Abstract
Purpose
The clinical significance and prognostic role of circulating plasma cells (CPCs) in multiple myeloma (MM) are still controversial. We conducted the first meta-analysis to clarify the correlation between CPCs and the clinicopathological features and prognosis of MM patients.
Methods
A comprehensive literary search for relevant studies was performed on PubMed, Embase, Medline, CNKI (Chinese) and Web of Science databases (January 1, 1950 to December 20, 2016). The associations between CPCs and survival rate and clinicopathological parameters, including International staging system (ISS) and Durie-Salm staging system (DS) stage, were evaluated. Then pooled hazard ratios (HRs) for survival with 95% confidence intervals (CIs), subgroup analysis, sensitivity analysis, and publication bias were conducted.
Results
11 studies covering a total of 2943 patients were included. Pooled hazard ratios (HRs) revealed that the presence of CPCs predicted aggressive disease progression (HR = 1.78, 95% CI = 1.57–2.03) and reduced overall survival (OS) (HR = 1.82, 95% CI = 1.59–2.08). Subgroup analyses demonstrated that CPCs positive patients also had poor disease progression and OS in detection methods and sampling time subsets. Moreover, the presence of CPCs was strikingly associated with increased ISS stage (OR = 2.78% CI = 1.69–4.56), but not with DS stage(OR = 1.60; 95% CI = 0.74–3.47).
Conclusions
CPCs status is associated with poorer survival outcome in multiple myeloma. Additionally, increased ISS stage could be significant risk factors for the presence of CPCs.
Introduction
Multiple myeloma (MM) is a hematological malignancy characterized by the proliferation of clonal plasma cells in the bone marrow [1], predominantly occurring in the elderly with an incidence of 10,000 deaths per year in the United States and Europe [2]. The advent of novel proteasome inhibitors and immunomodulatory drugs has significantly improved response rates and progression-free survival [3]. However, MM can often become refractory to treatment. Therefore, early identification of disease progression and relapse has become increasingly important for prognosis in MM patients.
As it is known, bone marrow plasma cells examination is a golden standard for evaluation the tumor burden and an indicator to assess the prognosis and response for MM patients [4]. Whereas, several studies utilizing various methods suggested that there were small numbers of plasma cells in peripheral blood [5], namely, circulating plasma cells (CPCs), and demonstrated that it had prognostic value among MM, MGUS [6], smoldering MM [7,8] and amyloidosis [9]. However, the prognostic value of CPCs in MM remains controversial. Some studies revealed that CPC status could predict poorer survival outcomes, while other studies failed to support this conclusion [10]. In addition, Peceliunas et al. [11] showed that CPCs status had prognostic relevance for time to tumor progression (TTP) but not for overall survival (OS) in MM patients. Similarly, Vagnoni et al. [12] proposed that CPCs status was associated with time to tumor progression (TTP) but not for overall survival (OS) in multivariate analysis. Interestingly, even in the same trial, CPCs detected at different time points indicated different prognoses of survival for MM participants [13]. These discrepancies may result from the small sample sizes used in these studies as well as differences in the sampling times and detection methods used.
The clinicopathological features such as ISS and DS stages of MM patients with CPCs have been analyzed in several studies, but controversies exist. Some studies [12,13] showed that the level of CPCs appeared to be largely independent of ISS and DS stages. However, in other studies, the presence of CPCs correlated more closely with DS [10] and ISS [10,14,15] stages. Hence, we also evaluated the association between CPCs and ISS, DS stages.
With the development of highly sensitive and specific diagnostic methods, including polymerase chain reaction (PCR), flow cytometry (FCM), slide-based immunofluorescence assay (IF) and conventional morphology (CM), it would be desirable to explore whether CPCs may serve as one biomarker for prognostic prediction and treatment option in MM. Here, we conducted the first meta-analysis to provide a better insight into the prognostic value of CPCs on disease progression and OS in MM patients. Furthermore, subgroup analyses were performed to evaluate whether the detection methods and time points of blood collection influence the prognostic value of CPCs.
Materials and methods
Search strategy
A literary search was performed on PubMed, Embase, Medline, CNKI (Chinese) and Web of Science databases (January 1, 1950 to December 20, 2016). Search term combinations were “multiple myeloma,” “myeloma,” “plasma cell myeloma,” “circulating plasma cell,” “circulating myeloma cell,” “peripheral blood plasma cell,” “monoclonal plasma cell,” in title/abstract. We also attempted to identify other potentially relevant articles by searching the reference sections of qualified manuscripts. we contacted authors by email if the data in studies was insufficient. If authors could not be contacted, the studies subsequently excluded.
Selection criteria and quality assessment
Studies were included only if they following the inclusion criteria: (1) focus on the correlations between CPCs status and clinicopathological features or survival outcomes (either disease progression or OS) in MM patients; (2) sufficient data was provided to extract HR with 95% confidence intervals (CIs); (3) when the same study cohort was published at several reports, only the most complete one was included in our meta-analysis; (4) each study enrolling more than 20 patients; (5) published in English. Studies such as reviews, letters, editorials, conference meeting, case reports abstracts and comments were excluded. We also excluded studies in which the outcomes of interest were not reported or if it was impossible to calculate outcomes from the originally published data. Two investigators independently evaluated the quality of included studies using the Newcastle-Ottawa Scale (NOS) for cohort study [16]. Any discrepancy was resolved by discussion or consultation with a third party if required.
Data extraction
Two of the authors independently collected the following data from each eligible study: first author’s name, publication year, study design, country, age, number of subjects analyzed, disease stage, median follow-up, sampling times, detection method, and cutoff of CPCs. HRs with 95% CIs for OS and disease progression including progression-free survival (PFS), relapse-free survival (RFS), time to progression (TTP), time to next therapy (TTNT) and event-free survival (EFS). HRs and 95% CIs were extracted from multivariable analyses. If the HR were not provided directly in the original study, we calculated these values from available reported data using software designed by Tierney et al. [17]. When CPCs was detected according to different time points in one study, such as before and after stem cell transplantation, both data were extracted.
Statistical analysis
The extracted information was analyzed using STATA software version 12.0. The disease progression and OS outcomes were evaluated by HR. When analyzing the association between CPCs and ISS, DS stage, Odd ratio (OR) was calculated. Simultaneously, subgroup analysis was performed on the basis of the detection methods and sample times. The pooled HRs using a fixed- or random-effect model was according to heterogeneity. Heterogeneity was tested using Cochran’s Q test and quantified by the I2 index, which is considered significant if P < 0.10 or I2 > 50% by convention. To evaluate the robustness of the results of our meta-analysis in the presence of uncertainty, we performed a sensitivity analysis estimating the average HR by omitting one study each time. Publication bias was evaluated with Begg’s and Egger’s test (a p-value < 0.05 was considered statistically significant).
Results
Description of included studies
A total of 661 potentially studies were identified using our search strategy. 612 studies were deemed ineligible after title and abstract screening. 46 potential studies were reviewed via the full-text. Then 36 studies were excluded because they were not meet the inclusion criteria, leaving 11 studies [10–15,18–22] that were eligible included in the meta-analysis (Fig 1).
Fig 1. Flow diagram showing the selection process for the including studies.
2943 MM patients were included and were conducted in 5 countries (China, Germany, USA, Lithuania and Italy), published between 1996 and 2016. Peripheral blood were analyzed and methods used to detect CPCs were FCM, PCR, IF and CM. The sampling times were divided into 4 time points (first diagnosis, before stem cell transplantation, after stem cell transplantation, and relapsed/refractory MM). 647 relapsed/refractory (RA) MM patients were included in Gonsalves’s study [22], but only 145 actively relapsing patients were analyzed in the Kaplan-Meier OS distributions. Korthal’s study [13] detected CPCs before and after stem cell transplantation (SCT) and both have the prognostic analysis, so two HRs were extracted. HRs with 95% CIs were directly extracted from original articles in 6 studies [12,14,15,18,20,22]. Five studies[10,11,13,19,21] did not report HRs with 95% CIs directly, so these values were calculated according to the method suggested by Tierney et al. [17]. The characteristics and the quality of the included studies evaluated with the NOS are summarized in Table 1.
Table 1. Main characteristics of included studies.
| Study | Study design | Country | No. of patients(M/F) | Age (median, range) | Stage | Detectionmethod | Cutoff of CPCs | Sampling times | Follow up | NOS | Outcome | HR | 95%CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Witzig 1996 | Pro | USA | 254(152/102) | 63.6(20.0–92.9) | NR | IF | 3×106/L | First diagnosis | NR | 6 | OS | 2.05 | 1.45–2.91 |
| Gertz 1997 | Pro | USA | 33(19/14) | 52(32–64) | NR | IF | 0.2×106/L | B-SCT | NR | 5 | RFS | 1.58 | 0.52–4.80 |
| OS | 1.26 | 0.34–4.68 | |||||||||||
| Nowakowski 2005 | Pro | USA | 302(180/123) | 65(29–94) | NR | FCM | 10 cells/50 000 | First diagnosis | 33.5 | 7 | OS | 1.42 | 1.01–1.99 |
| Dingli 2006 | Pro | USA | 246(155/91) | 57.2(30–74) | D-S I-III | FCM | 1 cells/50 000 | B-SCT | 34 | 8 | TTP | 1.48 | 1.09–1.99 |
| OS | 1.64 | 1.10–2.43 | |||||||||||
| Peceliunas 2012 | Pro | Lithuania | 42(NR) | 57(39–74) | NR | FCM | 20 cells/1 000 000 | RA | 21 | 7 | OS | 2.33 | 1.01–5.36 |
| Korthals 2013 | Pro | Germany | 21(NR) | 55(44–65) | ISS I-III | PCR | 0.01% | B-SCT | 45 | 8 | EFS | 1.53 | 0.18–13.36 |
| D-S I-III | OS | 1.76 | 0.12–25.79 | ||||||||||
| Korthals 2013* | Pro | Germany | 32(NR) | 55(42–62) | ISS I-III | PCR | 0.01% | A-SCT | 45 | 8 | EFS | 4.41 | 1.56–12.48 |
| D-S I-III | OS | 5.88 | 1.03–33.59 | ||||||||||
| Gonsalves 2014 | Retro | USA | 157(93/62) | 65(39–95) | ISS I-III | FCM | 400 cells/150 000 | First diagnosis | NR | 5 | TTNT | 1.85 | 1.07–3.16 |
| OS | 3.16 | 1.43–7.08 | |||||||||||
| Gonsalves WI 2014 | Retro | USA | 145(NR) | 63(43–80) | NR | FCM | 100 cells/150 000 | RA | 21 | 8 | OS | 2.67 | 1.37–5.19 |
| An 2015 | Retro | China | 767(471/296) | 59 | ISS I-III | CM | 2% | First diagnosis | 41 | 8 | PFS | 1.54 | 1.22–1.95 |
| D-S I-III | OS | 1.59 | 1.26–2.00 | ||||||||||
| Vagnoni 2015 | Pro | Italy | 104(52/52) | 72(45–85) | ISS I-III | FCM | 41 cells/50 000 | First diagnosis | 35.9 | 6 | PFS | 2.63 | 1.51–5.92 |
| D-S I-III | |||||||||||||
| Chakraborty 2016 | Retro | USA | 840(500/340) | 61.1(24.4–76.1) | ISS I-III | FCM | 1 cells/15 0000 | B-SCT | 44 | 8 | PFS | 2.03 | 1.64–2.50 |
| OS | 2.52 | 1.78–3.55 |
* Same literature but different sampling time
NR: Not Reported; HR: hazard ratio; CI: confidence intervals; NOS: Newcastle-Ottawa Scale; CPCs: circulating plasma cells; IF: immunofluorescence; PCR: polymerase chain reaction; FCM: flow cytometry; CM: conventional morphology; B-SCT: before stem cell transplantation; A-SCT: after stem cell transplantation; RA: relapsed or refractory multiple myeloma; ISS: international staging system; DS: Durie-Salm staging system; OS: overall survival; PFS: progression-free survival; RFS: relapse-free survival; TTP: time to progression; TTNT: time to next therapy; EFS: event-free survival; Pro: prospective; Retro: retrospective
Overall analyses
The HRs for disease progression were available in 8 studies [10–15,19,21]. In Korthal’s study [13], two HRs were extracted according to different sampling times. The estimated pooled HR showed an increased risk of disease progression in patients with CPC positive group (HR = 1.78, 95%CI = 1.57–2.03). The heterogeneity among studies was not noted (P = 0.327, I2 = 12.9%). Data on OS were available in 9 studies [10,13–15,18–22]. Two HRs were extracted in Korthal’s study for the same reason as mentioned above. The pooled HRs showed a significantly increased risk of mortality in patients with CPC positive group (HR = 1.82, 95%CI = 1.59–2.08). Heterogeneity among studies was not noted (P = 0.186 and I2 = 28.1%).
Subgroup analyses
When assessing the effects of CPCs status on outcomes for different detection methods, the “FCM” subgroup and “CM” subgroup indicated a worse prognosis for both disease progression (FCM: HR = 1.88, 95%CI = 1.60–2.20; CM: HR = 1.54, 95%CI = 1.22–1.94)(Fig 2) and OS (FCM: HR = 1.91, 95% CI = 1.58–2.31; CM: HR = 1.58, 95%CI = 1.25–2.00)(Fig 3). However, the “PCR” subgroup exhibited prognostic significance for disease progression (HR = 3.60, 95%CI = 1.41–9.19) but not for OS (HR = 4.11, 95% CI = 0.95–17.75). The “IF” subgroup showed prognostic significance for OS (HR = 1.99, 95% CI = 1.41–2.79) but not for disease progression (HR = 1.58, 95%CI = 0.52–4.84). Significant heterogeneity was not found in “FCM” subgroup, “CM” subgroup, “PCR” subgroup and “IF” subgroup.
Fig 2. Forest plot of HRs and 95%CIs for disease progression.
Subgroup analysis was according to different detection methods. The black boxes’ sizes are proportional to the study weight, with the lines indicating 95% confidence intervals (CIs).
Fig 3. Forest plot of HRs and 95%CIs for OS.
Subgroup analysis was according to different detection methods. The black boxes’ sizes are proportional to the study weight, with the lines indicating 95% confidence intervals (CIs). HR higher than 1 indicate that the presence of CPCs is associated with worse prognosis.
In addition, we explored the effects of CPCs status on outcomes for various sampling times. CPCs detected at first diagnosis and before SCT indicated an increased risk for both disease progression (First diagnosis: HR = 1.66, 95%CI = 1.35–2.04; Before SCT: HR = 1.81, 95% CI = 1.53–2.15) (Fig 4) and OS (First diagnosis: HR = 1.68, 95% CI = 1.42–1.98; Before SCT: HR = 2.03, 95% CI = 1.57–2.62) (Fig 5). CPCs detected at RA and After SCT exhibited prognostic significance for both disease progression (RA: HR = 2.34, 95% CI = 1.03–5.33; after SCT: HR = 4.39, 95%CI = 1.55–12.41) and OS (RA: HR = 2.66, 95% CI = 1.37–5.19; after SCT: HR = 5.87, 95% CI = 1.03–33.59), but both of subgroups only had 1 study.
Fig 4. Forest plot of HRs and 95%CIs for disease progression.
Subgroup analysis was according to different sampling time: four studies assessed CPCs before SCT; three studies assessed CPCs at first diagnosis. HR higher than 1 indicate that the presence of CPCs is associated with worse prognosis.
Fig 5. Forest plot of HRs and 95%CIs for OS.
Subgroup analysis was according to different sampling time: four studies assessed CPCs at first diagnosis; four studies assessed CPCs before SCT. HR higher than 1 indicate that the presence of CPCs is associated with worse prognosis.
CPCs and ISS, DS stage
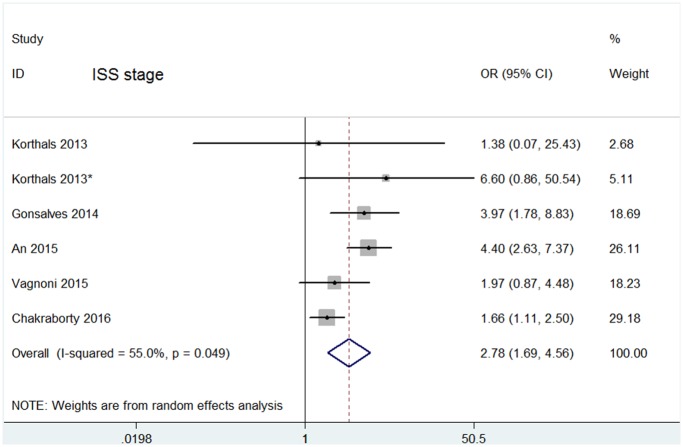
The ISS and DS stage are two criteria for the diagnosis of multiple myeloma. 5 studies[10,12–15] reported the relationship between CPCs positivity and ISS, while other 4 studies[10,12,13,21] reported DS stage separately. The overall positive rate of CPCs in ISS I~II stage group was 13.88% lower than the 26.61% of ISS III group, whereas in DS I~II stage and DS III stage are 18.34% and 22.97% respectively. Pooled analysis also showed that CPCs positivity in ISS III is greater than that of ISS I~II (OR = 2.78, 95% CI = 1.69–4.56, random effects), the heterogeneity among studies is present (I2 = 55.0%, p = 0.049) as shown in Fig 6, but not in the DS stage (OR = 1.60, 95% CI = 0.74–3.47, random effects), with significant heterogeneity between studies (I2 = 54.4%, p = 0.067) as shown in S1 Fig.
Fig 6. Forest plots of the association between CPCs and ISS stage.
Odd ratio (OR) higher than 1 indicate that CPCs were more frequently detected in patients with increased ISS stage.
Sensitivity analyses and publication bias
A sensitivity analysis was performed by removing one study each time and analyzing the heterogeneity across the remaining studies. Our result showed that no individual studies significantly influenced the HRs of disease progression (S2 Fig) and OS (S3 Fig). Begg’s test showed that no significant publication bias was found in the pooled analysis of disease progression (S4 Fig) and (S5 Fig). Furthermore, we also performed Egger’s test for disease progression and OS. The P value of the Egger’s test was 0.317 and 0.212, which were > 0.05, also demonstrating that there was no publication bias existed.
Discussion
Many studies have shown that the presence of CPCs was significantly associated with prognosis or other clinicopathologic parameters in MM patients. While, the lack of similar detection methods or consistent cutoff values in different study designs has hampered efforts to effectively treat and monitor disease progression. In an effort to prove the clinical significance of CPCs, we presented the first meta-analysis to evaluate the value of CPCs in MM patients. Overall, our results demonstrated that patients in CPC positive group had a worse OS and more aggressive disease progression compared with CPC negative group. Moreover, the presence of CPCs was associated with elevated ISS, but not with DS stage.
Subgroup analysis for OS and disease progression by detection methods showed that “FCM” subgroups presented significant associations with no heterogeneity, whereas it was not significant in the "PCR" subgroup. Though Paiva et al. [23] have revealed that PCR-based approach is more sensitive (10−6) as compared with FCM-based method (10−4), it has some limitations. PCR–based technique requires fixed and stable gene mutation or fusion gene fragment for individual follow-up of patients [24–27], so it can only be applied to approximately 75% of patients [25]. Moreover, cells detected by PCR requiring specific primers are difficult to reflect the corresponding amount of tumor cells in vivo, and this may explain why CPCs detected by PCR wasn’t associated with OS. FCM for the detection of CPCs is widely used with objectivity, high efficiency and strong reproducibility, since more than 90% of MM patients express plasma cell aberrant immunophenotype [24]. More importantly, the sensitivity of FCM can be improved through acquiring a large number of cells such as 150 000 events [14,15], 100 0000 events [11]. The “IF” subgroup showed prognostic significance for OS but not for disease progression. A slide-based immunofluorescence (IF) assay can be subjective, low sensitive depending on the ability of the morphologist consuming intense labor and much time to recognize CPCs, thus limiting its clinical application [14]. A rational explanation of An et al. Study [10] is that compared with the above methods, the sensitivity and specificity of conventional morphology (CM) are lowest, and the number of cells analyzed is least. Consequently, FCM used to detect CPCs was more likely to associate with OS according to our subgroup analysis.
One essential question was whether CPCs in peripheral blood at a single time point were of prognostic relevance. So further subgroup analysis based on sampling times including at the time of first diagnosis, RA, before SCT and after SCT were performed. Pooled HRs for OS and disease progression were fairly stable and not influenced by the sampling times. This suggested that the presence of CPCs in peripheral blood indicates poor prognosis in MM patients. Considering that “RA” and “After-SCT” subgroups only included one study, more research was required to assess the effectiveness and stability of these sampling times.
Our meta-analysis also underscored the presence of CPCs being closely associated with elevated ISS stage. This suggests that CPCs were more frequently detected in patients with increased ISS stage. Whereas DS stage failed to shows this association. Prognostic factors involve clinical features, laboratory parameters and imaging examinations. ISS stage based on serum albumin and β2-microglobulin, adopted by WHO as a unified system for MM, is mainly applied to determine the prognosis [28]. It has prognostic value for patients who received conventional treatments as well as autologous hematopoietic stem cell transplantation [29]. However, with the development of newer therapies, the DS system has less prognostic impact on survival [29]. This may be associated with the fact that these treatments can strikingly reduce tumor burden and the DS stage predominantly reflects tumor burden [28], thus weakening its significance. Kumar et al. [30]and Nowakowski et al. [20]have also supported that CPCs correlated with aggressive disease rather than tumor burden. CPCs have higher propensity to circulate, strong ability to migrate and invade, then spread the tumor cells to various parts of the bone marrow, and lead to metastasis or relapse [31]. This proposed model for spread shows intriguing similarities to epithelial–mesenchymal transformation (EMT) in solid tumors [32]. Significant heterogeneity was observed in the ISS and DS data, sample size might have contributed to the heterogeneity. More large-scale studies are warranted to validate the clinical power of CPCs status.
Our study has some limitations. First, our meta-analysis included only 11 eligible studies, of which were small retrospective case-series, which resulted in relatively insufficient statistical power with regard to estimating the prognostic role of CPCs in MM patients. Thus larger, prospective studies should be performed. Second, some HRs were extracted from the survival curves, which inevitably brought about small statistical errors. Third, CPCs detection methods and cutoff values were different among 11 included studies, but FCM was most broadly applied in 7 studies, and the ranges of cut-off values in First diagnosis, RA and B-SCT group were 2~26.67×10−4, 0.2~6.67×10−4 and 0.067~0.2×10−4 respectively, mainly fluctuating at 10−4. Some studies[23] [25]also demonstrate that the sensitivity of FCM to detect minimal residue disease is about 10−4. Certainly, a unified detection method and cutoff value still need to be established. Finally, we did not acquire all effective clinicopathological data such as β2-microglobulin, cytogenetic abnormality, and salvage treatment options from the included studies. If these data were available, our meta-analysis might have provided sufficient evidence to evaluate the impact of treatment intervention.
In conclusion, our meta-analysis indicates that the presence of CPCs was associated with aggressive disease course and poor OS in MM patients. CPCs status was strongly associated with elevated ISS stage and represents aggressive disease rather than tumor burden. Regardless of whether CPCs are detected in an early stage or in relapse patients, CPCs status may serve as a useful tool to guide the prognosis of MM patients. Considering the limitations of present analysis, further prospective multicentre studies designed with larger sample size and unified detection methods are needed.
Supporting information
(DOC)
(DOCX)
Odd ratio (OR) higher than 1 indicate that CPCs were more frequently detected in patients with increased DS stage.
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
Acknowledgments
We thank the Jie Lu PhD and Dr Yi Zhuang for their guide in the statistical method, and Dr Robert P. Seifert for critiquing the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Rajkumar SV (2009) Multiple myeloma. Curr Probl Cancer 33: 7–64. doi: 10.1016/j.currproblcancer.2009.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham Deepa MM, Jemal Ahmedin (2013) Cancer statistics, 2013. Ca A Cancer Journal for Clinicians 63: 11–30. doi: 10.3322/caac.21166 [DOI] [PubMed] [Google Scholar]
- 3.Kumar SK, Dingli D, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK, et al. (2008) Autologous stem cell transplantation in patients of 70 years and older with multiple myeloma: Results from a matched pair analysis. Am J Hematol 83: 614–617. doi: 10.1002/ajh.21191 [DOI] [PubMed] [Google Scholar]
- 4.Palumbo A, Anderson K. (2011)Multiple Myeloma. N Engl J Med 364:1046–1060. doi: 10.1056/NEJMra1011442 [DOI] [PubMed] [Google Scholar]
- 5.Witzig TE, Kyle RA, O'Fallon WM, Greipp PR. (1994) Detection of peripheral blood plasma cells as a predictor of disease course in patients with smouldering multiple myeloma. Br J Haematol 87:266–272. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, Rajkumar SV, Kyle RA, Lacy MQ, Dispenzieri A, Fonseca R, et al. (2005) Prognostic value of circulating plasma cells in monoclonal gammopathy of undetermined significance. J Clin Oncol 23: 5668–5674. doi: 10.1200/JCO.2005.03.159 [DOI] [PubMed] [Google Scholar]
- 7.Bianchi G, Kyle RA, Larson DR, Witzig TE, Kumar S, Dispenzieri A, et al. (2013) High levels of peripheral blood circulating plasma cells as a specific risk factor for progression of smoldering multiple myeloma. Leukemia 27: 680–685. doi: 10.1038/leu.2012.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonsalves WI, Rajkumar SV, Dispenzieri A, Dingli D, Timm MM, Morice WG, et al. (2017) Quantification of circulating clonal plasma cells via multiparametric flow cytometry identifies patients with smoldering multiple myeloma at high risk of progression. Leukemia 31: 130–135. doi: 10.1038/leu.2016.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pardanani A, Witzig TE, Schroeder G, McElroy EA, Fonseca R, Dispenzieri A, et al. (2003) Circulating peripheral blood plasma cells as a prognostic indicator in patients with primary systemic amyloidosis. Blood 101: 827–830. doi: 10.1182/blood-2002-06-1698 [DOI] [PubMed] [Google Scholar]
- 10.An G, Qin X, Acharya C, Xu Y, Deng S, Shi L, et al. (2015) Multiple myeloma patients with low proportion of circulating plasma cells had similar survival with primary plasma cell leukemia patients. Ann Hematol 94: 257–264. doi: 10.1007/s00277-014-2211-0 [DOI] [PubMed] [Google Scholar]
- 11.Peceliunas V, Janiulioniene A, Matuzeviciene R, Zvirblis T, Griskevicius L (2012) Circulating plasma cells predict the outcome of relapsed or refractory multiple myeloma. Leuk Lymphoma 53: 641–647. doi: 10.3109/10428194.2011.627481 [DOI] [PubMed] [Google Scholar]
- 12.Vagnoni D, Travaglini F, Pezzoni V, Ruggieri M, Bigazzi C, Dalsass A, et al. (2015) Circulating plasma cells in newly diagnosed symptomatic multiple myeloma as a possible prognostic marker for patients with standard-risk cytogenetics. Br J Haematol 170: 523–531. doi: 10.1111/bjh.13484 [DOI] [PubMed] [Google Scholar]
- 13.Korthals M, Sehnke N, Kronenwett R, Schroeder T, Strapatsas T, Kobbe G, et al. (2013) Molecular monitoring of minimal residual disease in the peripheral blood of patients with multiple myeloma. Biol Blood Marrow Transplant 19: 1109–1115. doi: 10.1016/j.bbmt.2013.04.025 [DOI] [PubMed] [Google Scholar]
- 14.Gonsalves WI, Rajkumar SV, Gupta V, Morice WG, Timm MM, Singh PP, et al. (2014) Quantification of clonal circulating plasma cells in newly diagnosed multiple myeloma: implications for redefining high-risk myeloma. Leukemia 28: 2060–2065. doi: 10.1038/leu.2014.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakraborty R, Muchtar E, Kumar SK, Jevremovic D, Buadi FK, Dingli D, et al. (2016) Risk stratification in myeloma by detection of circulating plasma cells prior to autologous stem cell transplantation in the novel agent era. Blood Cancer J 6: e512 doi: 10.1038/bcj.2016.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25: 603–605. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 17.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8: 16 doi: 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witzig TE, Gertz MA, Lust JA, Kyle RA, O'Fallon WM, Greipp PR (1996) Peripheral blood monoclonal plasma cells as a predictor of survival in patients with multiple myeloma. Blood 88:1780–1787. [PubMed] [Google Scholar]
- 19.Gertz MA, Witzig TE, Pineda AA, Greipp PR, Kyle RA, Litzow MR(1997) Monoclonal plasma cells in the blood stem cell harvest from patients with multiple myeloma are associated with shortened relapse-free survival after transplantation. Bone Marrow Transplant 19:337–342. doi: 10.1038/sj.bmt.1700670 [DOI] [PubMed] [Google Scholar]
- 20.Nowakowski GS, Witzig TE, Dingli D, Tracz MJ, Gertz MA, Lacy MQ, et al. (2005) Circulating plasma cells detected by flow cytometry as a predictor of survival in 302 patients with newly diagnosed multiple myeloma. Blood 106: 2276–2279. doi: 10.1182/blood-2005-05-1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dingli D, Nowakowski GS, Dispenzieri A, Lacy MQ, Hayman SR, Rajkumar SV, et al. (2006) Flow cytometric detection of circulating myeloma cells before transplantation in patients with multiple myeloma_ a simple risk stratification system. Blood. 107:3384–3388. doi: 10.1182/blood-2005-08-3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonsalves WI, Morice WG, Rajkumar V, Gupta V, Timm MM, Dispenzieri A, et al. (2014) Quantification of clonal circulating plasma cells in relapsed multiple myeloma. Br J Haematol 167: 500–505. doi: 10.1111/bjh.13067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paiva B, Vidriales MB, Cervero J, Mateo G, Perez JJ, Montalban MA, et al. (2008) Multiparameter flow cytometric remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantation. Blood 112: 4017–4023. doi: 10.1182/blood-2008-05-159624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rawstron AC, Orfao A, Beksac M, Bezdickova L, Brooimans RA, Bumbea H, et al. (2008) Report of the European Myeloma Network on multiparametric flow cytometry in multiple myeloma and related disorders. Haematologica 93: 431–438. doi: 10.3324/haematol.11080 [DOI] [PubMed] [Google Scholar]
- 25.Sarasquete ME, García-Sanz R, González D, Martínez J, Mateo G, Martínez P, et al. (2005) Minimal residual disease monitoring in multiple myeloma_ a comparison between allelic-specific oligonucleotide real-time quantitative polymerase chain reaction and flow cytometry. Haematologica 90:1365–1372. [PubMed] [Google Scholar]
- 26.Rawstron AC, Davies FE, DasGupta R, Ashcroft AJ, Patmore R, Drayson MT, et al. (2002) Flow cytometric disease monitoring in multiple myeloma: the relationship between normal and neoplastic plasma cells predicts outcome after transplantation. Blood 100: 3095–3100. doi: 10.1182/blood-2001-12-0297 [DOI] [PubMed] [Google Scholar]
- 27.San Miguel JF, Gutiérrez NC, Mateo G, Orfao A (2006) Conventional diagnostics in multiple myeloma. European Journal of Cancer 42: 1510–1519. doi: 10.1016/j.ejca.2005.11.039 [DOI] [PubMed] [Google Scholar]
- 28.Munshi NC, Anderson KC, Bergsagel PL, Shaughnessy J, Palumbo A, Durie B, et al. (2011) Consensus recommendations for risk stratification in multiple myeloma report of the International Myeloma Workshop Consensus Panel 2. Blood 117:4696–4700. doi: 10.1182/blood-2010-10-300970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, et al. (2005) International staging system for multiple myeloma. J Clin Oncol 23: 3412–3420. doi: 10.1200/JCO.2005.04.242 [DOI] [PubMed] [Google Scholar]
- 30.Kumar S, Witzig TE, Greipp PR, Rajkumar SV (2003) Bone marrow angiogenesis and circulating plasma cells in multiple myeloma. Br J Haematol 122(2):272–274. [DOI] [PubMed] [Google Scholar]
- 31.Paiva B, Paino T, Sayagues JM, Garayoa M, San-Segundo L, Martín M, et al. (2013) Detailed characterization of multiple myeloma circulating tumor cells shows unique phenotypic, cytogenetic, functional, and circadian distribution profile. Blood 122(22):3591–3598. doi: 10.1182/blood-2013-06-510453 [DOI] [PubMed] [Google Scholar]
- 32.Ghobrial IM (2012) Myeloma as a model for the process of metastasis: implications for therapy. Blood 120: 20–30. doi: 10.1182/blood-2012-01-379024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
Odd ratio (OR) higher than 1 indicate that CPCs were more frequently detected in patients with increased DS stage.
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.