Abstract
Objectives
Individual differences in adolescent exercise behavior are strongly influenced by genetic factors. The affective response to exercise is a potential source of these genetic influences. To test its role in the motivation to exercise, we estimated the heritability of the affective responses during and after exercise and the overlap with the genetic factors influencing regular voluntary exercise behavior.
Design
226 twin pairs and 38 siblings completed two submaximal exercise tests on a cycle ergometer and a treadmill and a maximal exercise test on a cycle ergometer. Affective responses were assessed by the Feeling Scale (FS), Borg’s Rating of Perceived Exertion (RPE) and the Activation-Deactivation Adjective Checklist (AD ACL).
Methods
Multivariate structural equation modeling was used to estimate heritability of the affective responses during and after submaximal and maximal exercise and the (genetic) correlation with self-reported regular voluntary exercise behavior over the past year.
Results
Genetic factors explained 15% of the individual differences in FS responses during the cycle ergometer test, as well as 29% and 35% of the individual differences in RPE during the cycle ergometer and treadmill tests, respectively. For the AD ACL scales, heritability estimates ranged from 17% to 37% after submaximal exercise and from 12% to 37% after maximal exercise. Without exception, more positive affective responses were associated with higher amounts of regular exercise activity (.15 < r < .21) and this association was accounted for by an overlap in genetic factors influencing affective responding and exercise behavior.
Conclusions
We demonstrate low to moderate heritability estimates for the affective response during and after exercise and significant (genetic) associations with regular voluntary exercise behavior. These innate individual differences in the affective responses to exercise should be taken into account in interventions aiming to motivate adolescents to adopt and maintain regular exercise.
Keywords: psychological response, heritability, twin study, physical activity, exercise behavior
INTRODUCTION
Regular physical activity is a key contributor to adolescents’ health (Janssen &Leblanc, 2010). However, the majority of youngsters does not engage in regular exercise at the recommended level, despite efforts of governments and health care organizations promoting exercise (Martinez-Gonzalez et al., 2001; Troiano et al., 2008). To create a successful intervention, one must have knowledge about the underlying predictors of a physically active lifestyle. One of the potential motivational mechanisms underlying exercise behavior is the affective response immediately during exercise and shortly after cessation of an exercise bout (Ekkekakis et al., 2013; Ekkekakis et al., 2011).
Affect refers to an individual’s core of all valenced states: good versus bad, pleasure and displeasure, positive and negative (Ekkekakis et al., 2013; Ekkekakis et al., 2011). In contrast to the persistent general belief that exercise is enjoyable for everyone, strong individual differences are found in the affective responses during and after exercise. Whereas some individuals indeed report an increase in pleasure or no change, others report reduced pleasure or negative changes in affect (Ekkekakis et al., 2005; Ekkekakis et al., 2011; Van Landluyt et al., 2000; Welch et al., 2007). Based on the principles of instrumental conditioning, the repeated affective responses to exercise activities could be a powerful determinant of the formation of stable behavioral habits. If the affective response is on balance positive, people are likely to maintain the behavior and become regular exercisers. However, if the net affective response is not favorable, people are at risk of dropping out and becoming non-exercisers. In keeping with this theoretical expectation, a more favorable affective response during exercise was found to be associated with the intention to engage in voluntary exercise (Kwan & Bryan, 2010; Ruby et al., 2011) and greater actual participation in (voluntary) moderate to vigorous exercise (Dunton & Vaughan, 2008; Rhodes & Kates, 2015; Schneider et al., 2009; Williams et al., 2008; Williams et al., 2012). A better understanding of the determinants of the affective response to exercise may therefore be paramount to creating successful exercise interventions.
The net affective response during and shortly after exercise may reflect a mixture of multiple aversive and appetitive effects. Examples of immediate aversive effects are exercise-related fatigue related to muscle pain, respiratory exertion and monoamine depletion (Davis & Bailey, 1997). After exercise, cardiovascular activation levels may be uncomfortably high for a prolonged period, paired to lingering muscle fatigue and central fatigue (Ament & Verkerke, 2009). More complex aversive effects may involve the fear for embarrassment and injuries (Huppertz et al., 2014b; Rhodes et al., 1999; Skelton & Beyer, 2003; Vartanian & Shaprow, 2008). These aversive effects may be balanced by the rewarding effects which are governed by the mesolimbic reward system that involves dopaminergic pathways (Beaulieu & Gainetdinov, 2011). More complex appetitive effects can involve a sense of accomplishment or distraction from worry or feelings of anxiety (Anderson & Shivakumar, 2013) during but also after exercise cessation. Shortly after exercise activities, sympathetic withdrawal may temporarily reduce the physiological sensitivity to stress (Chen & Bonham, 2010; Hsu et al., 2015).
De Geus and de Moor (2008) have hypothesized that these individual differences in part reflect differences in genetic sensitivity to the psychological effects of exercise (de Geus & de Moor, 2008). A significant genetic contribution of the affective responses to exercise could explain the now well-documented heritability of voluntary exercise behavior which peaks at 82% in late adolescence (Huppertz et al., 2012) and remains in play throughout adulthood with heritability estimates of around 42% (de Moor et al., 2011). Genetic variants influencing the affective exercise response could do so in part by an effect on the so-called ‘activity drive’ (Lerman et al., 2002; Lightfoot et al., 2004; Rowland, 1998). This activity drive can be conceptualized as an innate motivation to be physical active in the classical Hullian sense, not different from sex drive, hunger or thirst. Just as the glucostat cells and the baroreflex that keep sugar and blood pressure level constant at an optimal level, the activity-stat could keep a person’s energy expenditure at an optimal level, but that level may differ significantly across individuals dependent on genotype (Lightfoot et al., 2004; Swallow et al., 1998). The activity-stat could influence the net balance of positive and negative affective responses during and after a bout of exercise as the fulfillment of drives is intrinsically rewarding.
Other factors known to influence exercise behavior could further modulate the affective response to exercise. Positive attitudes and expected health benefits may lead the individual to endure an unfavorable balance between the aversive and appetitive effects, as may a strong ability to self-regulate. Both self-regulation traits and attitude are associated with exercise behavior and/or physical activity (Dishman et al., 2014; Dishman et al., 2015; Hagger et al., 2002; Rhodes et al., 2006). Moreover, attitudes are proven to be heritable (Huppertz et al., 2014b) and the psychological concept of self-regulation is also under substantial genetic control (Posner & Rothbart, 2009). Increased sensitivity to punishment as seen in neuroticism, aversion to arousal as seen in introversion, or reward-seeking behavior as seen in extraversion and sensation seeking, all heritable personality traits, may further modulate the affective response to exercise accounting for the association of personality with exercise behavior (de Moor et al., 2006).
A final important contributor to the net affective response to exercise is exercise ability and/or trainability. Activities that one is good at are likely to be pursued in leisure time. Performing better at exercise than others, or gaining more rapidly when exposed to comparable training regimes, will lead to feelings of competence, whereas lower levels of performance and trainability might lead to disappointment or shame (particularly when the exercise is performed in a competitive context). A large body of literature has confirmed self-efficacy, the belief and conviction that one can perform a given activity at an adequate level of performance, is a powerful determinant of whether someone engages in and adheres to an exercise program (Dishman et al., 2005; McAuley & Blissmer, 2000; Nigg, 2001). Self-efficacy may be an especially strong factor in adolescence, when the sensitivity to one’s own relative ranking among peers may be largest.
The present study aims to test the hypothesis that the affective responses during and after exercise show significant heritability in adolescence. Secondly, it aims to test the hypothesis by de Geus and de Moor (2007) that the genetic factors underlying this heritability partly overlap with the genetic factors underlying regular voluntary exercise behavior. To test these two hypotheses, the affective state was repeatedly measured in a large adolescent sample of twins and siblings during and after graded (sub)maximal exercise tests. Regular voluntary exercise behavior over the past year was characterized in these participants by a lifestyle interview. In a twin study, the intrapair resemblance for a trait is compared between genetically identical (monozygotic, MZ) and non-identical (dizygotic, DZ) twins. We expect that MZ twins resemble each other more than DZ twins in affective responses to exercise, providing evidence for genetic influences on this response. In a bivariate extension of the twin design, cross-trait/cross-twin correlations can be further used to compute the correlation between genetic factors influencing these two traits. We expect a significant genetic correlation between adolescent exercise behavior and the exercise-induced affective response showing that they are influenced by shared genetic factors.
METHODS
Subjects
Healthy adolescent twin pairs aged between 16 and 18 and their siblings (age range 12 – 25) from the Netherlands Twin Register (Van Beijsterveldt et al., 2013) were invited to participate in a study on the determinants of adolescent exercise behavior. A complete dataset was available for 499 subjects: 115 monozygotic pairs (MZ) and 111 dizygotic pairs (DZ), and 35 of their singleton siblings. Six additional non-twin sibling pairs participated. All subjects provided written consent and if the subjects were under 18 consent was given by both of their parents/guardians. All study procedures were reviewed and approved by the Medical Ethics Review Committee of the VU Medical Center Amsterdam (NL35634.029.10).
Measures
Regular voluntary exercise behavior was measured by a short lifestyle interview, in which the subjects indicated what types of regular exercise they were currently involved in. The questions in this interview were structured identical as in our longitudinal surveys used by the Netherlands Twin Register (van der Aa et al., 2010). Participants were asked 1) whether or not they currently participate in exercise activities in leisure time and if so, 2) for how many years, 3) how many months per year, 4) how many times a week, and 5) how many minutes each time. Activities that were related to transportation (walking and cycling) and compulsory education classes were excluded. As we were interested in regular voluntary exercise activities, we only included activities that were conducted for at least 3 months a year and since at least half a year, thereby excluded holiday specific exercise activities such as sailing camps and skiing. Each activity was recoded into a metabolic equivalent of task (MET) score, based on the compendium of energy expenditure published by Ainsworth. (Ainsworth et al., 2000). A MET is defined as the ratio of work metabolic rate to a standard resting metabolic rate i.e. the energy required to perform an activity relative to the energy that is expended during quiet rest. By multiplying the MET score, the frequency (how many times a week), and the duration of each exercise activity, weekly MET-hours spent on exercise activities were calculated.
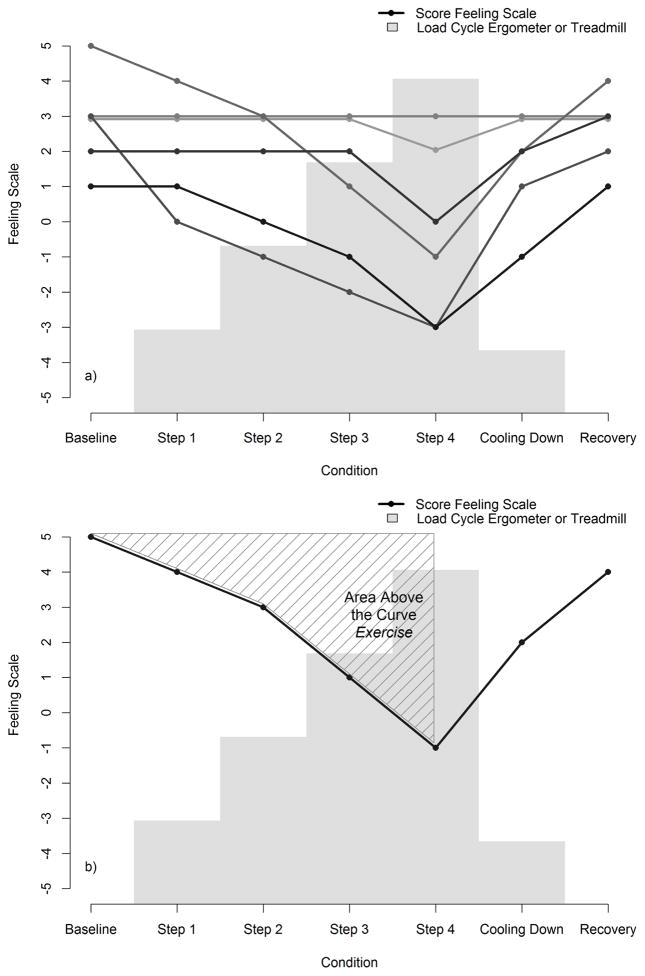
Affective responses to exercise were assessed by the Dutch versions of the Feeling Scale (Hardy & Rejeski, 1989) and the Activation-Deactivation Adjective Checklist (Thayer, 1986). The Feeling Scale (FS) is an 11-point bipolar measure of pleasure-displeasure. The scale ranges from −5 “very bad” to +5 “very good” and has been used as in many studies on the affective response to exercise (Ekkekakis et al., 2008; Ekkekakis et al., 2011; Hall et al., 2002; Parfitt et al., 2006; Schneider & Graham, 2009). Figure 1a shows the scores on the Feeling Scale of 6 randomly selected subjects for every step of a submaximal exercise test. The area above the curve was calculated for every participant during exercise (for details see Figure 1b) (using the polyarea function in Matlab (Matlab 2014a, The MathWorks Inc., Natick, Massachusetts, USA). These scores were recoded so that negative scores were associated with a larger decrease on the FS during the exercise tests. The Activation-Deactivation Adjective Checklist (AD ACL) is a multidimensional test of transitory arousal states using a four-point self-rating system: “definitely feel” (4), “slightly feel” (3), “cannot decide” (2) or “definitely do not feel” (1). As the subjects experienced some trouble with understanding three of the items “placid” and “wakeful” and “intense”, these items were left out of the analyses. This questionnaire is scored by averaging five scores for each subscale: Energy, Tiredness, Tension, and Calmness. Finally, to measure subjective exercise intensity The Borg’s Rating of Perceived Exertion (RPE) (Borg, 1970) was used: A 15-point scale ranging from 6 to 20, with marks at 7 (“very, very light”), 9 (“very light”), 11 (“fairly light”), 13 (“somewhat hard”), 15 (“hard”), 17 (“very hard”) and 19 (“very, very hard”). The sum of the scores for every submaximal exercise test was used for analyses. These scores were recoded so that more negative scores were associated with a higher perceived extertion.
Figure 1.
An example of the decline and increase in Feeling Scales responses during a submaximal exercise test. a) Feeling Scale response of 6 randomly selected subjects during a submaximal exercise test; b) Example of the quantification of the Feeling Scale response during a submaximal exercise test in a randomly selected subject. The hatched area is used as the Feeling Scale response for this subject.
To account for potential effects of differences in the relative intensity of exercise compared to a person’s maximal exercise capacity, oxygen uptake (V̇O2) and carbon dioxide production (V̇CO2) were recorded continuously by means of a telemetric gas exchange system (Cosmed K4b2, Roma, Italy) during the submaximal and maximal exercise tests. Breath-by-breath VO2 data were exported and deviant breaths were removed by excluding the breaths that were 3 times the standard deviation from the mean.
Procedure
On arrival at the laboratory, height and weight were measured, the life-style interview was completed, and baseline FS and AD ACL responses were obtained. Next, two submaximal exercise tests were conducted (in fixed order) on an electromechanically braked Lode cycle ergometer (type Corival) and a Lode treadmill (type Valiant) at fixed loads that are typically below the intensity of the ventilatory threshold (VT) for most adolescents. The first session on the cycle ergometer started with a 2-minute warming up period, followed by 4 incremental stages of 5 minutes each (males: 70 Watt (W), 90W, 110W, 130W; females: 40W, 60W, 80W, 100W). Subjects were instructed to pedal at fixed rounds per minute (RPM): between 60 and 70 RPM. The test ended with a 1-minute cooling down phase, followed by a 5-minute recovery period. The second session on the treadmill consisted of a 1-minute warm-up period, followed by 4 incremental stages of 5 minutes each (males: 6, 6.5, 7 and 8 km/h; females: 5.5, 6, 6.5 and 7 km/h). Again, the test ended with a 1-minute cooling-down phase, followed by a 5-minute recovery period. In order for the participants to reach a steady-state during the 4 steps of the submaximal exercise protocol, FS and RPE responses were collected in the last minute (after 4 minutes) of every step. During the cooling down phase (1 minute), FS response was collected in the last 15 seconds. During the recovery phases, the AD ACL and FS responses were collected after respectively ~2 and 5 minutes of sitting quietly.
To ensure that the participants did not exercise at vigorous intensities, the ratio of the oxygen consumption and carbon dioxide production (V̇O2/ V̇CO2) was monitored. This respiratory exchange ratio (RER) can be used to estimate the blood lactate-based anaerobic threshold (Solberg et al., 2005). This threshold is transcended when exhalation of CO2 exceeds inhalation of O2, which is visualized by a RER > 1.00. The load of each stage was adjusted when necessary to keep the intensity of the final stage of each submaximal test below an RER of 1. For subjects who showed an RER above 1.0 during the submaximal tests, FS and AD ACL responses for that submaximal were set to missing (including the cool down and recovery period).
Finally, an incremental maximal exercise test was conducted on a cycle ergometer to establish V̇O2max. The work rate was increased every minute until exhaustion (see Schutte et al., 2016 for measurement details on V̇O2max). On average 25.2 ± 7.6 minutes after the end of the maximal exercise test and a shower the AD ACL was filled out a final time.
Statistical analyses
The classical twin design compares the intrapair resemblance between two types of sibling relationships; genetically identical twins or monozygotic (MZ), the result of division of a single fertilized egg during an early stage in embryonic development, and non-identical twins or dizygotic (DZ), resulting from two separate fertilized eggs. Consequently, MZ twins are genetically identical, whereas DZ twins shared on average 50% of their genetic make-up. Twin studies decompose all phenotypic variance of a trait into sources of genetic influences (‘A’), shared environmental influences (influences shared with other family members e.g. upbringing; referred to as ‘C’), dominant genetic influences (‘D’) and person-specific influences (influences that are unique to the individual; referred to as ‘E’). An important assumption is that the shared environmental effects are independent of zygosity (and thus equal for both MZ and DZ twins).
Modeling of the twin and sibling data was performed using structural equation modeling (SEM) in OpenMx (Boker et al., 2011) under R (R Development Core Team, 2011) with the raw-data ML procedure for estimation of the parameters. For all analyses, a threshold of p < 0.05 was considered for statistical significance. Given the relative small sample size, with no power to test for sex-differences, and since (non-twin) siblings share, like DZ twins, on average 50% of their genes, parameter estimates were constrained to be equal for males and females and for DZ twins and siblings. Main effects of baseline measurements, sex and age and highest percentage of V̇O2max reached during the submaximal tests (see Figure 1b) on mean levels of the affective responses were considered in the model. In addition, for the AD ACL responses collected after the maximal test, we included the main effect of the time between the maximal exercise test and the final measurement of the AD ACL (in minutes) when modeling the mean AD ACL response.
First, twin-sibling correlations were estimated with univariate saturated models in OpenMx. In a saturated model, all parameters (means, variances) are estimated freely. Next, total phenotypic variance of FS, RPE and AD ACL responses was decomposed into sources of additive genetic variance (A), dominant genetic variance (D) or shared environmental variance (C) and person-specific environmental variance (E) to test which sources of variance significantly contribute to the phenotype and estimate their best value. Since C and D effects cannot be estimated simultaneously in the classical twin model, the ratio of the MZ correlations to the DZ correlations was used to determine which model (ACE or ADE) is most appropriate.
Significance of the variance components was tested by constraining them to zero (for instance, comparing model ACE versus a submodel AE, in which the C component was fixed to zero). These submodels were compared by hierarchic χ2 tests. The χ2 statistic is computed by subtracting log-likelihood (-2LL) of a submodel from the -2LL of the original model (χ2= −2LLoriginal model – −2LLsubmodel). This χ2 statistic is distributed with degrees of freedom (df) equal to the difference in the number of parameters estimated in the two models (Δdf = dforiginal model – dfsubmodel). If the difference test is significant, the constraints on the submodel cause a significant deterioration of the fit of the model (Rijsdijk & Sham, 2002).
The phenotypic and cross-trait/cross twin correlations for FS, RPE and AD ACL responses with regular exercise were estimated in bivariate models: an analysis of two variables to determine the relationship between them. When phenotypic correlations proved significant, genetic (rA) and environmental (rE) correlations were calculated to determine how much of the genetic influence on two variables is common to both. Finally, a multiple regression analysis was run in STATA to determine the amount of variance in exercise behavior explained by the FS, RPE and AD ACL responses while taking into account familial relatedness.
RESULTS
Descriptives
For 16 subjects affective responses collected during the submaximal cycle ergometer test (10) or submaximal treadmill (6) test were set to missing, and for 7 subjects affective responses collected during both tests were set to missing, because they showed an RER above 1.0 during these exercise tests. Table 1 shows the means and standard deviations (SDs) of age, body composition, regular exercise and V̇O2max. Tables 2 shows the means and SDs of the FS and RPE responses, V̇O2 expressed as a percentage of V̇O2max for every step of the experiment. As the intensity of the submaximal tests increased, the percentage of V̇O2max at which the subjects were exercising increased accordingly. The mean FS responses showed a decline when load was increasing, whereas the subjects reported a higher mean RPE. During the cool down and recovery phase, mean FS responses increased reflecting a return to a more positive affective state. The means and SDs and the Cronbach’s alpha for the four subscales of the AD ACL are shown in Table 3. The mean score for Energy increases during the recoveries of the submaximal tests, but decreases after the maximal exercise test. Calmness and Tiredness show a reverse pattern, whereas the scores for Tension seem to be stable over the course of the experiment. In the current study, Cronbach’s alpha was sufficient for Energy, Calmness and Tiredness (.60 – .87), but low for Tension (.34 – .48).
Table 1.
Means and standard deviations of age, body composition, regular exercise and V̇O2max.
| Male (N = 242) | Female (N = 257) | |||
|---|---|---|---|---|
|
| ||||
| Mean | SD | Mean | SD | |
| Age | 17.1 | 1.4 | 17.3 | 1.5 |
| Height (cm) | 180.5 | 8.1 | 168.1 | 6.9 |
| Weight (cm) | 67.6 | 10.8 | 61.7 | 10.0 |
| BMI (kg·m−1) | 20.7 | 2.5 | 21.8 | 3.2 |
| Voluntary Exercise Behavior (METs/week) | 25.8 | 22.6 | 19.5 | 21.9 |
| V̇O2max in mL/min | 3134.5 | 542.8 | 2232.1 | 329.2 |
| V̇O2max in mL/min/kg | 46.9 | 6.9 | 36.7 | 5.6 |
Table 2.
Means and standard deviations Feeling Scale, RPE and percentage of V̇O2max during and after submaximal exercise.
| Baseline | Feeling Scale | RPE | % of V̇O2max | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean | SD | Mean | SD | Mean | SD | interquartile range | |
|
| |||||||
| 3.53 | 1.21 | ||||||
| Cycle ergometer step 1 | 3.55 | 1.01 | 9.10 | 1.42 | 41.76 | 6.42 | 8.56 |
| Cycle ergometer step 2 | 3.25 | 1.07 | 10.27 | 1.51 | 48.52 | 7.33 | 9.31 |
| Cycle ergometer step 3 | 2.87 | 1.23 | 11.17 | 1.51 | 54.78 | 7.99 | 10.44 |
| Cycle ergometer step 4 | 2.56 | 1.42 | 11.97 | 1.78 | 60.64 | 8.89 | 11.60 |
| Cool down | 2.98 | 1.21 | 51.90 | 8.32 | 11.02 | ||
| Recovery | 3.36 | 1.06 | 19.01 | 3.63 | 4.70 | ||
|
| |||||||
| Treadmill step 1 | 3.43 | 1.14 | 8.87 | 1.53 | 45.27 | 7.80 | 10.17 |
| Treadmill step 2 | 3.11 | 1.21 | 10.10 | 1.77 | 49.42 | 8.37 | 10.49 |
| Treadmill step 3 | 2.78 | 1.41 | 11.14 | 1.88 | 54.64 | 9.77 | 12.32 |
| Treadmill step 4 | 2.54 | 1.50 | 12.06 | 1.96 | 61.98 | 11.36 | 13.95 |
| Cool down | 3.00 | 1.30 | 52.25 | 10.03 | 12.67 | ||
| Recovery | 3.50 | 1.18 | 19.62 | 4.11 | 5.43 | ||
Table 3.
Means and standard deviations of AD ACL subscales Energy, Calmness, Tiredness and Tension after (sub)maximal exercise.
| Energy | Calmness | Tiredness | Tension | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Mean | SD | Cronbach’s alpha | Mean | SD | Cronbach’s alpha | Mean | SD | Cronbach’s alpha | Mean | SD | Cronbach’s alpha | |
|
| ||||||||||||
| Baseline | 3.02 | 0.59 | 0.78 | 3.42 | 0.49 | 0.66 | 1.85 | 0.80 | 0.82 | 1.21 | 0.33 | 0.34 |
| Recovery cycle ergometer | 3.40 | 0.52 | 0.81 | 2.85 | 0.73 | 0.78 | 1.51 | 0.54 | 0.60 | 1.25 | 0.38 | 0.42 |
| Recovery treadmill | 3.40 | 0.55 | 0.83 | 2.83 | 0.80 | 0.65 | 1.59 | 0.59 | 0.84 | 1.23 | 0.39 | 0.48 |
| Recovery maximal exercise test | 3.11 | 0.71 | 0.87 | 3.29 | 0.60 | 0.68 | 1.74 | 0.67 | 0.78 | 1.15 | 0.31 | 0.41 |
Heritability of the affective responses to exercise
Table 4 shows the MZ and DZ/sibling correlations and genetic modelling results. For FS response during cycling, the dominant genetic factor (D) was not significant (p > .05) and heritability estimate (A) was 15% (95% CI: 0% – 31%). For FS responses during the submaximal treadmill test, the DZ/sibling correlation was higher than half the MZ correlation and the ACE model could be simplified by dropping either A or C, with a CE model giving the best fit. C explained 19% (95% CI: 8% – 30%) of the total variance in FS responses during the submaximal treadmill test. For RPE responses during both submaximal exercise tests, C could be dropped from the model and heritability estimates of 29% (95% CI: 13% – 43%) and 35% (95% CI: 20% – 48%) were found. Person-specific environmental influences explained a substantial portion of the variance in all FS and RPE responses.
Table 4.
Twin correlations (95% CI) and standardized estimates (95% CI) of additive (A) genetic influences, dominant (D) genetic influences or shared environmental (C), and unique environmental (E) influences on the Feeling Scale and RPE responses and AD ACL subscales Energy, Calmness, Tiredness and Tension
| rMZ | rDZ/sibling | ACE/ADE model | Best fitting | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| A | C | D | E | |||||||
|
|
||||||||||
| Cycle ergometer | Exercise | FS | .20 (.02, .36) | .01 (−.15, .16) | .00 (.00, .29) | .19 (.00, .35) | .81 (.65, .98) | A = .15 (.00, .31) | E = .85 (.69, 1) | |
| RPE | .25 (.06, .43) | .20 (.04, .34) | .08 (.00, .41) | .17 (.00, .34) | .75 (.59, .89) | A = .29 (.13, .43) | E = .71 (.57, .87) | |||
| Recovery | Energy | .35 (.17, .50) | .24 (.09, .38) | .25 (.00, .50) | .10 (.00, .36) | .65 (.50, .82) | A = .37 (.23, .51) | E = .63 (.49, .78) | ||
| Calmness | .24 (.05, .40) | .03 (−.11, .18) | .00 (.00, .32) | .22 (.00, .38) | .78 (.62, .95) | A = .19 (.02, .34) | E = .81 (.66, .98) | |||
| Tiredness | .10 (−.10, .29) | −.02 (−.17, .13) | .00 (.00, .23) | .09 (.00, .27) | .91 (.73, 1) | E = 1 | ||||
| Tension | .26 (.08, .41) | .10 (−.05, .25) | .31 (.00, .38) | .09 (.00, .41) | .75 (.59, .91) | A = .24 (.08, .38) | E = .76 (.62, .92) | |||
|
| ||||||||||
| Treadmill | Exercise | FS | .23 (.07, .38) | .14 (−.01, .29) | .12 (.00, .38) | .11 (.00, .30) | .77 (.62, .92) | C = .19 (.08, .30) | E = .81 (.70, .92) | |
| RPE | .33 (.20, .48) | .20 (.05, .34) | .23 (.00, .47) | .10 (.00, .36) | .67 (.53, .83) | A = .35 (.20, .48) | E = .65 (.52, .80) | |||
| Recovery | Energy | .23 (.04, .40) | .23 (.09, .36) | .01 (.00, .39) | .21 (.00, .33) | .78 (.61, .90) | C = .22 (.10, .33) | E = .78 (.67, .90) | ||
| Calmness | .21 (.04, .34) | .09 (−.06, .24) | .16 (.00, .35) | .05 (.00, .37) | .79 (.63, .94) | A= .21 (.05, .35) | E = .79 (.65, .95) | |||
| Tiredness | .11 (−.07, .27) | .13 (−.02, .27) | .00 (.00, .28) | .13 (.00, .24) | .87 (.72, .99) | E = 1 | ||||
| Tension | .17 (−.02 .34) | .03 (−.17, .12) | .00 (.00, .25) | .13 (.00, .30) | .87 (.70, 1) | E = 1 | ||||
|
| ||||||||||
| Maximal Exercise | Recovery | Energy | .39 (.24 .52) | .07 (−.09, .22) | .06 (.00, .48) | .33 (.00, .52) | .61 (.48, .76) | A = .37 (.22, .49) | E = .63 (.51, .78) | |
| Calmness | .38 (.23, .51) | .14 (−.03, .29) | .11 (.00, .48) | .27 (.00, .51) | .62 (.49, .78) | A = .36 (.21, .49) | E = .64 (.51, .79) | |||
| Tiredness | .33 (.15, .48) | .16 (.01, .30) | .32 (.00, .46) | .00 (.00, .44) | .68 (.54, .83) | A = .32 (.17, .46) | E = .68 (.54, .83) | |||
| Tension | .09 (−.10 .27) | .11 (−05, .27) | .00 (.00, .27) | .11 (.00, .23) | .89 (.73, 1) | E = 1 | ||||
For energy and Calmness the MZ correlations were higher than DZ/sibling correlations and the AE model provided the best fit, except for Energy measured during the recovery of the treadmill test for which a significant C component was found (22%). For Energy measured after the cycle test and the maximal exercise test, heritability estimates were 37%. For Calmness, heritability estimates increased over the course of the experiment, ranging from 19% after the submaximal cycle ergometer test to 36% after the maximal exercise test. Tiredness showed no evidence of genetic influences after the submaximal tests, but 32% of the differences in Tiredness after the maximal exercise test could be explained by genetic factors. Finally, heritability estimate of Tension after the submaximal cycle ergometer tests was 24%, but no evidence of genetic influences were found for Tension after the other two exercise tests.
Correlations with regular exercise behavior
Table 5 shows the phenotypic correlations between the responses on the FS and the RPE and regular exercise. Significant correlations were found for the FS responses and the RPE responses with voluntary exercise behavior (.15 < r < .21). A larger decrease in scores on the FS during the exercise test was associated with lower values of regular exercise. Significant genetic correlations for FS and RPE with voluntary exercise behavior were rG = .34 (FS during cycle ergometer test), rG = .22 (RPE, cycle ergometer), and rG = .36 (RPE, treadmill). Environmental correlations were small (.04 < rE < .14) and not significant.
Table 5.
Correlations of voluntary regular exercise with the Feeling Scale and RPE responses and AD ACL subscales Energy, Calmness, Tiredness and Tension
| Correlation with voluntary exercise behavior | |||
|---|---|---|---|
|
| |||
| Cycle ergometer | Exercise | FS | .18 (.08, .27) |
| RPE | .15 (.06, .25) | ||
| Recovery | Energy | .15 (.05, .25) | |
| Calmness | .12 (.03, .21) | ||
| Tiredness | −.11 (−.20, −.01) | ||
| Tension | −11 (−.20, −.00) | ||
|
| |||
| Treadmill | Exercise | FS | .21 (.11, .30) |
| RPE | .21 (.11, .30) | ||
| Recovery | Energy | .11 (.01, .21) | |
| Calmness | .17 (.08, .26) | ||
| Tiredness | −.06 (−.15, .03) | ||
| Tension | −.08 (−.17, .01) | ||
|
| |||
| Maximal Exercise | Recovery | Energy | .01 (−.09, .10) |
| Calmness | .12 (.03, .22) | ||
| Tiredness | −.05 (−.15, .04) | ||
| Tension | −.07 (−.17, .02) | ||
Note. Significant correlations in bold.
Significant correlations were found for voluntary exercise behavior with the four subscales of the AD ACL measured during the recovery of the cycle ergometer test. Subjects with higher exercise status reported higher values of Energy (r = .15) and Calmness (r = .12) and lower values of Tiredness (r = −.11) and Tension (r = −.11). After the submaximal treadmill test, only Energy and Calmness correlated significantly with voluntary exercise behavior (r = .11 and .17 respectively). After the maximal exercise test, only Calmness showed a significant correlation with exercise behavior (r = .12). That the same genetic variants may influence both exercise behavior and affective responding to exercise was confirmed by examination of the genetic correlation. Significant genetic correlations were found for voluntary exercise behavior with Energy measured during the recovery of the submaximal cycle ergometer test (rG =.28), Calmness measured during the recovery of all three exercise tests (rG = .27, rG = .41, and rG = .22 for the cycle ergometer, treadmill and maximal exercise test respectively) and Tension measured during the recovery of the submaximal cycle ergometer (rG = −24). Again, environmental correlations were small (−.05 < rE < .03) and not significant.
A multiple regression analysis (corrected for familial relatedness) showed that all FS, RPE and AD ACL responses, explained 11.1% of the variance in exercise behavior. When including only the FS, RPE and AD ACL responses collected during and after the submaximal cycle ergometer test, 6.3% of the variance in exercise behavior could be explained. For the treadmill test and maximal exercise test this was 9.5%.
DISCUSSION
The main aims of this study were to test the significance of a genetic contribution to the affective response to exercise and a genetic contribution to its relationship with regular exercise behavior. Results confirmed that the individual differences in affective responses in our adolescent sample during and after two submaximal exercise tests and a maximal exercise test could partly be explained by genetic factors. Heritability of the affective exercise response varied between 12% and 37%. This suggests that the well-documented individual differences in exercise-induced affective responses at moderate to vigorous (but not severe) intensities (Ekkekakis et al., 2005; Ekkekakis et al., 2011; Van Landluyt et al., 2000; Welch et al., 2007) should not solely be sought in environmental factors. In addition, more positive affective responses were associated with higher amounts of regular exercise activity (.15 < r < .21) and significant genetic correlations were found between higher amounts of regular voluntary exercise behavior and affective responses measured with the Feeling Scale during exercise and the AD ACL subscales Energy and Calmness after cessation of exercise. This supported our hypothesis that there is an overlap in the genetic variants causing favorable affective exercise responding and the genetic variants influencing voluntary exercise behavior.
The results of this study are in keeping with de Geus and de Moor (2007) who predicted a role for the genetic variants influencing the affective response to exercise in the heritability of exercise behavior. However, as longitudinal follow-up of long-term exercise behavior of these adolescents was not available, reverse causality cannot be ruled out. In reverse causality, the genetic correlation arises because the genetic variants influencing exercise behavior could become part of the heritability of the affective response if regular exercise itself sensitizes regular exercisers to the appetitive effects of exercise or desensitizes them to the aversive effects. Furthermore, a third scenario is that the same genetic variants independently influence the affective response and the tendency to become a regular exerciser. An example of such genetic pleiotropy would be genetic variants influencing vagally mediated heart rate recovery from exercise. Such recovery may be an important factor determining both the affective response to exercise as well as exercise ability which in turn will reinforce regular exercise behavior.
Mixtures of these three causal scenarios may be at play as well, e.g. there may be bidirectional causality in the presence of pleiotropy. Training studies could help resolve causality, but might suffer from selection bias, as they are typically conducted in sedentary individuals (regular exercisers would not show meaningful changes). Twin studies can resolve causality in unselected population-based samples if the sample size is sufficiently large to detect environmental correlations (de Moor et al., 2008), but might be a challenging undertaking for the relatively involved experimental protocol used here. Below 5000 twin pairs, the power to detect a significant environmental correlation between affective responses and exercise behavior is poor (Stubbe & de Geus, 2009). Mendelian Randomization would be a very good alternative strategy to resolve causality as this technique detects causal effects in an unbiased manner (Davey Smith & Hemani, 2014; Lawlor et al., 2008). However, this approach would need (a set of) genetic variants influencing only the affective response to exercise and ideally also a set of genetic variants influencing only exercise behavior. As many large cohorts have genetic data paired to data on voluntary exercise behavior, the latter will become available in time through a meta-analysis of cohort-specific genome-wide association analyses. For genetic variants influencing the affective response to exercise, a candidate gene approach seems the only feasible approach.
A future challenge is to identify the specific genes underlying the heritability of the affective response to exercise, to test their predictive value for the adoption of regular exercise behavior and their usefulness in personalizing exercise intervention. To do so studies need to have measured affective exercise responses in designs as used in the present study as well as having collected DNA materials. A study by Bryan et al. (2007) reported that the brain-derived neurotrophic factor (BDNF) gene, a peptide with a broad influence on the vascular, muscular and central nervous system, moderated the effect of exercise on mood, heart rate, and perceived exertion in a sample of healthy exercisers. Moreover, the BDNF gene might also be associated with intrinsic motivation during exercise (Caldwell Hooper et al., 2014). In inactive but healthy adults, Karoly et al. (2012) found two single nucleotide polymorphisms (SNPs) in the fat mass and obesity-associated protein gene (FTO) gene related to positive affect change during exercise (Karoly et al., 2012). Other candidate gene studies aimed at exercise behavior have already focused on the feelings of reward that are governed by the dopaminergic and cannabinoid reward systems in mesolimbic circuits. Genetic variation in these circuits might indeed explain the heritability of affective responses to exercise. Previous studies reported an effect of genetic variants in dopaminergic genes on voluntary physical activity in animals (Knab & Lightfoot, 2010), but for humans the dopaminergic connection is less well established (Huppertz et al., 2014a; Jozkow et al., 2013; Simonen et al., 2003)
However, exercise also generates aversive responses. Genetic variation in brain circuits governing punishment or pain and fatigue may be as relevant as reward (Ekkekakis, 2003) but they have been much less studied to date. Our study was no exception: here we deliberately chose to measure affective states below or close to the VT, i.e. the range where displeasure is not yet very strong. As many participants engaged in regular leisure time exercise activities will stay below this intensity threshold, individual differences in (dis)pleasure experienced at such intensities could be important determinants of voluntary maintenance of regular exercise behavior. However, this neglects the potential importance of the increase in interindividual variation in affective responding at intensities just above the VT, when the supply of energy through oxygen must be supplemented by anaerobic metabolism and the physiological steady-state is challenged (Ekkekakis, 2003). We confirm the emergence of stronger individual differences with increased intensity as is reflected by the increase in standard deviations of the FS (Table 2). Exercising above the VT (but below the maximum steady-state lactate concentration) may therefore increase the genetic variance in affective response beyond that seen below the VT. Indeed, when calculating MZ and DZ/sibling correlations for FS for every step of the submaximal test separately, the difference between these correlations was increasing with intensity (with MZ correlations increasing; data not shown). Future studies should confirm the expectation that affective responses to exercise above the VT exercise is to a substantial part driven by genetic factors.
Some further limitations of the study must be addressed. Two different submaximal tests were performed on a cycle ergometer and a treadmill. Although the use of more than one exercise mode adds to the robustness of the findings and increase external validity, these laboratory conditions still do not reflect daily settings in which an individual is exercising. The type of exercise e.g. aerobic or anaerobic exercise, individual or in teams, time of the day and whether it is done outdoor rather than indoor as in the current study, might all have an influence on how one feels during and after exercise. Furthermore placing the treadmill test always in fixed order after the cycling test could have influenced the affective responses during the treadmill test even if Dutch adolescents are very used to cycling and the recovery time was enough to reach resting V̇O2 values. Most importantly, affective responses may have been influenced by the prospect of a maximal exercise test that would have to be completed at the end of the session. Many other studies on this topic use a separate day for maximal exercise testing which has the added advantage that workloads can be standardized exactly as a percentage of V̇O2max. However, our participants were not students recruited in the typical way from a single high school or college, but came from the entire country as they were selected from a nation-wide twin register. This meant substantial travel for most of them in a period when many of them were engaged in their final school year (with closing examination determining their further careers). To reduce burden on the participants and also for logistic reasons only a single measurement day was therefore deemed possible. Apart from creating a potential foreshadowing influence on the affective response to the submaximal exercise tasks, this may have led participants to have been too exhausted to perform optimally at the maximal exercise test. However, comparison of the V̇O2max predicted from the submaximal tests to the actual peak V̇O2 attained during the maximal exercise test suggest that such underestimation will have been mild and only mildly affect rank order of aerobic fitness levels (Schutte et al., 2016).
The acknowledgement of the existence of individual differences in affective response to exercise is key to the innovation of exercise programs. Moderate heritability estimates of these parameters do show that it may be harder to engage some people in exercise than others, but does not suggests that we should stop trying. It simply suggests that we should not close our eyes to human genetic variation. In the population at large, regular leisure time exercise seems associated with better mental health largely through pleiotropic genetic effects (Schutte et al., 2014). The longer term beneficial psychological effects of exercise appear to be more easily unlocked by some genetic profiles than by others. This may well be linked to the heritability of the psychological responses for a single bout of exercise, as tested in the present study in adolescents. Favourable genetic profiles may for instance cause a larger sensitivity to the rewarding or a smaller sensitivity to the punishing effects of broad classes of activities, including exercise. For some individuals, exercising may be associated with a strong ‘feel good’ experience and constitute an excellent short-term coping strategy that helps to unwind more rapidly from daily pressures experienced in the school, job or home environment. For others, the aversive effects of exercise, at least in the forms that they tried so far, may greatly overwhelm the rewarding effects, causing them to drop-out. These individuals might benefit more from an individualized exercise intervention, in which the appetitive aspects for an individual should be emphasized and the aversive aspects reduced as much as possible.
As the motivation to adopt and maintain regular exercise is key to a better public health, genetic pathways underlying individual differences in the affective responses to exercise should remain an important target for research. A main future challenge is to identify the specific genes underlying the heritability of the affective response to exercise, to test their predictive value for the adoption of regular exercise behavior in adolescence and in other age ranges as well as their usefulness in personalizing exercise intervention.
Highlights.
An important determinant of exercise behavior is the affective response to exercise
Genetic factors explain up until 37% of the variance in this affective response
This affective response shows significant (genetic) associations exercise behavior
Acknowledgments
We thank the members of the twin families registered with The Netherlands Twin Register for their continued support of scientific research. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases under Grant number RO1DK092127 and the Netherlands Organization for Scientific Research under Grant number 022.003.010.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O’Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, Jacobs DR, Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- Ament W, Verkerke GJ. Exercise and fatigue. Sports Med. 2009;39(5):389–422. doi: 10.2165/00007256-200939050-00005. [DOI] [PubMed] [Google Scholar]
- Anderson E, Shivakumar G. Effects of exercise and physical activity on anxiety. Front Psychiatry. 2013;4:27. doi: 10.3389/fpsyt.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63(1):182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, Spies J, Estabrook R, Kenny S, Bates T, Mehta P, Fox J. OpenMx: an open source extended structural equation modeling framework. Psychometrika. 2011;76(2):306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2(2):92–98. [PubMed] [Google Scholar]
- Bryan A, Hutchinson KE, Seals DR, Allen DL. A transdisciplinary model integrating genetic, physiological, and psychological correlates of voluntary exercise. Health Psychol. 2007;26(1):30–39. doi: 10.1037/0278-6133.26.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell Hooper AE, Bryan AD, Hagger MS. What keeps a body moving? The brain-derived neurotrophic factor val66met polymorphism and intrinsic motivation to exercise in humans. J Behav Med. 2014;37(6):1180–1192. doi: 10.1007/s10865-014-9567-4. [DOI] [PubMed] [Google Scholar]
- Chen CY, Bonham AC. Postexercise hypotension: central mechanisms. Exerc Sport Sci Rev. 2010;38(3):122–127. doi: 10.1097/JES.0b013e3181e372b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89–R98. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Bailey SP. Possible mechanisms of central nervous system fatigue during exercise. Med Sci Sports Exerc. 1997;29(1):45–57. doi: 10.1097/00005768-199701000-00008. [DOI] [PubMed] [Google Scholar]
- de Geus EJC, de Moor MHM. A genetic perspective on the association between exercise and mental health. Mental Health and Physical Activity. 2008;1(2):53–61. [Google Scholar]
- de Moor MH, Beem AL, Stubbe JH, Boomsma DI, de Geus EJ. Regular exercise, anxiety, depression and personality: a population-based study. Prev Med. 2006;42(4):273–279. doi: 10.1016/j.ypmed.2005.12.002. [DOI] [PubMed] [Google Scholar]
- de Moor MH, Boomsma DI, Stubbe JH, Willemsen G, de Geus EJ. Testing causality in the association between regular exercise and symptoms of anxiety and depression. Arch Gen Psychiatry. 2008;65(8):897–905. doi: 10.1001/archpsyc.65.8.897. [DOI] [PubMed] [Google Scholar]
- de Moor MH, Willemsen G, Rebollo-Mesa I, Stubbe JH, De Geus EJ, Boomsma DI. Exercise participation in adolescents and their parents: evidence for genetic and generation specific environmental effects. Behav Genet. 2011;41(2):211–222. doi: 10.1007/s10519-010-9415-4. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Jackson AS, Bray MS. Self-regulation of exercise behavior in the TIGER study. Ann Behav Med. 2014;48(1):80–91. doi: 10.1007/s12160-013-9573-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishman RK, McIver KL, Dowda M, Saunders RP, Pate RR. Motivation and Behavioral Regulation of Physical Activity in Middle School Students. Med Sci Sports Exerc. 2015;47(9):1913–1921. doi: 10.1249/MSS.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishman RK, Motl RW, Sallis JF, Dunn AL, Birnbaum AS, Welk GJ, Bedimo-Rung AL, Voorhees CC, Jobe JB. Self-management strategies mediate self-efficacy and physical activity. Am J Prev Med. 2005;29(1):10–18. doi: 10.1016/j.amepre.2005.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunton GF, Vaughan E. Anticipated affective consequences of physical activity adoption and maintenance. Health Psychol. 2008;27(6):703–710. doi: 10.1037/0278-6133.27.6.703. [DOI] [PubMed] [Google Scholar]
- Ekkekakis P. Pleasure and displeasure from the body: Perspectives from exercise. Cognition and Emotion. 2003;17(2):213–239. doi: 10.1080/02699930302292. [DOI] [PubMed] [Google Scholar]
- Ekkekakis P, Hargreaves E, Parfitt G. Envisioning the next fifty years of research on the exercise-affect relationship. Psychol Sport Exerc. 2013;14:751–758. [Google Scholar]
- Ekkekakis P, Hall EE, Petruzzello SJ. Variation and homogeneity in affective responses to physical activity of varying intensities: an alternative perspective on dose-response based on evolutionary considerations. J Sports Sci. 2005;23(5):477–500. doi: 10.1080/02640410400021492. [DOI] [PubMed] [Google Scholar]
- Ekkekakis P, Hall EE, Petruzzello SJ. The relationship between exercise intensity and affective responses demystified: to crack the 40-year-old nut, replace the 40-year-old nutcracker! Ann Behav Med. 2008;35(2):136–149. doi: 10.1007/s12160-008-9025-z. [DOI] [PubMed] [Google Scholar]
- Ekkekakis P, Parfitt G, Petruzzello SJ. The pleasure and displeasure people feel when they exercise at different intensities: decennial update and progress towards a tripartite rationale for exercise intensity prescription. Sports Med. 2011;41(8):641–671. doi: 10.2165/11590680-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Hagger M, Chatzisarantis N, Biddle S. A meta-analytic review of the theories of reasoned action and planned behavior in physical activity: predictive validity and the contribution of additional variables. Journal of Sport and Exercise Psychology. 2002;24(1):3–32. [Google Scholar]
- Hall EE, Ekkekakis P, Petruzzello SJ. The affective beneficence of vigorous exercise revisited. Br J Health Psychol. 2002;7(Pt 1):47–66. doi: 10.1348/135910702169358. [DOI] [PubMed] [Google Scholar]
- Hardy C, Rejeski W. Not what, but how one feels: the measurement of affect during exercise. J Sport Exerc Psychol. 1989;11:304–317. [Google Scholar]
- Hsu YC, Tsai SF, Yu L, Chuang JI, Wu FS, Jen CJ, Kuo YM. Long-term moderate exercise accelerates the recovery of stress-evoked cardiovascular responses. Stress. 2015;19(1):125–132. doi: 10.3109/10253890.2015.1108305. [DOI] [PubMed] [Google Scholar]
- Huppertz C, Bartels M, Groen-Blokhuis MM, Dolan CV, de Moor MH, Abdellaoui A, Van Beijsterveldt CE, Ehli EA, Hottenga JJ, Willemsen G, Xiao X, Scheet P, Davies GE, Boomsma DI, Hudziak JJ, de Geus EJ. The dopaminergic reward system and leisure time exercise behavior: a candidate allele study. Biomed Res Int 2014. 2014a;2014:591717. doi: 10.1155/2014/591717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz C, Bartels M, Jansen IE, Boomsma DI, Willemsen G, de Moor MH, de Geus EJ. A twin-sibling study on the relationship between exercise attitudes and exercise behavior. Behav Genet. 2014b;44(1):45–55. doi: 10.1007/s10519-013-9617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz C, Bartels M, Van Beijsterveldt CE, Boomsma DI, Hudziak JJ, De Geus EJ. Effect of shared environmental factors on exercise behavior from age 7 to 12 years. Med Sci Sports Exerc. 2012;44(10):2025–2032. doi: 10.1249/MSS.0b013e31825d358e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I, Leblanc AG. Systematic review of the health benefits of physical activity and fitness in school-aged children and youth. Int J Behav Nutr Phys Act. 2010;7:40. doi: 10.1186/1479-5868-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozkow P, Slowinska-Lisowska M, Laczmanski L, Medras M. DRD2 C313T and DRD4 48-bp VNTR polymorphisms and physical activity of healthy men in Lower Silesia, Poland (HALS study) Ann Hum Biol. 2013;40(2):186–190. doi: 10.3109/03014460.2012.748829. [DOI] [PubMed] [Google Scholar]
- Karoly HC, Stevens CJ, Magnan RE, Harlaar N, Hutchison KE, Bryan AD. Genetic Influences on Physiological and Subjective Responses to an Aerobic Exercise Session among Sedentary Adults. J Cancer Epidemiol. 2012;2012:540563. doi: 10.1155/2012/540563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knab AM, Lightfoot JT. Does the difference between physically active and couch potato lie in the dopamine system? Int J Biol Sci. 2010;6(2):133–150. doi: 10.7150/ijbs.6.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan BM, Bryan A. In-task and post-task affective response to exercise: translating exercise intentions into behaviour. Br J Health Psychol. 2010;15(Pt 1):115–131. doi: 10.1348/135910709X433267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey SG. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133–1163. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- Lerman I, Harrison BC, Freeman K, Hewett TE, Allen DL, Robbins J, Leinwand LA. Genetic variability in forced and voluntary endurance exercise performance in seven inbred mouse strains. J Appl Physiol. 2002;92(6):2245–2255. doi: 10.1152/japplphysiol.01045.2001. [DOI] [PubMed] [Google Scholar]
- Lightfoot JT, Turner MJ, Daves M, Vordermark A, Kleeberger SR. Genetic influence on daily wheel running activity level. Physiol Genomics. 2004;19(3):270–276. doi: 10.1152/physiolgenomics.00125.2004. [DOI] [PubMed] [Google Scholar]
- Martinez-Gonzalez MA, Varo JJ, Santos JL, De IJ, Gibney M, Kearney J, Martinez JA. Prevalence of physical activity during leisure time in the European Union. Med Sci Sports Exerc. 2001;33(7):1142–1146. doi: 10.1097/00005768-200107000-00011. [DOI] [PubMed] [Google Scholar]
- McAuley E, Blissmer B. Self-efficacy determinants and consequences of physical activity. Exerc Sport Sci Rev. 2000;28(2):85–88. [PubMed] [Google Scholar]
- Nigg CR. Explaining adolescent exercise behavior change: a longitudinal application of the transtheoretical model. Ann Behav Med. 2001;23(1):11–20. doi: 10.1207/S15324796ABM2301_3. [DOI] [PubMed] [Google Scholar]
- Parfitt G, Rose EA, Burgess WM. The psychological and physiological responses of sedentary individuals to prescribed and preferred intensity exercise. Br J Health Psychol. 2006;11(Pt 1):39–53. doi: 10.1348/135910705X43606. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Toward a physical basis of attention and self regulation. Phys Life Rev. 2009;6(2):103–120. doi: 10.1016/j.plrev.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes RE, Kates A. Can the Affective Response to Exercise Predict Future Motives and Physical Activity Behavior? A Systematic Review of Published Evidence. Ann Behav Med. 2015;49(5):715–731. doi: 10.1007/s12160-015-9704-5. [DOI] [PubMed] [Google Scholar]
- Rhodes RE, Martin AD, Taunton JE, Rhodes EC, Donnelly M, Elliot J. Factors associated with exercise adherence among older adults. An individual perspective. Sports Med. 1999;28(6):397–411. doi: 10.2165/00007256-199928060-00003. [DOI] [PubMed] [Google Scholar]
- Rhodes RE, Smith NE. Personality correlates of physical activity: a review and meta-analysis. Br J Sports Med. 2006;40(12):958–965. doi: 10.1136/bjsm.2006.028860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijsdijk FV, Sham PC. Analytic approaches to twin data using structural equation models. Brief Bioinform. 2002;3(2):119–133. doi: 10.1093/bib/3.2.119. [DOI] [PubMed] [Google Scholar]
- Rowland TW. The biological basis of physical activity. Med Sci Sports Exerc. 1998;30(3):392–399. doi: 10.1097/00005768-199803000-00009. [DOI] [PubMed] [Google Scholar]
- Ruby MB, Dunn EW, Perrino A, Gillis R, Viel S. The invisible benefits of exercise. Health Psychol. 2011;30(1):67–74. doi: 10.1037/a0021859. [DOI] [PubMed] [Google Scholar]
- Schneider M, Dunn A, Cooper D. Affective, exercise, and physical activity among healthy adolescents. J Sport Exerc Psychol. 2009;31(6):706–723. doi: 10.1123/jsep.31.6.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ML, Graham DJ. Personality, physical fitness, and affective response to exercise among adolescents. Med Sci Sports Exerc. 2009;41(4):947–955. doi: 10.1249/MSS.0b013e31818de009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte NM, Bartels M, de Geus EJ. Genetic modification of the effects of exercise behavior on mental health. Front Psychiatry. 2014;5:64. doi: 10.3389/fpsyt.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte NM, Nederend I, Hudziak JJ, Bartels M, de Geus EJ. Twin-sibling study and meta-analysis on the heritability of maximal oxygen consumption. Physiol Genomics. 2016;48(3):210–219. doi: 10.1152/physiolgenomics.00117.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonen RL, Rankinen T, Perusse L, Leon AS, Skinner JS, Wilmore JH, Rao DC, Bouchard C. A dopamine D2 receptor gene polymorphism and physical activity in two family studies. Physiol Behav. 2003;78(4–5):751–757. doi: 10.1016/s0031-9384(03)00084-2. [DOI] [PubMed] [Google Scholar]
- Skelton DA, Beyer N. Exercise and injury prevention in older people. Scand J Med Sci Sports. 2003;13(1):77–85. doi: 10.1034/j.1600-0838.2003.00300.x. [DOI] [PubMed] [Google Scholar]
- Solberg G, Robstad B, Skjonsberg OH, Borchsenius F. Respiratory gas exchange indices for estimating the anaerobic threshold. J Sports Sci Med. 2005;4(1):29–36. [PMC free article] [PubMed] [Google Scholar]
- Stubbe J, de Geus E. Genetics of Exercise Behavior. In: Kim YK, editor. Handbook of Behavior Genetics. New York, NY, USA: Springer Science+Business Media; 2009. pp. 343–358. [Google Scholar]
- Swallow JG, Carter PA, Garland T., Jr Artificial selection for increased wheel-running behavior in house mice. Behav Genet. 1998;28(3):227–237. doi: 10.1023/a:1021479331779. [DOI] [PubMed] [Google Scholar]
- Thayer RE. Activation-Deactivation Adjective Check List: Current overview and structural analysis. Psychol Rep. 1986;58:607–614. [Google Scholar]
- Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- Van Beijsterveldt CE, Groen-Blokhuis M, Hottenga JJ, Franic S, Hudziak JJ, Lamb D, Huppertz C, de ZE, Nivard M, Schutte N, Swagerman S, Glasner T, van FM, Brouwer C, Stroet T, Nowotny D, Ehli EA, Davies GE, Scheet P, Orlebeke JF, Kan KJ, Smit D, Dolan CV, Middeldorp CM, de Geus EJ, Bartels M, Boomsma DI. The Young Netherlands Twin Register (YNTR): longitudinal twin and family studies in over 70,000 children. Twin Res Hum Genet. 2013;16(1):252–267. doi: 10.1017/thg.2012.118. [DOI] [PubMed] [Google Scholar]
- van der Aa N, de Geus EJ, van Beijsterveldt TC, Boomsma DI, Bartels M. Genetic Influences on Individual Differences in Exercise Behavior during Adolescence. Int J Pediatr. 2010;2010:138345. doi: 10.1155/2010/138345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Landluyt L, Ekkekakis P, Hall E, Petruzzello S. Throwing mountains into the lakes: On the perils of nomothetic conceptions of the exercise-affect relationship. J Sport Exerc Psychol. 2000;22:208–234. [Google Scholar]
- Vartanian LR, Shaprow JG. Effects of weight stigma on exercise motivation and behavior: a preliminary investigation among college-aged females. J Health Psychol. 2008;13(1):131–138. doi: 10.1177/1359105307084318. [DOI] [PubMed] [Google Scholar]
- Welch A, Hulley A, Ferguson C, Beauchamp M. Affective responses of inactive women to a maximal incremental execise test: A test of the dual-mode model. Psychol Sport Exerc. 2007;8(4):401–423. [Google Scholar]
- Williams DM, Dunsiger S, Ciccolo JT, Lewis BA, Albrecht AE, Marcus BH. Acute Affective Response to a Moderate-intensity Exercise Stimulus Predicts Physical Activity Participation 6 and 12 Months Later. Psychol Sport Exerc. 2008;9(3):231–245. doi: 10.1016/j.psychsport.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DM, Dunsiger S, Jennings EG, Marcus BH. Does affective valence during and immediately following a 10-min walk predict concurrent and future physical activity? Ann Behav Med. 2012;44(1):43–51. doi: 10.1007/s12160-012-9362-9. [DOI] [PMC free article] [PubMed] [Google Scholar]