Kajanoja P, Widholm O. Pregnancy and delivery in women aged 40 and over. Obstetrics & Gynecology. 1978. January; 51: 47–51. [PubMed] [Google Scholar]
- 39.Naeye RL. The duration of maternal cigarette smoking, fetal and placental disorders. Early Human Development. 1979. September; 3: 229–237. [DOI] [PubMed] [Google Scholar]
- 40.Hassold T, Chiu D. Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy. Human genetics. 1985. May; 70: 11–17. [DOI] [PubMed] [Google Scholar]
- 41.Cano F, Simon C, Remohi J, Pellicer A. Effect of aging on the female reproductive system: evidence for a role of uterine senescence in the decline in female fecundity. Fertility and sterility. 1995. September 30; 64: 584–589. [DOI] [PubMed] [Google Scholar]
- 42.Shaikh BT, Hatcher J. Health seeking behaviour and health service utilization in Pakistan: challenging the policy makers. Journal of public health. 2005. March; 27: 49–54. doi: 10.1093/pubmed/fdh207 [DOI] [PubMed] [Google Scholar]
- 43.Chakraborty N, Islam MA, Chowdhury RI, Bari W, Akhter HH. Determinants of the use of maternal health services in rural Bangladesh. Health promotion international. 2003. December 1; 18: 327–337. [DOI] [PubMed] [Google Scholar]
- 44.Cripe SM, Phung T, Nguyen T, Williams MA. Risk factors associated with stillbirth in Thai Nguyen Province, Vietnam. Journal of tropical paediatrics. 2007. October 1; 53: 366–367. [DOI] [PubMed] [Google Scholar]
- 45.Auger N, Delezire P, Harper S, Platt RW. Maternal education and stillbirth: Estimating gestational-age-specific and cause-specific associations. Epidemiology. 2012. March; 23: 247–254. doi: 10.1097/EDE.0b013e31824587bc [DOI] [PubMed] [Google Scholar]
- 46.Luo ZC, Wllkins R, Kramer MS. Effect of neighbourhood income and maternal education on birth outcomes: a population-based study. Canadian Medical Association Journal. 2006. May 9; 174: 1415–1420. doi: 10.1503/cmaj.051096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olsen O, Madsen M. Effects of maternal education on infant mortality and stillbirths in Denmark. Scandinavian Journal of Public Health. 1999. June 16; 2: 128–136. [PubMed] [Google Scholar]
- 48.Gonzales GF, Tapia V, Carrillo CE. Stillbirth rates in Peruvian populations at high altitude. International Journal of Gynecology and Obstetrics. 2008. March; 100: 221–227. doi: 10.1016/j.ijgo.2007.08.009 [DOI] [PubMed] [Google Scholar]
- 49.Lawn JE, Lee CC, Kinney M, Sibley L, Carlo WA, Paul MK, et al. Two million intrapartum-related stillbirths and neonatal deaths: where, why, and what can be done? International Journal of Gynecology & Obstetrics. 2009. October; 107: S5–S19. [DOI] [PubMed] [Google Scholar]
- 50.Guillot M, Allendorf K. Hindu-Muslim differentials in child mortality in India. Genus. 2010. May 1; 66: 43–68. [Google Scholar]
- 51.Thind A, Mohani A, Banerjee K, Hagigi F. Where to deliver? Analysis of choice of delivery location from a national survey in India. BMC Public Health. 2008. January 24; 8: 29 doi: 10.1186/1471-2458-8-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012. December 15; 380: 2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Padhi BK, Baker KK, Dutta A, Cumming O, Freeman MC, Satpathy R, et al. Risk of Adverse Pregnancy Outcomes among Women Practicing Poor Sanitation in Rural India: a Population-Based Prospective Cohort Study. Plos Medicine. 2015. July 7; 12: e1001851 doi: 10.1371/journal.pmed.1001851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.World Health Organization. Every Newborn: An action plan to end preventable deaths. 2014
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data was obtained from http://www.dhsprogram.com/data/available-datasets.cfm.