Abstract
Purpose
The purpose of this study was to evaluate and compare the MP-1S (Nidek Technologies, Padova, Italy) and the S-MAIA (CenterVue, Padova, Italy) for mesopic and scotopic fundus-controlled perimetry (FCP) in age-related macular degeneration (AMD).
Methods
Eleven eyes from 11 patients underwent mesopic and, after 30 minutes of dark adaptation, scotopic (MP-1S: Goldmann V, 200 ms, background luminance 0.0032 cd/m2; S-MAIA: Goldman III, 200 ms, background luminance <0.0001 cd/m2) FCP. For the S-MAIA device, cyan (505 nm) and red (627 nm) scotopic FCP were performed. For both devices, a grid of 56 stimulus points covering 16° of the central macula was used. Examination time, fixation stability, and threshold values were analyzed.
Results
The upper end of the dynamic range (≤4 dB of lowest threshold) was frequently reached by the MP-1S for mesopic testing (median 34 of 56 stimuli), while threshold values within the lower 4 dB of the dynamic range were occasionally found with the S-MAIA for scotopic testing (median 3 for cyan, median 2 for red). After correction of the stimulus intensity for the S-MAIA results, the median difference for all stimuli between both devices for mesopic testing was −2.0 dB (interquartile range [−4;0], range –14 to 6).
Conclusions
The results indicate that robust testing of mesopic and scotopic function is feasible with both devices in patients with AMD, although both devices are susceptible to floor and ceiling effects.
Translational Relevance
The interpretation and particularly the comparison of both scotopic and mesopic FCP results between the MP-1S and the S-MAIA in AMD eyes need to consider variable susceptibility of floor and ceiling effects. Further software updates are desirable as FCP captures visual functional loss that is not noted with best-corrected central visual acuity and is important for clinical trials in AMD.
Keywords: fundus-controlled perimetry, microperimetry, scotopic, mesopic, MP-1S, S-MAIA, drusen, age-related macular degeneration
Introduction
Age-related macular degeneration (AMD) is a leading cause of irreversible vision loss.1–3 In order to reduce the burden of late AMD, novel interventions that stop or delay progression from intermediate to late AMD are needed.
With the development of high-resolution imaging, an improved detection and monitoring of structural changes in different retinal diseases, including AMD, has become possible.4–6 Although retinal imaging appears to be an important tool for the assessment of efficacy and safety of new therapeutic interventions, there is an unmet need for robust, reliable, and meaningful tests that allow the direct assessment of functional impairment and that are also accepted by regulatory agencies, health technology assessment bodies, and payers.
The gold standard for functional testing in clinical studies is the assessment of best-corrected central visual acuity (BCVA).7, 8 Obviously, BCVA does not reflect the complete visual function. In nonexudative AMD, BCVA is considered to be particularly insensitive to both early visual impairment and progressive decline in visual function over time. This is because visual disabilities under dim light condition, delayed dark adaptation, and paracentral scotomas—due to initial development of photoreceptor degeneration typically outside the central macula (“foveal sparing”)—are not captured by BCVA.7, 9–12
Fundus-controlled perimetry (FCP), which allows for precise assessment of retinal sensitivity thresholds at specific retinal areas, represents a potentially attractive tool to assess visual impairment in AMD that may be used in clinical trials to evaluate the efficacy of novel interventions. Previous studies evaluating FCP in AMD patients have reported reduced mesopic sensitivities over drusen, pigmentary changes, or geographic atrophy.8,13 Furthermore, using the MP-1S device (Nidek Technologies, Padova, Italy), it has been reported that scotopic dysfunction appeared to occur earlier and to a larger extent as compared to mesopic sensitivity impairment in eyes with AMD.14,15 We have recently shown that the presence of reticular drusen is spatially confined to an impairment of scotopic function and associated with outer retinal thinning on spectral-domain optical coherence tomography (SD-OCT).16
Recently, another FCP device for scotopic testing of retinal sensitivity, the S-MAIA (Macular Integrity Assessment, S-MAIA; CenterVue, Padova, Italy) has been introduced. This instrument allows for mesopic testing with achromatic stimuli as well as dark-adapted two-color scotopic perimetry with cyan (505 nm) and red (627 nm) stimuli.17 Early data confirmed the assumption that scotopic cyan testing is largely derived from rod photoreceptor-mediated function while scotopic red testing stimulates both the cone-mediated and the rod-mediated responses.18 In patients with glaucoma, different visual field instruments (MAIA, MP-3, and Humphrey field analyzer) revealed similar structure–function relationships.19
While particularly scotopic as compared to mesopic FCP appears to be meaningful in AMD patients, the assessment of scotopic function is obviously more complex and time-consuming, requires dark adaptation and a completely darkened room, and is more prone to confounding factors. Under these circumstances, it is of interest, beyond the pure technical setup and settings, to gain further knowledge of the practical application and possible differences between the two commercially available FCP systems to be used in the context of larger prospective clinical studies in AMD.
The aim of this pilot study was to compare the MP1-S and the S-MAIA system in eyes with AMD with regard to examination duration, fixation stability, and threshold values.
Methods
Subjects
Participants were recruited from the outpatient clinics at the Department of Ophthalmology, University of Bonn, Germany. Informed written consent was obtained from each subject after explanation of the nature and possible consequences of participating in retinal imaging research and functional testing. The study followed the tenets of the Declaration of Helsinki and was approved by the local ethics committee (Ethik-Kommission, Medizinische Fakultät, Rheinische Friedrich-Wilhelms-Universität; Lfd. Nr. 125/14 and Nr. 191/16).
One eye per subject was included in the study. For inclusion, the study eye had to be diagnosed with AMD (according to the classification system by Ferris et al.)20 and a minimum BCVA of 0.1 logMAR (Snellen 0.8). Only eyes with clear media to allow central visual function testing and retinal imaging were enrolled in the study. Exclusion criteria were refractive errors >5.00 diopters of spherical equivalent and >1.50 diopters of astigmatism, as well as history of glaucoma, retinal diseases other than AMD, uveitis, diabetes, history of intravitreal injections within the last 3 months, prior enrollment, or any history of prior vitreoretinal surgery and retinal laser treatment. If both eyes met the inclusion criteria, the better seeing eye was assigned as the study eye.
All subjects underwent a complete ophthalmological examination, including BCVA, slit-lamp examination, and fundoscopy. In addition, imaging and functional tests were performed.
Imaging Protocol
Retinal imaging was performed according to standardized operating procedures. After dilation of the pupils with 1.0% tropicamide and 2.5% phenylephrine, color fundus camera photography of the central macula was obtained using the Visucam 500 (Carl Zeiss Meditec AG, Jena, Germany). High-speed combined and simultaneous confocal scanning laser ophthalmoscopy (cSLO) and SD-OCT imaging (768 × 768 pixel) was performed with the Spectralis HRA+OCT (Heidelberg Engineering, Heidelberg, Germany) device and included the acquisition of central 30° × 30° near-infrared reflectance (IR; λ = 820 nm, ART [automatic real time] at least 15 frames) and fundus autofluorescence (FAF; excitation λ = 488 nm, emission λ = 500–800 nm, at least 15 frames) images. In addition, single-horizontal and vertical combined cSLO+SD-OCT scans through the fovea (30°, ART at least 15 scans) and a raster scan (30° × 25°, ART at least four frames, 61 B-scans, distance 120 μm) were recorded.
Fundus-Controlled Perimetry
All subjects underwent mesopic and scotopic FCP examinations of the central retina in the study eye following pupil dilatation using both the MP-1S device (Nidek Technologies) and the modified Macular Integrity Assessment device (S-MAIA, CenterVue).22 Examinations were split into two different visits; that is, subjects underwent MP-1S and S-MAIA exams on 2 different days. Prior to testing, instructions regarding the testing procedure were given, and the correct operation of the subject response trigger was practiced. For both devices, the same main test grid of 56 stimulus points, centered on the fovea, was used. The specific settings for both devices and the protocols for all types of functional testing have been described previously and are listed in Table 1.8,15,17,21,22 Briefly, FCP using the MP-1S device started with mesopic testing under light-adapted conditions, followed by dark adaptation of 30 minutes, the filter selection exam, and finally two consecutive scotopic tests. Only eyes with the 2.0 neutral density filter and a difference between the total mean values of both scotopic assessments ≤3 dB were included for the additional testing with the S-MAIA device. In addition, only subjects with an exceptional motivation were chosen.
Table 1.
Specific Settings for Both Fundus-Controlled Perimetry Devices
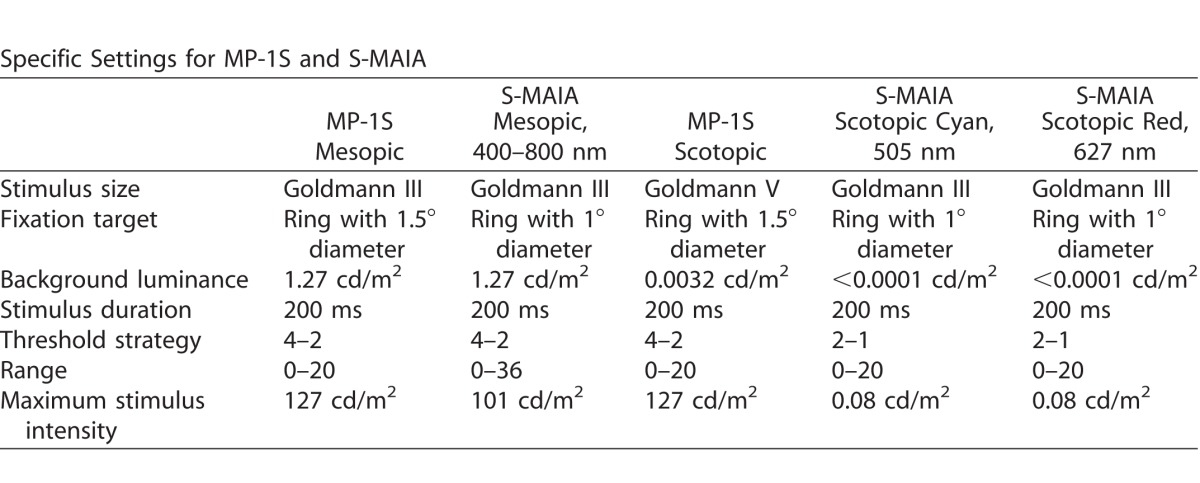
Fundus-controlled perimetry using the modified S-MAIA device also started mesopic testing under light-adapted conditions, followed by dark adaptation of 30 minutes, then scotopic cyan, and finally scotopic red testing using the same previously mentioned grid.
Statistical Analysis
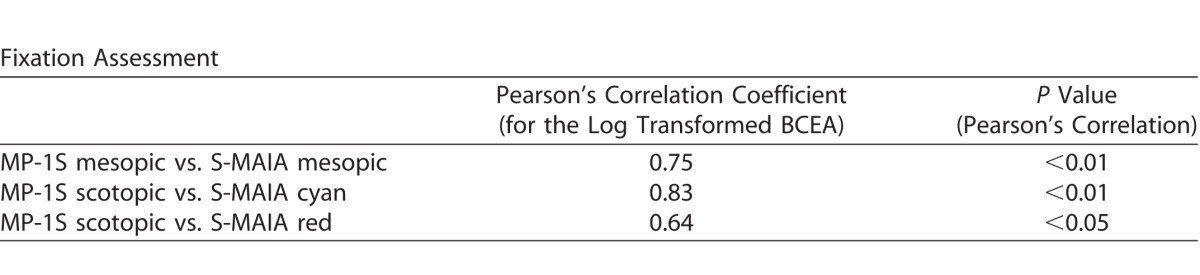
Statistical analysis included frequency and descriptive statistics. Results for BCVA were converted to the logMAR equivalent.23 Test duration and fixation data were recorded during all perimetry sessions and manually entered into Excel (Microsoft, Bellevue, WA). Fixation data was summarized as single overall measure by calculating the area of an ellipse that encompasses 63% of the fixation points (bivariate contour ellipse area [BCEA], 63%).21 Bivariate contour ellipse area values were log transformed to obtain normally distributed data. Pearson's correlation coefficient was used to determine the correlation of the fixation assessment among the two devices.
The threshold values of mesopic and scotopic examinations for both devices were evaluated and compared to each other. Firstly, the mean values of all 56 threshold values were separately calculated per eye. Secondly, due to the fact that drusen and other AMD-related changes are mostly found within the central 4° whereas the periphery is often spared, the central 16 stimulus points were compared to the 40 peripheral stimulus points.24 Thirdly, in order to evaluate the number of stimulus points reaching the border area of the sensitivity range (floor and ceiling effects), the number of test points within the upper and lower 4 dB of the sensitivity range were counted for each eye. Finally, the absolute retinal sensitivity values at each stimulus location for mesopic testing of the two systems were directly compared by subtracting 4 dB of each individual threshold value of S-MAIA testing. This correction was based on the previous report by Vujosevic and Casciano17 who have reported that the intensity scale (differential luminance) was approximately 4 dB higher for the S-MAIA as compared to the MP-1S.
Results
Eleven eyes of 11 patients (six men and five women) were included. The median age was 74.4 years (interquartile range [IQR] [67;79], range 59–86). The median visual acuity was 0.0 logMAR (IQR 0.0–0.1, range 0.0–0.1; Snellen 1.0–0.8). According to the AMD classification system proposed by Ferris et al.,20 nine eyes were classified as intermediate AMD and two eyes as late AMD (both inactive extrafoveal choroidal neovascularization, i.e., outside the central subfield of the Early Treatment in Diabetes Retinopathy Study [ETDRS] grid). Five eyes were pseudophakic and six phakic, respectively.
The difference in the median duration of testing between both devices (24.0 minutes, IQR [23;26], range 19–30) for the MP1-S device (mesopic, filter test, and scotopic examination) and a median of 25.4 minutes (IQR [24;26], range 22–27) for the S-MAIA device (mesopic, scotopic cyan, scotopic red examination) was not statistically significant (for details see Fig. 1; P = 0.328). The results of the fixation assessment (63% BCEA) were correlated for different types of testing between the two devices (Table 2) and showed similar results for both devices.
Figure 1.
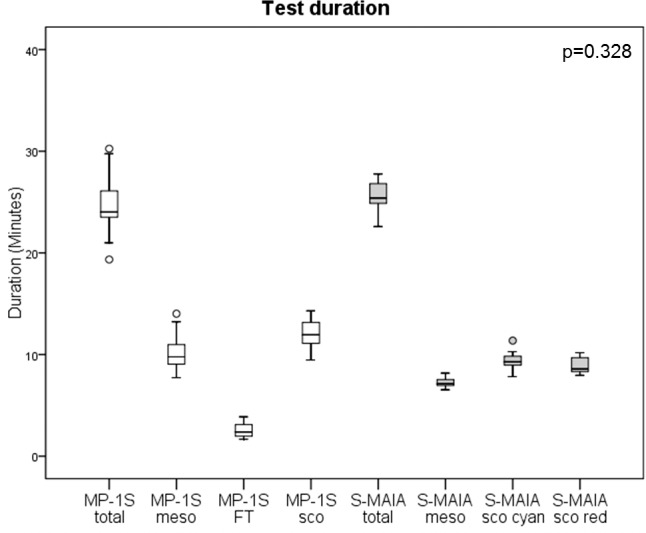
Test duration for the MP-1S device (total time, mesopic, filter test, and scotopic examination) and the S-MAIA device (total time, mesopic, scotopic cyan, and scotopic red examination).
Table 2.
Results of the Fixation Assessment
The median mesopic sensitivity was 17.2 dB (IQR [16;17], range 13–18), using the MP-1S and 22.7 dB (IQR [22;24], range 20–24; P = 0.003) using the S-MAIA. The median sensitivities for the first scotopic examination of the MP-1S (15.1 dB; IQR [14;15], range 12–17) was higher than the median sensitivity for the scotopic examinations of the S-MAIA device (scotopic cyan: 10.0 dB; IQR [9;11], range 7–13; scotopic red: 9.3 dB; IQR [8;10], range 5–11) (both P = 0.003). Figure 2 and 3 show representative examples.
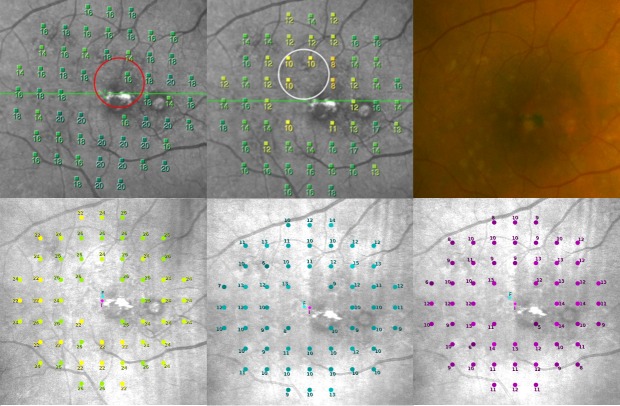
Figure 2.
A representative example of the FCP of a patient with drusen in the central macula (color image upper right, upper left MP-1S mesopic, lower left S-MAIA mesopic, upper middle MP-1S scotopic, lower middle and right S-MAIA scotopic cyan and red).
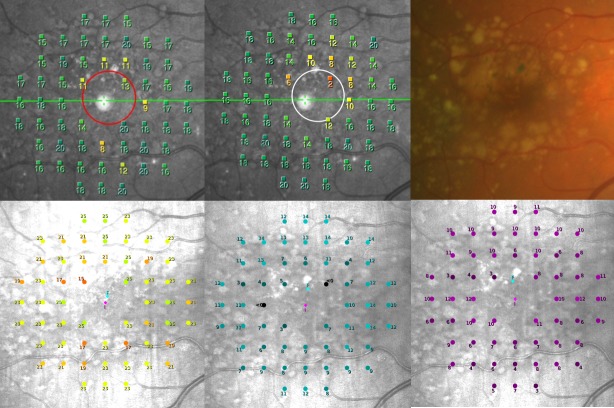
Figure 3.
A representative example of FCP of a patient with confluent drusen and pigmentary changes (color image upper right, upper left MP-1S mesopic, lower left S-MAIA mesopic, upper middle MP-1S scotopic, lower middle and right S-MAIA scotopic cyan and red).
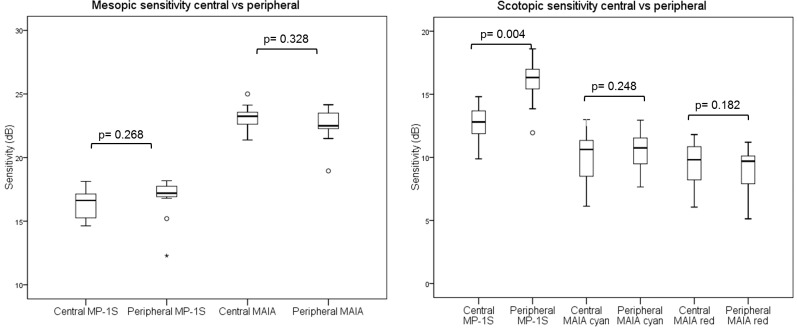
For both devices, no significant differences were found for mesopic testing between central and peripheral stimulus points (Fig. 4, left, MP-1S: P = 0.268; S-MAIA P = 0.328). Using the MP-1S device, a significant reduction of scotopic sensitivity for central testing (median 12.8 dB; IQR [11;13], range 9–14) compared to peripheral analysis (median 16.3 dB; IQR [15;17], range 11–18) was found (P = 0.004).
Figure 4.
The central and peripheral sensitivity values for mesopic (left) and scotopic (right) testing.
For the scotopic cyan and red examinations of the S-MAIA device (Fig. 4, right), no significant differences between central and peripheral locations were found, respectively (P = 0.248 and P = 0.182).
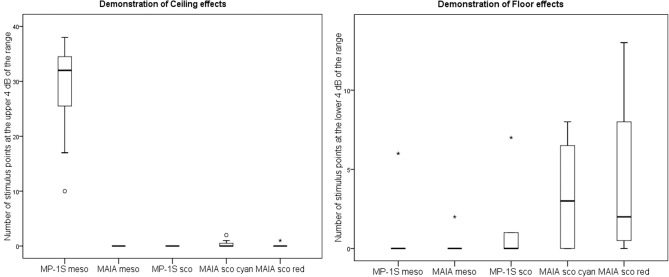
The analysis for ceiling effects revealed that the upper 4 dB of the dynamic range was frequently reached for mesopic testing with the MP-1S device (median 34 stimulus points, IQR [28;35], range 10–40) (Fig. 5, left). By contrast, mesopic testing using the S-MAIA device did not show any stimulus point at the upper 4 dB of the dynamic range (e.g., no point with a value of 33–36 dB). For scotopic testing with both devices, there were only very few points in individual eyes reaching the upper 4 dB of the dynamic range (i.e., only 1–2 stimulus points showed a sensitivity value of 17–20).
Figure 5.
Both devices (MP-1S and S-MAIA) are susceptible to ceiling (left) and floor (right) effects for different types of mesopic and scotopic testing.
The analysis for floor effects disclosed that the lower 4 dB of the dynamic range was seldom reached for mesopic testing for both devices (MP-1S: median 0 stimulus points, IQR [0;0], range 0–6); S-MAIA: median 0 stimulus points (IQR [0;0], range 0–2) (Fig. 5, right). For scotopic testing with the MP-1S, a median of 0 stimulus points (IQR [0;1.5], range 0–7) was detected, whereas using the S-MAIA, a median of 3 stimulus points (IQR [0;6], range 0–8) was seen for scotopic cyan and a median of 2 stimulus points (IQR [0.5;8], range 0–13) was found for scotopic red testing.
The direct comparison of absolute sensitivity values for mesopic testing of each stimulus location between both systems by the subtraction of 4 dB for each stimulus of the S-MAIA assessment (according to Vujosevic and Casciano17) disclosed a median difference of −2.0 dB (IQR [−4;0], range −14 to 6).
Discussion
The results of this study suggest that both robust testing of both mesopic and scotopic FCP is well feasible with both systems in eyes with AMD and good visual acuity.
Beyond the differences in the technical setup, the variability of the overall sensitivity values and sensitivity ranges for different types of testing indicate that not only the direct comparison of threshold values between the two systems are limited. Further, these findings suggest that the devices are susceptible to floor and ceiling effects to different degrees. In this context, sensitivity values within the upper limit of the dynamic range were particularly evident and occurred in every eye with mesopic testing using the MP-1S device. This observation confirms that the MP-1S system is not able to reliably detect slight reductions in mesopic retinal function while the likelihood for ceiling effects with the S-MAIA system for mesopic function appears to be negligible, given the median sensitivity of 22.7 dB (maximum 24 dB) and the upper limit of 36 dB.8,15,25 The advantage of an increased dynamic range of the mesopic S-MAIA was already noticed by Parodi et al.26 in patients with retinal dystrophies.
The lower end of the dynamic range for scotopic function tended to be more often reached by both scotopic cyan and scotopic red testing of the S-MAIA device as compared to scotopic testing of the MP-1S. For the latter, only a few points were within the lower range of 4 dB. However, it must be noted that MP-1S scotopic testing initially involved a filter selection exam and only eyes with the 2.0 neutral density filter were included. Therefore, the limited dynamic range, particularly for scotopic testing, remains a major limitation for both devices. We believe that it will be of great benefit if further developments of the S-MAIA device would lead to an extension toward the lower thresholds for stimulus intensities.
The median difference for individual mesopic threshold values of the MP-1S was approximately 2 dB lower than those of the S-MAIA, which is in the range of the approximate individual difference of 4 dB for stimuli intensities as reported by Vujosevic and Casciano.17 This finding suggests that individual threshold values for mesopic testing between the two systems can be roughly compared to each other in AMD eyes, provided floor and ceiling effects are considered (i.e., values close to the upper and lower dynamic range require particular attention). For scotopic testing, already due to the predefined difference in stimuli size, such a mathematical correction appears not to be reasonable. As previously recommended, follow-up examinations should be performed by the same FCP device.27
Interestingly, only the scotopic testing of the MP-1S revealed a significant reduction in overall sensitivity for the central stimulus points, which are mostly affected by AMD changes compared to the peripheral stimulus points that represent most often an unaffected retina. Only slight changes were found for mesopic testing. Along with the observation that such differences were not indicated by S-MAIA testing, we would be reluctant to interpret that the MP-1S findings clearly suggested that rod function would be more severely affected as compared to cone function in the central macula. Another possible explanation for this difference in scotopic and mesopic function between central and peripheral retinal locations could be caused by the limited dynamic range toward the upper limit for mesopic testing of the MP1-S. Of note, this pilot study was not designed to topographically correlate changes in retinal sensitivity to structural alterations as detected by retinal imaging in eyes with AMD.
The test duration with an average of 24 to 25 minutes (excluding the time of dark adaptation) was comparable to previous reports that used either one of the two devices.15,25 Although FCP is time-consuming, the current study demonstrates that this functional assessment may be used in clinical trials. However, for the S-MAIA device, two different scotopic testings were performed, which offers the potential advantage to better differentiate between cone and rod function by employing both cyan and red scotopic testing. For scotopic MP-1S testing, the use of different neutral density filters–depending on the overall scotopic retinal sensitivity of individual eyes as determined by the initial filter selection exam–represents a disadvantage. We are not aware of any reliable and meaningful method to mathematically correct and then compare test results of individual threshold values that have been determined using different neutral density filters.
Both devices yielded similar results with regard to fixation stability as shown by the strong correlation of the BCEA measurements. However, the size of the fixation target differed between the S-MAIA (1° diameter) and the MP1-S (1.5° diameter). The difference in size of the fixation target most likely explains the small differences in the BCEA measurements. Indeed, Bellmann and coworkers28 have demonstrated previously that fixation stability is dependent on the type of fixation target.
Only a relatively small number of subjects were included in this pilot study. As only patients with good BCVA (median logMAR 0.0) were included in the study, this study cannot indicate if additional differences between the two devices might occur in patients with worse BCVA. However, testing with both systems was considerably time-consuming and required exceptional patient motivation. While we cannot exclude that the results of this study might have been different if other settings had been chosen, most of these factors were not modifiable by the operator. Overall, we believe that this pilot study is helpful for the design of future large-scale studies in AMD patients looking at more detail in impairment of both mesopic and scotopic FCP.
In summary, herein we compared two different commercially available FCP systems that allow for both mesopic and scotopic retinal sensitivity testing of eyes with AMD. Differences in sensitivity values and major challenges concerning the sensitivity ranges resulting in ceiling and floor effects were demonstrated. Examination time and fixation stability were similar with both devices. The utility of dark-adapted two-color perimetry in other macular diseases remains to be shown. Of note, Fraser and coworkers29 recently reported with another device that the cyan–red difference might represent a relevant biomarker in the context of age-related macular disease. Future hardware and software updates of both devices might become available and improve the performance so that functional results may get an outcome measure in future clinical trials.
Acknowledgments
Supported by Gertrud Kusen Foundation; University of Bonn Bonfor Grant O-137.0021(to JSS) and Grant O-137.0022 (to MP); DFG Grant FL 658/4-1 and FL 658/4-2.
The sponsors or funding organizations had no role in the design or conduct of this research.
Disclosure: J.S. Steinberg, Carl Zeiss Meditec (F), Heidelberg Engineering (F), Optos (F); M. Saßmannshausen, Carl Zeiss Meditec (F), Heidelberg Engineering (F), Optos (F); M. Pfau, Carl Zeiss Meditec (F), Heidelberg Engineering (F), Optos (F); M. Fleckenstein, Alcon/Novartis (C, F), Bayer (R), Carl Zeiss MediTec (F), Heidelberg Engineering (F, R), Optos (F), Genentech/Roche (C, F, R F), R. P. Finger Bayer (C), Novartis (C), Santen (C), Opthea (C), QLTC; Novartis (F); F.G. Holz, Acucela (C, F), Allergan (F, R), Bayer (C, F, R), Bioeq (C, F), Boehringer-Ingelheim (C), Carl Zeiss MediTec (F, R), Genentech/Roche (C, F, R), Heidelberg Engineering (C, F, R), Merz (F), NightstarX (F), Novartis (C, F, R), Optos (F), Thea (C, S); S. Schmitz-Valckenberg, Alcon/Novartis (C, F), Allergan (F, R), Bayer (F, R), Carl Zeiss MediTec (F), Formycon (F), Heidelberg Engineering (F, R), Optos (F), Genentech/Roche (C, F, R)
References
- 1. Bourne RR,, Jonas JB,, Flaxman SR,, et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe: 1990-2010. Br J Ophthalmol. 2014; 98: 629– 638. [DOI] [PubMed] [Google Scholar]
- 2. Congdon N,, O'Colmain B,, Klaver CC,, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004; 122: 477– 485. [DOI] [PubMed] [Google Scholar]
- 3. Resnikoff S,, Pascolini D,, Etya'ale D,, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004; 82: 844– 851. [PMC free article] [PubMed] [Google Scholar]
- 4. Khanifar AA,, Koreishi AF,, Izatt JA,, Toth CA. Drusen ultrastructure imaging with spectral domain optical coherence tomography in age-related macular degeneration. Ophthalmology. 2008; 115: 1883– 1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmitz-Valckenberg S,, Alten F,, Steinberg JS,, et al. Reticular drusen associated with geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011; 52: 5009– 5015. [DOI] [PubMed] [Google Scholar]
- 6. Leuschen JN,, Schuman SG,, Winter KP,, et al. Spectral-domain optical coherence tomography characteristics of intermediate age-related macular degeneration. Ophthalmology. 2013; 120: 140– 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lindner M,, Boker A,, Mauschitz MM,, et al. Directional kinetics of geographic atrophy progression in age-related macular degeneration with foveal sparing. Ophthalmology. 2015; 122: 1356– 1365. [DOI] [PubMed] [Google Scholar]
- 8. Midena E,, Vujosevic S,, Convento E,, Manfre A,, Cavarzeran F,, Pilotto E. Microperimetry and fundus autofluorescence in patients with early age-related macular degeneration. Br J Ophthalmol. 2007; 91: 1499– 1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schmitz-Valckenberg S,, Bultmann S,, Dreyhaupt J,, Bindewald A,, Holz FG,, Rohrschneider K. Fundus autofluorescence and fundus perimetry in the junctional zone of geographic atrophy in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci 2004; 45: 4470– 4476. [DOI] [PubMed] [Google Scholar]
- 10. Forte R,, Querques G,, Querques L,, Leveziel N,, Benhamou N,, Souied EH. Multimodal evaluation of foveal sparing in patients with geographicatrophy due to age-related macular degeneration. Retina. 2013; 33: 482– 489. [DOI] [PubMed] [Google Scholar]
- 11. Sunness JS,, Gonzalez-Baron J,, Applegate CA,, et al. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology. 1999; 106: 1768– 1779. [DOI] [PubMed] [Google Scholar]
- 12. Sunness JS,, Rubin GS,, Zuckerbrod A,, Applegate CA. Foveal-sparing scotomas in advanced dry age-related macular degeneration. J Vis Impair Blind. 2008; 102: 600– 610. [PMC free article] [PubMed] [Google Scholar]
- 13. Pilotto E,, Guidolin F,, Convento E,, et al. Fundus autofluorescence and microperimetry in progressing geographic atrophy secondary to age-related macular degeneration. Br J Ophthalmol. 2013; 97: 622– 626. [DOI] [PubMed] [Google Scholar]
- 14. Nebbioso M,, Barbato A,, Pescosolido N. Scotopic microperimetry in the early diagnosis of age-related macular degeneration: preliminary study. Biomed Res Int. 2014; 2014: 671529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steinberg JS,, Fitzke FW,, Fimmers R,, Fleckenstein M,, Holz FG,, Schmitz-Valckenberg S. Scotopic and photopic microperimetry in patients with reticular drusen and age-related macular degeneration. JAMA Ophthalmol. 2015; 133: 690– 697. [DOI] [PubMed] [Google Scholar]
- 16. Steinberg JS,, Sassmannshausen M,, Fleckenstein M,, et al. Correlation of partial outer retinal thickness with scotopic and mesopic fundus-controlled perimetry in patients with reticular drusen. Am J Ophthalmol. 2016; 168: 52– 61. [DOI] [PubMed] [Google Scholar]
- 17. Vujosevic SC,, Casciano M. Microperimetry: technical remarks. : Midena E, Microperimetry and Multimodal Retinal Imaging. Berlin: Springer; 2014: 13– 22. [Google Scholar]
- 18. Sloan LL. The threshold gradients of the rods and the cones; in the dark-adapted and in the partially light-adapted eye. Am J Ophthalmol. 1950; 33: 1077– 1089. [DOI] [PubMed] [Google Scholar]
- 19. Hirooka K,, Misaki K,, Nitta E,, Ukegawa K,, Sato S,, Tsujikawa A. Comparison of Macular Integrity Assessment (MAIA) , MP-3, and the Humphrey Field Analyzer in the evaluation of the relationship between the structure and function of the macula. PLoS One. 2016; 11: e0151000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferris FL,, 3rd,, Wilkinson CP,, Bird A,, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013; 120: 844– 851. [DOI] [PubMed] [Google Scholar]
- 21. Crossland MD,, Dunbar HM,, Rubin GS. Fixation stability measurement using the MP1 microperimeter. Retina. 2009; 29: 651– 656. [DOI] [PubMed] [Google Scholar]
- 22. Pfau M,, Lindner M,, Fleckenstein M,, et al. Test-retest reliability of scotopic and mesopic fundus-controlled perimetry using a modified MAIA (Macular Integrity Assessment) in normal eyes. Ophthalmologica. 2017; 237: 42– 54. [DOI] [PubMed] [Google Scholar]
- 23. Holladay JT. Proper method for calculating average visual acuity. J Refract Surg. 1997; 13: 388– 391. [DOI] [PubMed] [Google Scholar]
- 24. Rogala J,, Zangerl B,, Assaad N,, Fletcher EL,, Kalloniatis M,, Nivison-Smith L. In vivo quantification of retinal changes associated with drusen in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015; 56: 1689– 1700. [DOI] [PubMed] [Google Scholar]
- 25. Crossland MD,, Luong VA,, Rubin GS,, Fitzke FW. Retinal specific measurement of dark-adapted visual function: validation of a modified microperimeter. BMC Ophthalmol. 2011; 11: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parodi MB,, Triolo G,, Morales M,, et al. Mp1 and maia fundus perimetry in healthy subjects and patients affected by retinal dystrophies. Retina. 2015; 35: 1662– 1669. [DOI] [PubMed] [Google Scholar]
- 27. Wong EN,, Mackey DA,, Morgan WH,, Chen FK. Inter-device comparison of retinal sensitivity measurements: the CenterVue MAIA and the Nidek MP-1. Clin Exp Ophthalmol. 2016; 44: 15– 23. [DOI] [PubMed] [Google Scholar]
- 28. Bellmann C,, Feely M,, Crossland MD,, Kabanarou SA,, Rubin GS. Fixation stability using central and pericentral fixation targets in patients with age-related macular degeneration. Ophthalmology. 2004; 111: 2265– 2270. [DOI] [PubMed] [Google Scholar]
- 29. Fraser RG,, Tan R,, Ayton LN,, Caruso E,, Guymer RH,, Luu CD. Assessment of retinotopic rod photoreceptor function using a dark-adapted chromatic perimeter in intermediate age-related macular degeneration. Invest Ophthalmol Vis Sci. 2016; 57: 5436– 5442. [DOI] [PubMed] [Google Scholar]