Abstract
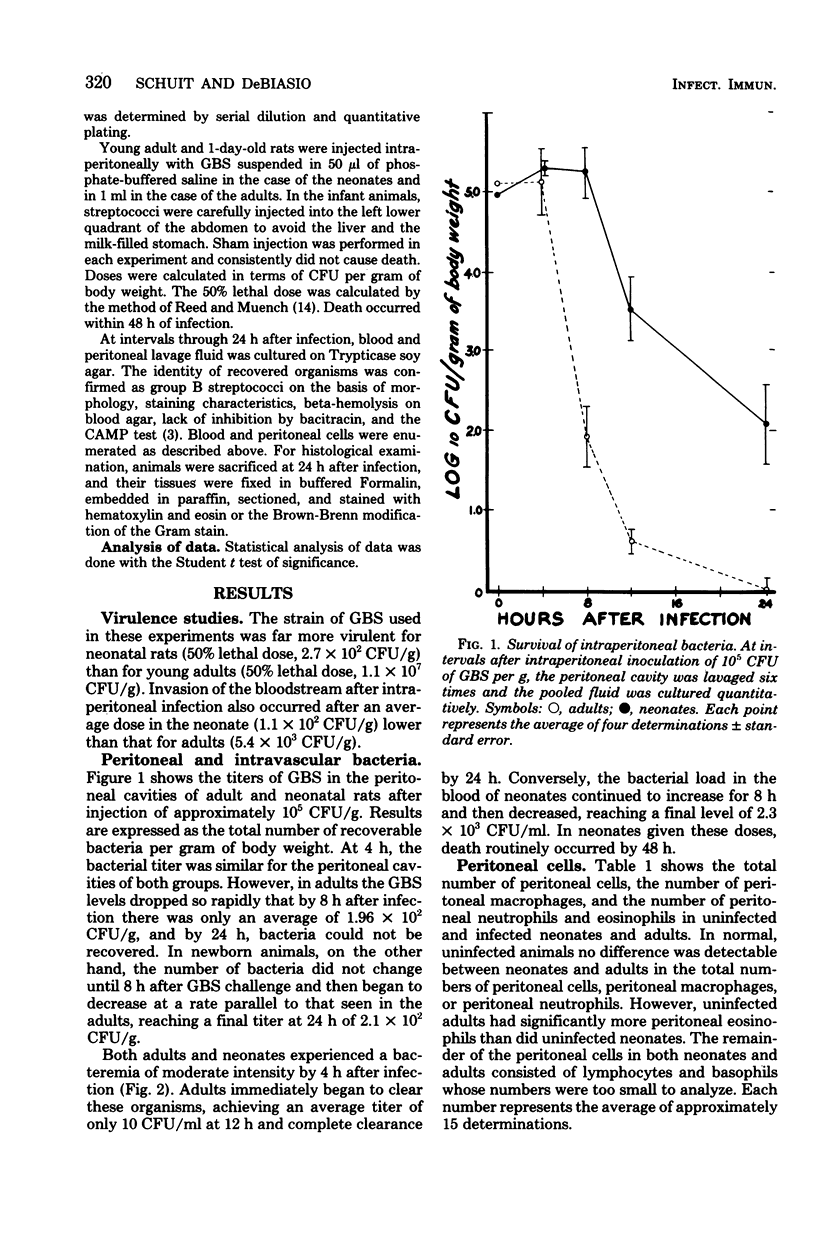
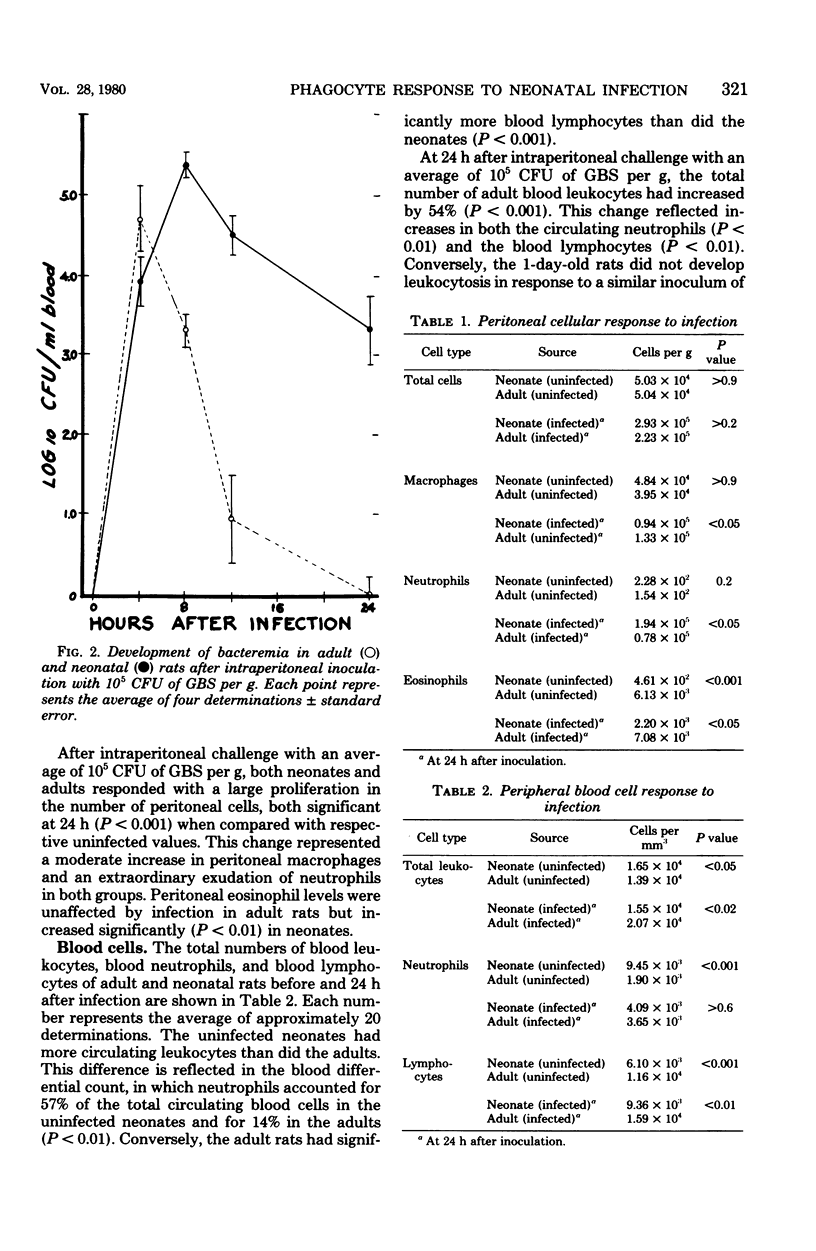
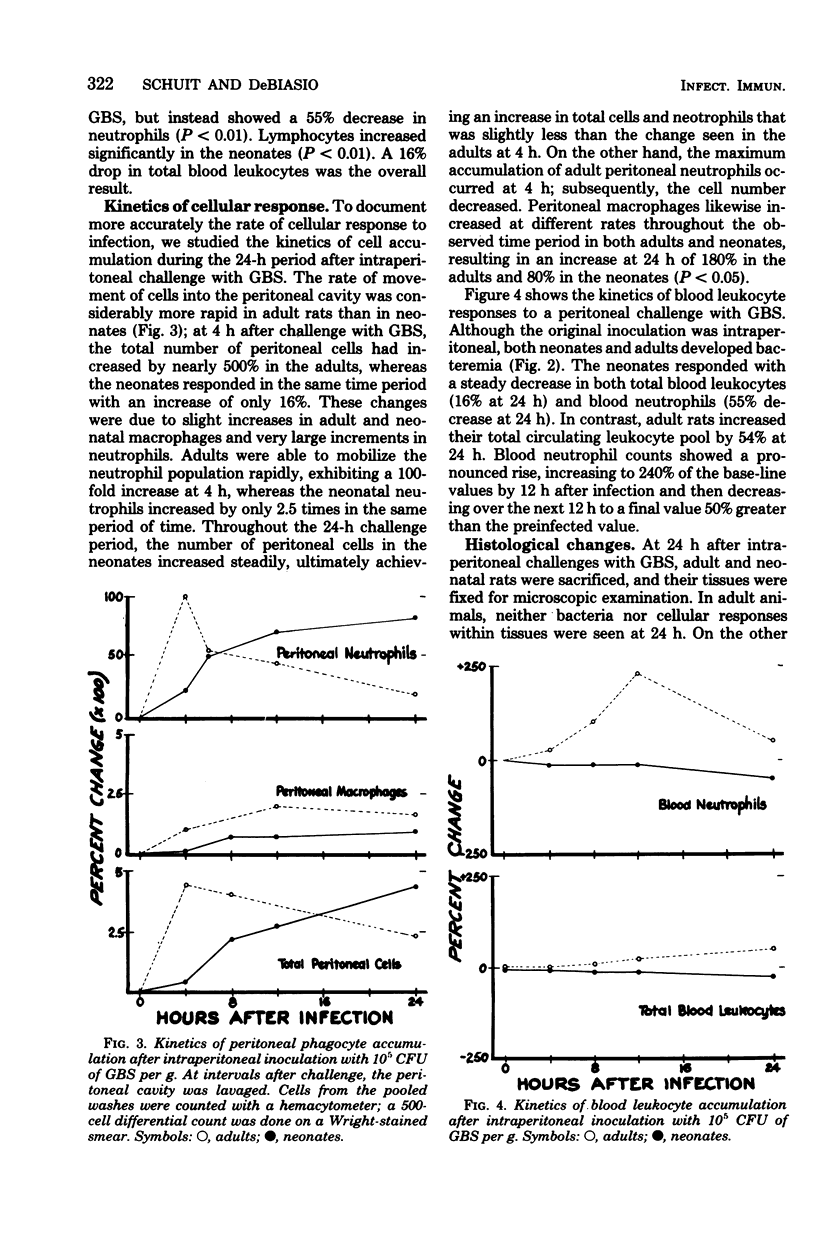
To test the hypothesis that inadequate in vivo mobilization of leukocytes may contribute to the unique susceptibility of neonates to infection, we studied the kinetics of phagocyte response to neonatal and adult rats to intraperitoneal infection with group B streptococcus, type Ia. The 50% lethal dose was considerably greater for adults than for neonates (1.1 x 10(7) colony-forming units per g versus 2.7 x 10(2) colony-forming units per g). After challenge with group B streptococcus, type Ia, the number of neonatal peritoneal leukocytes increased more slowly than did those of adult rats. For example, at 4 h, the adult neutrophil count was 41 times greater than that of the neonate, but at 24 h, neonatal peritoneal neutrophils had not yet reached the adult 4-h level. Peritoneal macrophages also increased more rapidly in adults than in neonates. After intraperitoneal infection, both adults and neonates developed bacteremia, but adults cleared the bacteria with greater efficiency. Adult blood neutrophils increased 247% by 12 h and then decreased; neonatal neutrophils steadily decreased to a 57% reduction by 24 h. These data suggest that the neonatal neutrophil response to group B streptococcus, type Ia, infection is inadequate and may contribute to the high mortality associated with this infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cline M. J., Moore M. A. Embryonic origin of the mouse macrophage. Blood. 1972 Jun;39(6):842–849. [PubMed] [Google Scholar]
- Dossett J. H., Williams R. C., Jr, Quie P. G. Studies on interaction of bacteria, serum factors and polymorphonuclear leukocytes in mothers and newborns. Pediatrics. 1969 Jul;44(1):49–57. [PubMed] [Google Scholar]
- Dretschmer R. R., Stewardson R. B., Papierniak C. K., Gotoff S. P. Chemotactic and bactericidal capacities of human newborn monocytes. J Immunol. 1976 Oct;117(4):1303–1307. [PubMed] [Google Scholar]
- Fireman P., Zuchowski D. A., Taylor P. M. Development of human complement system. J Immunol. 1969 Jul;103(1):25–31. [PubMed] [Google Scholar]
- Forman M. L., Stiehm E. R. Impaired opsonic activity but normal phagocytosis in low-birth-weight infants. N Engl J Med. 1969 Oct 23;281(17):926–931. doi: 10.1056/NEJM196910232811704. [DOI] [PubMed] [Google Scholar]
- Kay A. B. The eosinophil in infection diseases. J Infect Dis. 1974 May;129(5):606–613. doi: 10.1093/infdis/129.5.606. [DOI] [PubMed] [Google Scholar]
- Klein R. B., Fischer T. J., Gard S. E., Biberstein M., Rich K. C., Stiehm E. R. Decreased mononuclear and polymorphonuclear chemotaxis in human newborns, infants, and young children. Pediatrics. 1977 Oct;60(4):467–472. [PubMed] [Google Scholar]
- McCracken G. H., Jr, Eichenwald H. F. Leukocyte function and the development of opsonic and complement activity in the neonate. Am J Dis Child. 1971 Feb;121(2):120–126. doi: 10.1001/archpedi.1971.02100130074008. [DOI] [PubMed] [Google Scholar]
- Miller M. E. Pathology of chemotaxis and random mobility. Semin Hematol. 1975 Jan;12(1):59–82. [PubMed] [Google Scholar]
- Mills E. L., Thompson T., Björkstén B., Filipovich D., Quie P. G. The chemiluminescence response and bactericidal activity of polymorphonuclear neutrophils from newborns and their mothers. Pediatrics. 1979 Mar;63(3):429–434. [PubMed] [Google Scholar]
- Pahwa S. G., Pahwa R., Grimes E., Smithwick E. Cellular and humoral components of monocyte and neutrophil chemotaxis in cord blood. Pediatr Res. 1977 May;11(5):677–680. doi: 10.1203/00006450-197705000-00010. [DOI] [PubMed] [Google Scholar]
- Wright W. C., Jr, Ank B. J., Herbert J., Stiehm E. R. Decreased bactericidal activity of leukocytes of stressed newborn infants. Pediatrics. 1975 Oct;56(4):579–584. [PubMed] [Google Scholar]
- Yang H. Y., Skinsnes O. K. Peritoneal macrophage response in neonatal mice. J Reticuloendothel Soc. 1973 Aug;14(2):181–191. [PubMed] [Google Scholar]
- Zeligs B. J., Nerurkar L. S., Bellanti J. A., Zeligs J. D. Maturation of the rabbit alveolar macrophage during animal development. I. Perinatal influx into alveoli and ultrastructural differentiation. Pediatr Res. 1977 Mar;11(3 Pt 1):197–208. doi: 10.1203/00006450-197703000-00011. [DOI] [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968 Sep 1;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]


